Significance
Selection of actions that allow the seeking of rewards and avoidance of uncomfortable environments is a fundamental animal behavior. Here, we report an in vivo method, in which the activities of PKA and ERK were optically recorded by microendoscopy of Förster resonance energy transfer responses of biosensors in distinct D1 and D2 dopamine receptor-expressing neurons of the dorsal striatum. The PKA and ERK were coordinately but reciprocally regulated not only by rewarding and aversive stimuli but also between the two parallel projection neurons. Importantly, the cell type-specific regulation of PKA and ERK was causally linked to active and indifferent mating reactions of male mice. The dynamic modulation of PKA and ERK in the striatum underlies the selection of alternative actions.
Keywords: in vivo FRET imaging, microendoscope, dorsal striatum, action selection, mating behavior
Abstract
The selection of reward-seeking and aversive behaviors is controlled by two distinct D1 and D2 receptor-expressing striatal medium spiny neurons, namely the direct pathway MSNs (dMSNs) and the indirect pathway MSNs (iMSNs), but the dynamic modulation of signaling cascades of dMSNs and iMSNs in behaving animals remains largely elusive. We developed an in vivo methodology to monitor Förster resonance energy transfer (FRET) of the activities of PKA and ERK in either dMSNs or iMSNs by microendoscopy in freely moving mice. PKA and ERK were coordinately but oppositely regulated between dMSNs and iMSNs by rewarding cocaine administration and aversive electric shocks. Notably, the activities of PKA and ERK rapidly shifted when male mice became active or indifferent toward female mice during mating behavior. Importantly, manipulation of PKA cascades by the Designer Receptor recapitulated active and indifferent mating behaviors, indicating a causal linkage of a dynamic activity shift of PKA and ERK between dMSNs and iMSNs in action selection.
In changing environments, animals are forced to choose actions among several alternatives to survive and to keep offspring for the next generation. A brain region critical for initiation and selection of actions is the striatum (1–3), and its dysfunction leads to devastating neurological and psychiatric disorders such as Parkinson’s disease and drug addiction (4–7). The striatum receives convergent glutamatergic inputs from virtually all cortical areas and the thalamus, and dopaminergic inputs from the substantia nigra pars compacta (SNc) and the ventral tegmental area (8, 9). The glutamatergic inputs convey various sensory, motor, and cognitive information and drive striatal medium spiny projection neurons (MSNs) to fire, whereas the dopaminergic inputs strongly influence synaptic transmission and excitability of MSNs (10, 11). MSNs are divided into two subpopulations, the direct pathway MSN (dMSN) expressing Gs-coupled dopamine D1 receptors and sending axons directly to the internal segment of the globus pallidus (GPi) and the substantia nigra pars reticulata (SNr), and the indirect pathway MSN (iMSN) expressing Gi-coupled D2 receptors and sending axons indirectly to the SNr via the external segment of the globus pallidus (GPe) and subthalamic nucleus (12, 13).
Intracellular signaling cascades operating in these two types of MSNs are important for plastic modification of synaptic transmission and excitability (14, 15). Among them, protein kinase A (PKA) and extracellular signal-regulated kinase (ERK) have been shown to be the key molecules in these signaling cascades (10, 14, 16). PKA is positively and negatively regulated by Gs-coupled D1 and Gi-coupled D2 receptors, respectively, and contributes to the regulation of a wide range of cellular substrates (10, 11, 16). ERK has been shown to sense coincidental dopaminergic and glutamatergic activations to induce protein synthesis and synaptic modification (17).
Although both the ventral and dorsal striatum are essential for naturally occurring reward-seeking and aversive behaviors, such as eating, mating, and escaping from uncomfortable environments (7, 18, 19), the dorsal striatum plays a key role in efficiently choosing suitable outcomes of actions (1, 2). Among naturally occurring rewarding behaviors, male mating behavior is particularly interesting, because it represents innate rapid selection behavior, sometimes actively seeking a female animal but at other times becoming impassive toward it (20, 21). However, little is known about how dMSNs and iMSNs are involved in such action selection. The poor understanding of dynamic intracellular signaling mechanisms in action selection behavior is mainly due to the lack of effective techniques to monitor time-lapsed changes in such activities in behaving animals. In this study, we developed an in vivo methodology in which genetically encoded Förster resonance energy transfer (FRET) biosensors of PKA and ERK (22) were specifically expressed in either dMSNs (D1-PKA and D1-ERK) or iMSNs (D2-PKA and D2-ERK), and cell-specific fluorescence changes in the active and inactive forms of PKA and ERK of the dorsal striatum were monitored by newly designed microendoscopy in freely moving mice (23, 24). We revealed that the activities of PKA and ERK in dMSNs and iMSNs rapidly shifted when male mice became active or impassive toward a female mouse during mating behavior and that the dynamic activity shift of PKA and ERK between dMSNs and iMSNs was causally linked to the selection of alternative actions.
Results
Development of FRET Biosensor-Expressing Transgenic Mice and FRET Microendoscopy.
We previously established a method to generate transgenic mice expressing FRET biosensors, with which the time lapse of activity changes in protein kinases can be imaged (25, 26). To achieve dMSN- and iMSN-specific labeling of the PKA and ERK FRET biosensors, we created two flox lines of transgenic mice, floxed-AKAR3EV (PKA) and floxed-EKAREV (ERK) (27), both of which contained a nuclear export signal to localize these kinases in the cytoplasm (Fig. 1A). Crossing of the two flox lines with D1-Cre and D2-Cre BAC transgenic mice (28) generated four species of transgenic mice: D1-PKA mice expressing AKAR3EV in dMSNs, D1-ERK mice expressing EKAREV in dMSNs, D2-PKA mice expressing AKAR3EV in iMSNs, and D2-ERK mice expressing EKAREV in iMSNs. We confirmed that the FRET biosensors in D1-PKA and D1-ERK mice were specifically expressed in dMSNs whose axons projected to the two main output nuclei of the basal ganglia: the GPi and the SNr (Fig. S1A). In D2-PKA and D2-ERK mice, the FRET biosensors were selectively expressed in iMSNs whose axons terminated in the GPe (Fig. S1A).
Fig. 1.
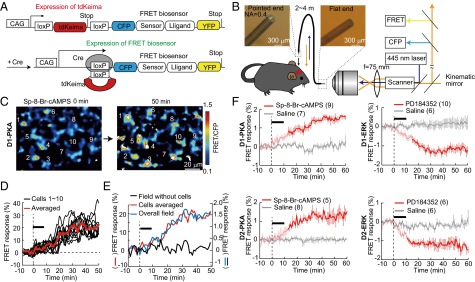
Live imaging of PKA and ERK activities in dMSNs and iMSNs of transgenic mice expressing FRET biosensors. (A) Construct for Cre-dependent expression of FRET biosensors. (B) Schema of the microendoscope system. (C) A representative microendoscopic image of FRET responses of 10 cellular structures of D1-PKA mice. (D) Changes in FRET responses of 10 cellular structures were calculated by subtracting background fluorescence levels and averaged (red). The black bar indicates the local application of Sp-8-Br-cAMPS (2 mM). (E) Changes in FRET responses of an entire field of view (blue) as calculated without subtracting background fluorescence (black) paralleled those of the averaged responses of individual cells (red). (F) FRET responses to local application (black bars) of Sp-8-Br-cAMPS (2 mM) and PD184352 (10 μM) into the dorsal striatum of anesthetized mice. In this and other figures, error bars (vertical lines) show SEM; deep colors in traces indicate statistically significant changes (P < 0.05; Mann–Whitney U test) from 0 min. The numbers of animals analyzed are given in parentheses.
FRET imaging of the striatum of freely moving mice was achieved by developing a fiber bundle-based microendoscope technique and using a confocal laser scanning microscope equipped with a 445-nm laser for excitation and a pair of band-path filters, 483 ± 16 nm for CFP and 542 ± 13 nm for FRET (Fig. 1B, Fig. S2, and Movie S1). On insertion of the endoscopic probe into the brain, excitation of the FRET biosensors was exclusively detected in the striatum but not in the cerebral cortex covering the striatum (Fig. S1B). When FRET responses (changes in the FRET/CFP ratio) were calculated for the individual cellular structures of the D1-PKA mice by subtracting background levels, the increase in FRET responses by application of a cAMP analog (8-bromoadenosine-3′,5′-cyclic monophosphorothioate, Sp isomer—Sp-8-Br-cAMPS) through a cannula attached to the side of the endoscope exceeded 20% and was comparable to that reported for cultured cells (22, 29) (Fig. 1 C and D and Fig. S3). Furthermore, the FRET responses calculated for an entire field of view corresponded well to the averaged FRET responses of each cellular structure (Fig. 1E). So this simple and practical approach to measure FRET responses in the entire field of view was adopted for quantification of FRET excitation in subsequent analyses.
In vivo administration of Sp-8-Br-cAMPS progressively increased FRET responses in both D1-PKA and D2-PKA mice (Fig. 1F, Left), but not in the striatum expressing phosphorylation site-mutated, FRET-negative PKA biosensors (Fig. S3D). Conversely, the MEK inhibitor 2-(2-chloro-4-iodophenylamino)-N-cyclopropylmethoxy-3, 4-difluorobenzamide (PD184352) gradually decreased FRET responses in both D1-ERK and D2-ERK mice (Fig. 1F, Right). These results showed that activities of PKA and ERK could be monitored by the FRET microendoscopic observation. No alteration in cell number, shape, or striatal organization was detected by direct visualization (Movie S1) or histological analysis with DAPI staining and anti-GFP and antityrosine hydroxylase immunostaining of the endoscope-implanted striatum (Fig. S2F).
PKA and ERK Responses of dMSNs and iMSNs to Cocaine Injection.
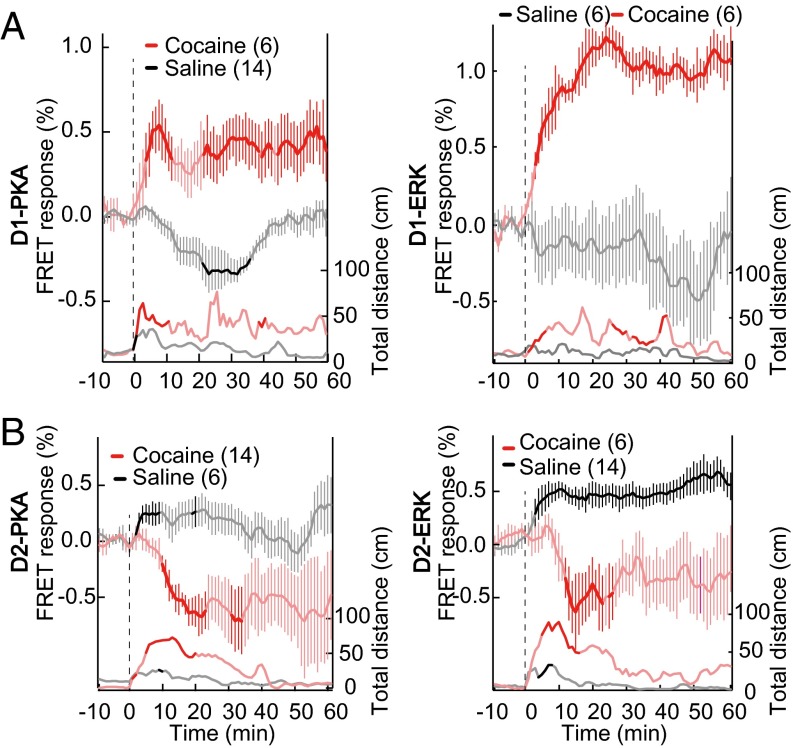
To explore molecular activities underlying rewarding action, we monitored activities of PKA and ERK in dMSNs and iMSNs upon cocaine injection. This stimulus is known to activate PKA and ERK of dMSNs and to induce hyperlocomotion by blocking dopamine uptake (30). Upon cocaine administration (25 mg/kg, i.p.), the PKA and ERK activities in dMSNs rapidly increased concomitantly with the increased locomotion (Fig. 2A). Interestingly, this increase in PKA and ERK activities continued up to at least 60 min after cocaine administration. Surprisingly, upon cocaine administration, both PKA and ERK activities decreased in the iMSNs (Fig. 2B). Previous immunohistochemical analyses did not find such a decrease in the activities of PKA and ERK (30), implying that the FRET biosensors were more sensitive, affording detection of a decrease from the baseline activities. In this series of experiments, cocaine was administered by i.p. injection. As a control for this cocaine administration, we injected saline without cocaine. Both D2-PKA and D2-ERK activities showed a significant increase in response to saline injection. The mesencephalic dopaminergic neurons are known to show transient suppression of firing in response to the aversive stimuli (2, 31), and this suppression of dopaminergic inputs supposedly leads to the activation of PKA and ERK in iMSNs through Gi-coupled D2 receptors. We thus hypothesized that the procedure of i.p. injection may have served as an aversive stimulus to induce D2-PKA and D2-ERK activation in iMSNs. We tested this hypothesis more directly by monitoring striatal PKA and ERK activities in response to aversive footshock stimuli (Fig. 3).
Fig. 2.
Reciprocal regulation of PKA and ERK activities between dMSNs and iMSNs in response to cocaine or saline injection. PKA and ERK activities of dMSNs (A) and iMSNs (B) (upper traces) and locomotor activities (lower traces) in response to cocaine (red) or saline (gray) injection. There was no significant difference in locomotor activities in response to cocaine or saline injection among four lines of transgenic mice (P > 0.05; Mann–Whitney U test).
Fig. 3.
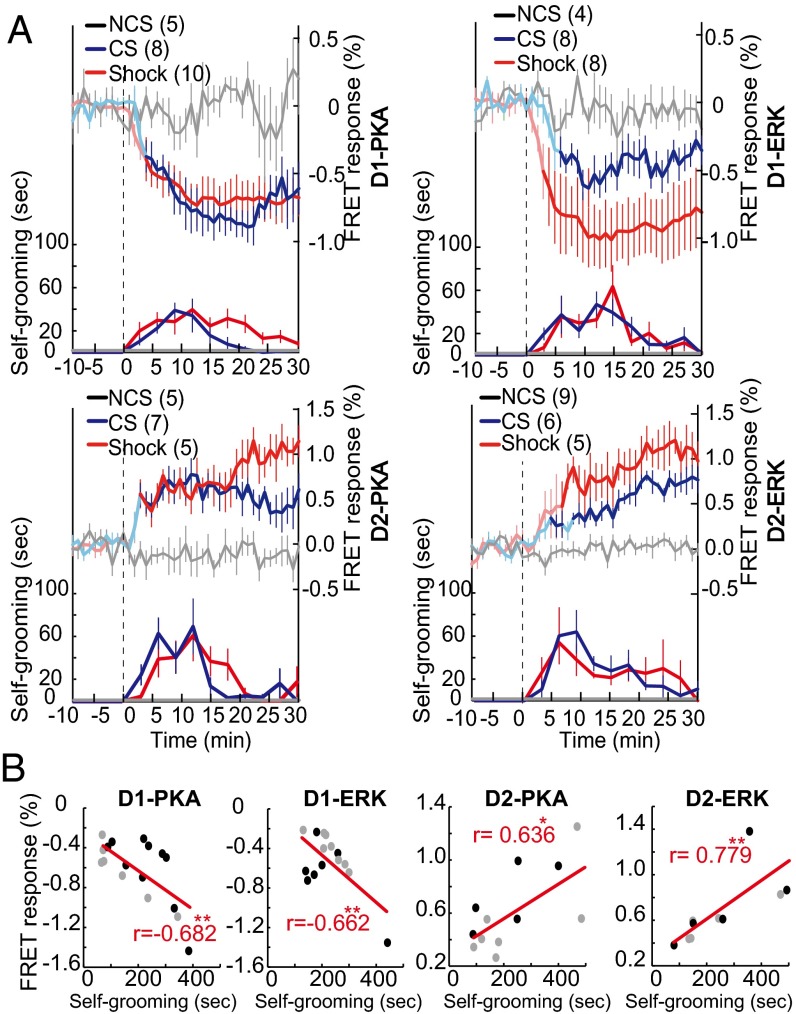
Reciprocal regulation of PKA and ERK activities between dMSNs and iMSNs in response to electric footshocks. (A) PKA and ERK activities (upper traces) and aversive self-grooming behavior (lower traces) in response to electric footshocks (Shock), bell sound without unconditioned stimulus (nonconditioned stimulus, NCS), and bell sound after conditioning with electric shocks (conditioned stimulus, CS). The time spent exhibiting self-grooming was measured every 3 min. The vertical dashed lines indicate a starting time point of these stimuli. (B) Average changes in FRET responses of PKA and ERK of dMSNs and iMSNs were plotted against the time spent exhibiting self-grooming behavior during the 30-min period. Black and gray circles represent electric shock and conditioned bell sound data, respectively, taken from A. The r value was calculated by use of Pearson correlation analysis; *P < 0.05, **P < 0.01.
PKA and ERK Responses of dMSNs and iMSNs Against Aversive Footshock Stimuli.
As for aversive reactions to electric footshock, we measured the time spent exhibiting a self-grooming behavior, which is a typical behavioral readout of an aversive reaction (32, 33). We then examined how PKA and ERK responses were correlated with the duration of self-grooming after electric footshocks. Electric footshocks (2 mA, 40 Hz for 2 s) markedly activated D2-PKA and D2-ERK and inactivated D1-PKA and D1-ERK (Fig. 3A, upper red), together with the concomitant emergence of a self-grooming behavior (Fig. 3A, lower red). Self-grooming was never observed in those mice not receiving the electric footshocks. In general, the duration of PKA and ERK activation and inactivation was longer than that of the self-grooming behavior.
In addition to an acute aversive reaction against a single electric footshock, we also addressed whether PKA and ERK could be involved in the induction of aversive learning by pairing electric shocks with the sound of a bell (Fig. 3A, blue). After habituation with repeated exposure to a bell sound (2 s in duration), an additional bell sound neither influenced the activities of PKA and ERK in dMSNs and iMSNs nor evoked a self-grooming behavior, indicating that a bell sound per se served as neutral information (Fig. 3A, gray). Notably, when the bell sound was conditioned with electric footshocks, this conditioned stimulus induced both PKA and ERK activation and inactivation in iMSNs and dMSNs, respectively, together with the concomitant expression of self-grooming behavior (Fig. 3A, blue). Importantly, the sum of duration exhibiting self-grooming behavior was correlated positively with the D2-PKA and D2-ERK activities and negatively with the D1-PKA and D1-ERK activities, respectively (Fig. 3B). Saline injection, which mildly activated D2-PKA and D2-ERK (Fig. 2B), also induced less but significant levels of self-grooming behavior (Figs. S4 and S5). However, there was no difference in extents of self-grooming behavior among the four lines of transgenic mice (Fig. S5). These results thus indicated a tight linkage between the D2-PKA and D2-ERK activation and the expression of aversive behaviors. Noteworthy also is that transient self-grooming was evoked immediately after cocaine injection but disappeared thereafter (Fig. S4). This finding strongly suggested that the opposing effects of cocaine administration on the PKA and ERK signaling cascades counteracted the aversive reaction induced by i.p. injection. Collectively, the FRET microendoscopy analysis thus allowed us to monitor dynamic and cell type-specific changes in the activities of PKA and ERK and demonstrated that the activities of PKA and ERK were reciprocally controlled not only by rewarding and aversive reactions but also between dMSNs and iMSNs.
PKA and ERK Responses in Male Mating Behavior.
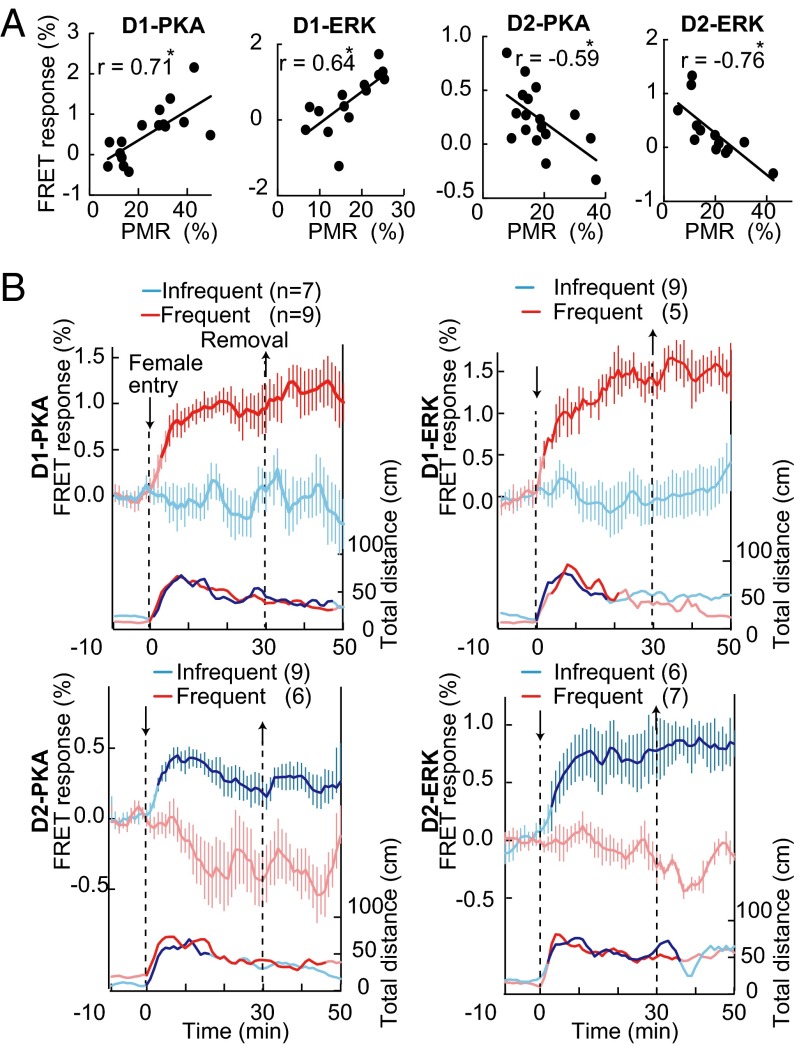
Mating reactions representing a naturally occurring reward-seeking behavior (18, 34) have been shown to induce an increase in dopamine concentrations in both ventral and dorsal striatum (35). The mating reactions were quantified by determining the percentages of time in which the male mouse exhibited characteristic mating behaviors (sniffing, grooming, and mounting) toward a female mouse (Movies S2–S9) (34) every 1 min during a 30-min period (% positive mating reaction, % PMR). A significant positive correlation was noted between the extent of D1-PKA and D1-ERK activation and % PMR (Fig. 4A), whereas a negative correlation was found in the case of D2-PKA and D2-ERK (Fig. 4A). When mating reactions were arbitrarily divided into frequent and infrequent interactions by a value of 20% PMR (Fig. 4B), the D1-PKA and D1-ERK activities significantly increased in the frequently interacting mice but not in the infrequently interacting ones. In clear contrast, the D2-PKA and D2-ERK activities increased in the infrequently interacting ones (Fig. 4B). Notably, there was no significant difference in locomotive distance between frequent and infrequent interaction groups (Fig. 4B, Bottom), suggesting that the mice in the infrequent interaction group avoided or escaped from the female with an extent of locomotion comparable to that of the male mice in the frequent interaction group that chased the female. These results indicated that the activation of PKA and ERK of iMSNs was also critically involved in impassive and avoiding behavior toward a female mouse.
Fig. 4.
PKA and ERK activities of dMSNs and iMSNs during sexual interaction. (A) A male mouse was exposed to an unfamiliar female mouse for 30 min. Average changes in FRET responses of PKA and ERK during the 30-min period were plotted against % PMR. The r value was calculated by use of Pearson correlation analysis; *P < 0.05. (B) Changes in activities of PKA and ERK (upper traces) and locomotor activities (lower traces) of frequently or infrequently interacting male mice.
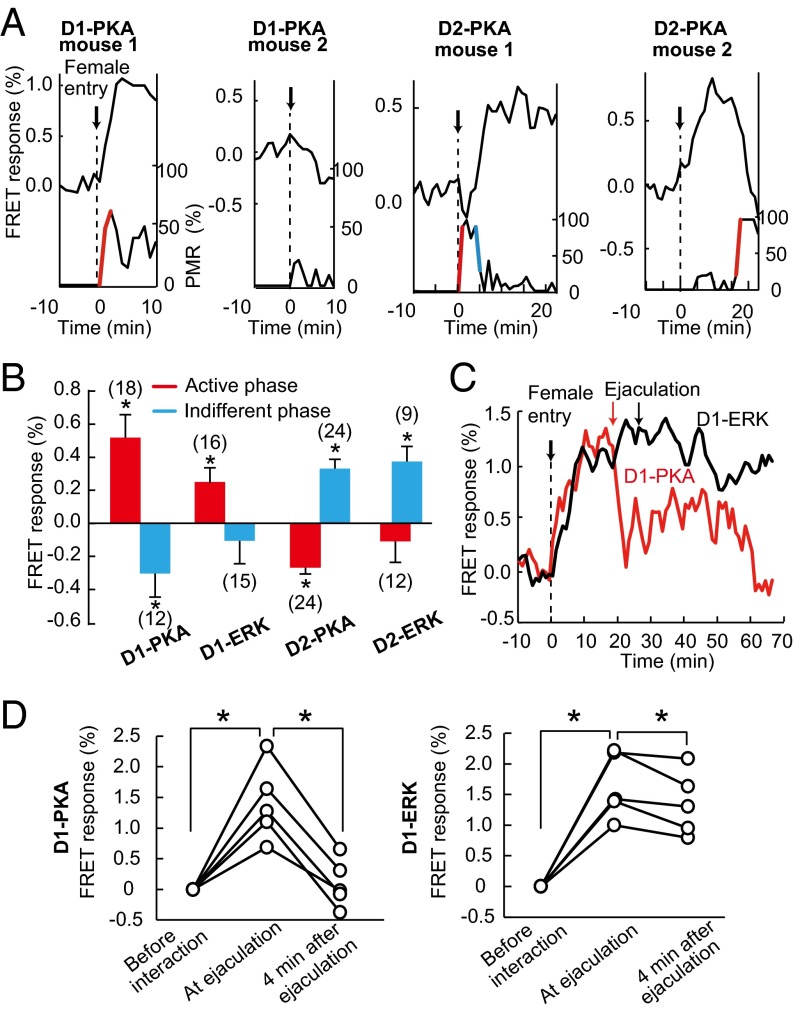
We also noticed that the successive active and indifferent/avoiding phases of a male mouse in mating behavior resulted in the frequent and infrequent mating reactions. We thus more carefully pursued temporal changes in the PKA and ERK activities during the mating reaction of individual mice. The rapid changes in active or indifferent phases of mating reactions were defined as more than a 50% PMR increase or decrease within 1–4 min, respectively (Fig. 5 A and B and Fig. S6). Fig. 5A illustrated four representative mice indicating close association of PKA activity with mating behavior. D1-PKA mouse 1 started sniffing immediately after female entry and showed a rapid concomitant increase in PKA activity. D1-PKA mouse 2 did not interact with the female mouse and showed no obvious increase in PKA activity. D2-PKA mouse 1 showed a strong interest toward the female mouse during the first 4 min but stopped PMR thereafter (Movie S8). The D2-PKA activity of this mouse concomitantly increased and remained high during the indifferent phase of mating reaction. D2-PKA mouse 2 showed no interest for the first 17 min and then started sniffing and grooming thereafter (Movie S9). Concomitantly, the D2-PKA activity shifted from activation to inactivation. Statistical analysis of the rapid change of PKA and ERK activities during mating behaviors explicitly indicated that the activities of D1-PKA and D1-ERK were markedly elevated during the active phase, whereas those of D2-PKA and D2-ERK were increased when a male mouse became indifferent to or escaped from a female mouse (Fig. 5B and Fig. S6). Thus, rapid change in the activities of both PKA and ERK reflected a shift between active and indifferent phases of the mating reaction. To substantiate this observation, we paired a male mouse with a hormonally primed female mouse to facilitate ejaculation by the male mouse (Fig. 5 C and D and Movie S10). The D1-PKA and D1-ERK activities were elevated during the mating reaction, but the D1-PKA activity was rapidly and significantly reduced to lower levels within 4 min after ejaculation, suggesting that PKA in the dMSNs was closely associated with the rapid shift in the mating reaction.
Fig. 5.
PKA and ERK responses of dMSNs and iMSNs upon a rapid shift in mating behavior. (A) Four representative mice indicating close association of PKA activity (upper traces) with mating behavior (lower traces). The red and blue lines in the lower traces show a rapid shift between the active and indifferent phases of mating reaction, which were defined as more than a 50% increase and decrease, respectively, in % PMR within 1–4 min. (B) Up- and down-regulation of PKA and ERK activities at the active and indifferent phases of the mating reaction is summarized from the data of Fig. S6 (*P < 0.05; Mann–Whitney U test). The numbers in parentheses indicate the sample numbers from six to nine animals. (C) Examples of changes in the PKA and ERK activities in dMSNs after ejaculation. Ejaculation is marked with the arrow in the D1-PKA (red) and D1-ERK (black) mouse. (D) Rapid inactivation of PKA in the D1-PKA mice after ejaculation (*P < 0.05; n = 5; Wilcoxon signed-rank test).
Pathway-Specific PKA Modification by DREADD and Its Effect on Mating Behavior.
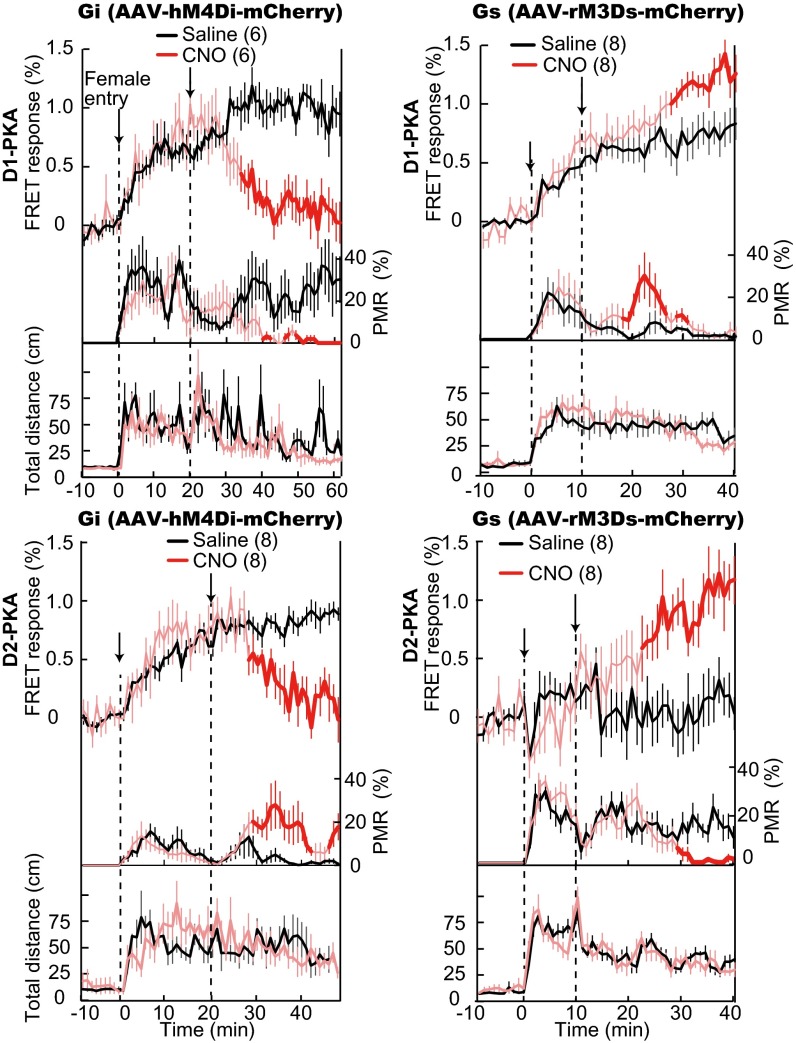
Finally, we examined the causal linkage of the PKA signaling cascade in induction and suppression of mating reactions by activation of Gs and Gi specific for either dMSNs or iMSNs by using the Designer Receptors Exclusively Activated by a Designer Drug (DREADD) method (36). We used two lines of flox adeno-associated viruses (AAVs) in which Gs-activating rM3Ds-mCherry and Gi-activating hM4Di-mCherry were bounded by loxP sequences (Fig. S7A). The transfection of D1- or D2-PKA mice with the AAV–rM3Ds-mCherry or the AAV–hM4Di-mCherry resulted in specific and exclusive expression of the respective designer receptors in either dMSNs or iMSNs of the transgenic mice (Fig. S7B). In the experiments using Gi-activated D1-PKA and Gs-activated D2-PKA male mice, we paired a male mouse with a hormonally primed BALB/c female mouse to increase the basal level of mating reactions of the male mouse (21). In the experiments using Gs-activated D1-PKA and Gi-activated D2-PKA male mice, we used a large-sized ICR female mouse to decrease the basal level of the mating reaction of the male mouse. This procedure helped in clarifying the effect of the cell-specific activation of Gs and Gi in the male mating reaction. When the designer receptor agonist clozapine-N-oxide (CNO) was injected 10–20 min after the female entry, this treatment considerably decreased FRET responses of PKA in the hM4Di-expressing MSNs of both D1-PKA and D2-PKA mice (Fig. 6, Left). In accordance with this decrease in PKA activity, these mice exhibited significant suppression and enhancement, respectively, of % PMR 10–20 min after CNO treatment (Fig. 6, Left). The effect of CNO treatment was reversed in the rM3Ds-expressing D1-PKA and D2-PKA mice (Fig. 6, Right). The increase in PKA activity in the D1-PKA and the D2-PKA mice significantly enhanced and suppressed, respectively, the % PMR of the corresponding PKA mice, although the enhancement of % PMR of the Gs-activated D1-PKA mice was transient after CNO treatment. Importantly, the CNO effect was specific for the mating reaction, as no difference in locomotor activity was detected following CNO treatment in the four lines of transgenic mice. These results indicated a causal linkage between the dorsal striatal PKA activity of dMSNs and iMSNs and the mating reaction of male mice.
Fig. 6.
PKA response and change in mating reactions induced by Gi or Gs activation by DREADD. Gi (Left) and Gs (Right) of the D1-PKA and D2-PKA mice were activated by CNO injection 10–20 min after female entry. Upper, middle, and lower traces show FRET response, % PMR, and locomotive distance, respectively. The numbers in parentheses indicate the sample numbers in which FRET responses were measured by alternately treating Gi-activated D1-PKA (n = 3) and other transgenic mice (n = 4 each) with either saline or CNO 1 d after each treatment and averaged. Deep red color in traces with CNO injection indicates a statistically significant change (P < 0.05; Mann–Whitney U test) from those with saline injection.
Discussion
This study demonstrated that PKA and ERK were coordinately but oppositely regulated between dMSNs and iMSNs in the dorsal striatum under different behavioral conditions. A sensory stimulus with bell sound had no effect on the PKA and ERK activities of either dMSNs or iMSNs of mice after habituation with a repeated bell sound, but rewarding and aversive stimuli markedly and reciprocally modulated the activities of both kinases in dMSNs and iMSNs. Animal behaviors can thus be orderly aligned from rewarding to aversive/impassive behaviors by the extent of activation and inactivation of PKA and ERK (Fig. S8). Remarkably, the rapid shift in the PKA and ERK activities of both dMSNs and iMSNs was tightly associated with the active and indifferent phases of naturally occurring mating reactions, indicating that reciprocal but highly synergistic activation and inactivation of the PKA and ERK cascades in dMSNs and iMSNs play a key role in selection of actions in the male mating behavior. This conclusion was verified by use of the DREADD technique, in which the activation of Gs and Gi induced active and impassive mating reactions in an MSN cell type-dependent manner. Thus, the modulation of the PKA cascade in the dorsal striatal MSNs is directly linked to the action selection in male mating behaviors.
The D2-PKA and D2-ERK in iMSNs were activated by aversive stimuli and in the impassive phase of male mating reaction. This finding is consistent with the predominant expression of high-affinity (nM order) D2 receptors in iMSNs, which are capable of sensing basal changes in synaptic dopamine concentrations in the striatum (10, 35, 37). The iMSN transmission would thus greatly contribute to the innate tendency of animals to be more concerned about avoiding uncomfortable and dangerous environments and escaping from a predator. Noteworthy also is that the D2 receptor antagonists are widely used as an effective therapeutic drug for some psychiatric disorders such as schizophrenia (38). The modulatory disturbance in the D2 receptor signaling in iMSNs may cause an imbalance of mental activity and could result in possible emergence of some forms of psychiatric disorders. Conversely, when animals encounter rewarding stimuli such as sexual interaction, an increase in dopamine levels in the striatum would reach the threshold for low-affinity (μM order) D1 receptor activation in dMSNs (10, 35, 37). Although other neurotransmitters and striatal interneurons may also be involved in reward-seeking and aversive behaviors (10, 39), the D1 and D2 receptors could play a key role in distinctly controlling striatum-mediated action selection via common but pathway-dependent PKA and ERK signaling cascades (37).
The use of deep brain-inserted fiber optics combined with measurement of FRET responses of signaling cascades enabled us to explore the regulation of signaling mechanisms in specific subgroups of MSNs that control action selection. Although detailed analysis of FRET responses of individual cells in behaving animals was not done in this study, multiple optic fibers of the microendoscope could allow investigation of spatiotemporal interactions in the cell assembly of a local circuit. Thus, this methodology could also be used to study regulatory mechanisms of neural circuits involved in a wide range of animal behaviors.
Materials and Methods
Essential methodological information needed for a basic understanding of the text has been described in the main text at the appropriate places. SI Materials and Methods contains detailed information on generation of transgenic mice expressing cell type-specific PKA and ERK biosensors, fabrication of microendoscope, imaging measurements and analysis by the microendoscope system, and animal behavioral analyses including locomotion, cocaine administration, electric footshock, and mating behavior. All animal experiments were approved by the animal research committee of Osaka Bioscience Institute.
Supplementary Material
Acknowledgments
We thank H. Hioki for the D1-Cre mouse and N. Yamatani for technical assistance. This research was supported by Research Grants-in-Aid 22220005 (to S.N.) and 24111552, 22300136, and 26560470 (to K.F.); by Grant-in-Aid for Scientific Research on the Innovative Area “Fluorescence Live imaging” 22113002 (to M.M.); and by a Grant-in-Aid for Japan Society for the Promotion of Science Fellows (to A.G.), from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507121112/-/DCSupplemental.
References
- 1.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci. 2012;15(9):1281–1289. doi: 10.1038/nn.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66(6):896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 5.Chesselet MF, Delfs JM. Basal ganglia and movement disorders: An update. Trends Neurosci. 1996;19(10):417–422. doi: 10.1016/0166-2236(96)10052-7. [DOI] [PubMed] [Google Scholar]
- 6.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 7.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 8.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Wilson CJ. The synaptic organization of the brain. In: Shepherd GM, editor. Basal Ganglia. 5th Ed. Oxford Univ Press; Oxford: 2004. pp. 361–414. [Google Scholar]
- 10.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76(1):33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerfen CR, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250(4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 13.Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86(2):353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 14.Girault JA. Signaling in striatal neurons: The phosphoproteins of reward, addiction, and dyskinesia. Prog Mol Biol Transl Sci. 2012;106:33–62. doi: 10.1016/B978-0-12-396456-4.00006-7. [DOI] [PubMed] [Google Scholar]
- 15.Shiflett MW, Balleine BW. Molecular substrates of action control in cortico-striatal circuits. Prog Neurobiol. 2011;95(1):1–13. doi: 10.1016/j.pneurobio.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30(5):228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Girault JA, Valjent E, Caboche J, Hervé D. ERK2: A logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7(1):77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76(3):470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Doherty J, et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304(5669):452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 20.Dickson BJ. Wired for sex: The neurobiology of Drosophila mating decisions. Science. 2008;322(5903):904–909. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- 21.Park JH. Assessment of male sexual behavior in mice. In: Gould T, editor. Mood and Anxiety Related Phenotypes in Mice. Vol 63. New York: Springer Science; 2011. pp. 357–373. [Google Scholar]
- 22.Komatsu N, et al. Development of an optimized backbone of FRET biosensors for kinases and GTPases. Mol Biol Cell. 2011;22(23):4647–4656. doi: 10.1091/mbc.E11-01-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent P, et al. Live imaging of neural structure and function by fibred fluorescence microscopy. EMBO Rep. 2006;7(11):1154–1161. doi: 10.1038/sj.embor.7400801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flusberg BA, et al. Fiber-optic fluorescence imaging. Nat Methods. 2005;2(12):941–950. doi: 10.1038/nmeth820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goto A, et al. GDNF and endothelin 3 regulate migration of enteric neural crest-derived cells via protein kinase A and Rac1. J Neurosci. 2013;33(11):4901–4912. doi: 10.1523/JNEUROSCI.4828-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamioka Y, et al. Live imaging of protein kinase activities in transgenic mice expressing FRET biosensors. Cell Struct Funct. 2012;37(1):65–73. doi: 10.1247/csf.11045. [DOI] [PubMed] [Google Scholar]
- 27.Sumiyama K, Kawakami K, Yagita K. A simple and highly efficient transgenesis method in mice with the Tol2 transposon system and cytoplasmic microinjection. Genomics. 2010;95(5):306–311. doi: 10.1016/j.ygeno.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Valjent E, Bertran-Gonzalez J, Hervé D, Fisone G, Girault JA. Looking BAC at striatal signaling: Cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32(10):538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Allen MD, Zhang J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem Biophys Res Commun. 2006;348(2):716–721. doi: 10.1016/j.bbrc.2006.07.136. [DOI] [PubMed] [Google Scholar]
- 30.Bertran-Gonzalez J, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28(22):5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilango A, Shumake J, Wetzel W, Scheich H, Ohl FW. The role of dopamine in the context of aversive stimuli with particular reference to acoustically signaled avoidance learning. Front Neurosci. 2012;6:132. doi: 10.3389/fnins.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Angio M, Serrano A, Driscoll P, Scatton B. Stressful environmental stimuli increase extracellular DOPAC levels in the prefrontal cortex of hypoemotional (Roman high-avoidance) but not hyperemotional (Roman low-avoidance) rats. An in vivo voltammetric study. Brain Res. 1988;451(1-2):237–247. doi: 10.1016/0006-8993(88)90768-8. [DOI] [PubMed] [Google Scholar]
- 33.Giorgi O, Lecca D, Piras G, Driscoll P, Corda MG. Dissociation between mesocortical dopamine release and fear-related behaviours in two psychogenetically selected lines of rats that differ in coping strategies to aversive conditions. Eur J Neurosci. 2003;17(12):2716–2726. doi: 10.1046/j.1460-9568.2003.02689.x. [DOI] [PubMed] [Google Scholar]
- 34.Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30(5):203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: Comparison with novelty and locomotion. Behav Neurosci. 1992;106(1):181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- 36.Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63(2):291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakanishi S, Hikida T, Yawata S. Distinct dopaminergic control of the direct and indirect pathways in reward-based and avoidance learning behaviors. Neuroscience. 2014;282C:49–59. doi: 10.1016/j.neuroscience.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 38.Seeman P. Dopamine D2 receptors as treatment targets in schizophrenia. Clin Schizophr Relat Psychoses. 2010;4(1):56–73. doi: 10.3371/CSRP.4.1.5. [DOI] [PubMed] [Google Scholar]
- 39.Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.