Singular roles of checkpoint proteins
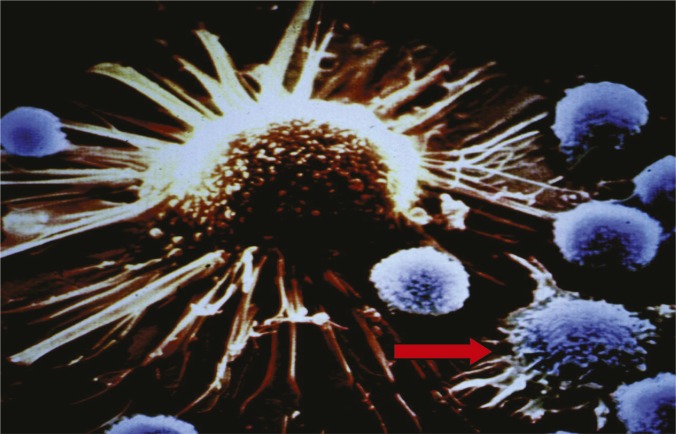
SEM of killer T cell recognizing a tumor cell (blue). Image courtesy of Drew Pardoll (PNAS, 2002).
Checkpoint proteins, which adorn the surface of some immune cells, help keep autoimmune diseases at bay by fine-tuning the activation of T cells, the immune system’s foot soldiers. Jun Liu et al. (pp. 6682–6687) tested whether two checkpoint proteins—the long-established PD-1 and the recently discovered VISTA, which is found on antigen-presenting cells as well as helper, killer, and regulatory T cells—play redundant roles in immune regulation. Compared with both wild-type mice and mice lacking either checkpoint protein, mice lacking both proteins showed increased signs of chronic inflammation in several organs, including the lungs, liver, and pancreas. VISTA-deficient mice that also lacked either PD-1 or the ligand PD-L1 displayed spontaneous activation of helper and killer T cells, suggesting that the two checkpoint proteins perform essential and singular functions in immune control. Compared with mouse models of colon cancer and melanoma that were treated with monoclonal antibodies against either checkpoint protein, mice that were administered antibodies against both proteins benefited from greater tumor shrinkage and prolonged survival, suggesting that VISTA and PD-1 independently control tumor-specific T-cell responses. Because checkpoint blockers targeting PD-1 and other such proteins have previously shown promise in cancer immunotherapy, a combinatorial approach to block VISTA and PD-1 might improve the efficacy of immunotherapy while minimizing adverse events, according to the authors. — P.N.
Face neurons and gender recognition
The primate brain contains “face neurons,” which aid in distinguishing faces from nonface objects. Face neurons are also thought to play a role in distinguishing among faces, but there is little direct evidence for this role. Arash Afraz et al. (pp. 6730–6735) report that face neurons influence face discrimination in monkeys using a technique called optogenetics, in which small clusters of neurons can be switched on or off by certain wavelengths of light. Monkeys were trained to identify various images of human faces as either male or female. Subsequently, the monkeys repeated the face identification task, but in half of the trials some of their face neurons were optogenetically switched off. When the face neurons were switched off, the monkeys were consistently less accurate at distinguishing male faces from female faces, compared with when the neurons functioned normally. Some groups of neurons responded more selectively than others to the gender of the face, and switching off these neurons led to a relatively higher loss of accuracy of face gender recognition. Similar results were obtained when the activity of the neurons was chemically suppressed. However, turning off nonface neurons in the same region of the brain had no effect on the monkeys’ performance. The authors suggest that the optogenetic approach might be useful for future studies of visual functions. — B.D.
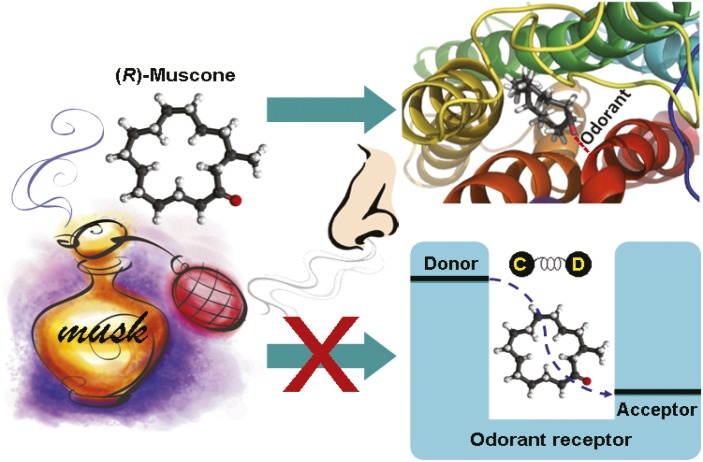
Dismantling the vibrational theory of smell
Odor sensing involves odorant–receptor interactions, not molecular vibrations.
The molecular mechanisms that allow humans and other animals to smell remain unclear. Researchers have proposed various theories of olfaction, the most controversial of which—known as the vibrational theory of olfaction (VTO)—posits that odorants are detected via their vibrational frequencies rather than through hand-in-glove-like substrate–receptor interactions. Eric Block et al. (pp. E2766–E2774) tested the VTO by comparing how different human and mouse odorant receptors respond to isotopomers, atomic-scale isotopic isomers, of various odorants. According to the VTO, electron transfer should occur across odorants at the active sites of odorant receptors, and different electron transfer rates should be observed for isotopomers, which differ in their vibrational frequencies. Studies by VTO proponents showed that humans can distinguish among the smells of various isotopomers. However, the authors found no differences in receptor response to the odorants tested. Furthermore, the current study demonstrates that the proposed electron transfer mechanism can be suppressed by quantum effects of nonodorant molecular vibrational modes. The authors suggest that the ability of humans to distinguish isotopomers reflects impurities or isotope effects occurring in the nasal mucus surrounding the receptor, rather than receptor-level vibrational effects. According to the authors, the study provides experimental and theoretical evidence to contradict the VTO at the receptor level, and suggests that the theory is implausible. — A.G.
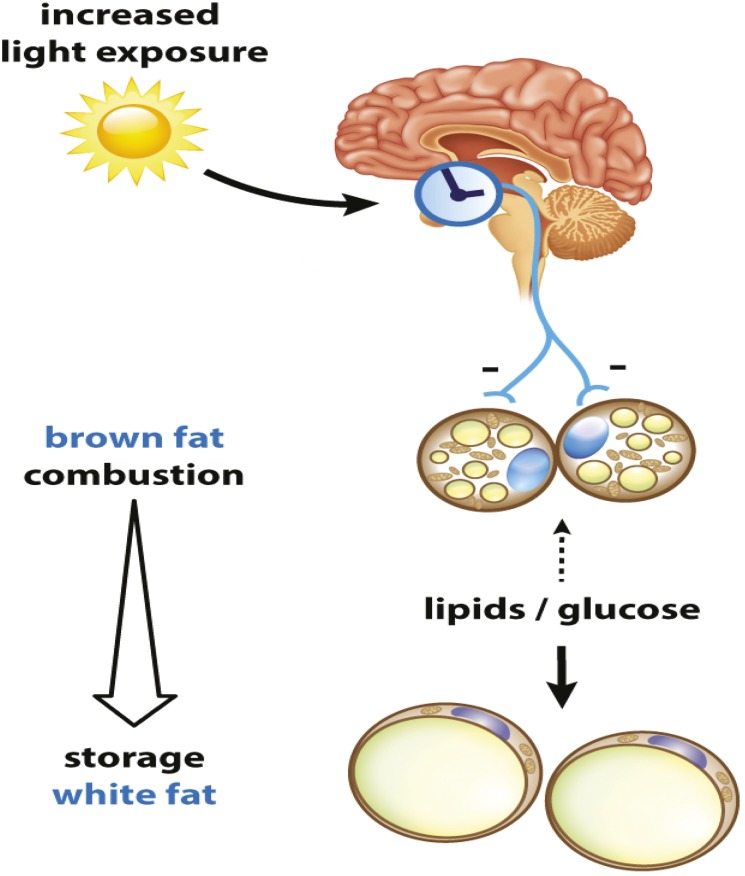
Artificial light exposure and obesity
Increased light exposure might induce adiposity.
Environmental light pollution contributes to a number of obesity-related illnesses, including type 2 diabetes and cardiovascular disease. Recent studies suggest constant light exposure disrupts the circadian rhythm, thereby decreasing energy expenditure and promoting weight gain. Sander Kooijman et al. (pp. 6748–6753) explored the effects of day length on the activity of brown adipose tissue (BAT) in mice, noting that BAT plays a central role in energy expenditure by converting energy from food into heat. The authors exposed mice to artificial light for 12, 16, or 24 hours per day for 5 weeks, and subsequently assessed the body weight and composition of the mice. Mice exposed to artificial light for 24 hours, compared with 12 hours, had significantly higher fat composition even as their daily food intake remained at baseline. The authors suggest that prolonging daily light exposure might promote obesity by decreasing energy expenditure, rather than increasing food intake or modulating physical activity. Further investigation revealed that increased light exposure decreases noradrenergic activation of BAT and thereby reduces conversion of fatty acids and glucose into heat. According to the authors, the study suggests that restoring impaired BAT activity may help overcome the negative consequences of increased light exposure. — A.G.
Fruit flies may be lefties or righties
A fly enters a locomotor decision-making point.
Animals such as octopi, tortoises, mice, and humans display handedness, a phenomenon underlying individual predilections for left- or right-sided maneuvers while performing certain tasks. To probe the basis of movement-related handedness, Sean Buchanan et al. (pp. 6700–6705) placed fruit flies (Drosophila melanogaster) in custom-designed Y-shaped mazes and monitored individual flies’ preferences to turn left or right at forks, while controlling for visual and olfactory cues and the flies’ activity levels. Analysis of 16,000,000 turns by 25,000 flies revealed that many individual flies were strongly predisposed to turn either left or right, though the typical response for most flies was roughly 50% for each direction. Turn preference persisted throughout the flies’ lifetimes but was unrelated to other natural asymmetries, such as the turn of the gut, and uncorrelated with other idiosyncrasies, such as whether resting flies placed their right or left wing on top. Crosses between flies revealed that turn preference was not heritable. However, genetic analysis suggested that the strength of the turn preference might be controlled by brain cells called PFN neurons in a region involved in planning and executing movement; suppressing the neurons resulted in extreme “leftiness” or “rightiness.” In a related article, Julien Ayroles et al. (pp. 6706–6711) found that a gene called Ten-a, implicated in synaptic wiring during fruit fly brain development, was associated with the magnitude of the behavioral variability associated with the flies’ turn preference. In addition, the authors found that flies of different genetic backgrounds display different levels of behavioral variability. The findings raise the possibility that genes and brain circuits might influence individual differences in similarly idiosyncratic human behaviors, according to the authors. — P.N.