Significance
This study shows how an animal actively and functionally controls its sensory field of view by means of changing its emitter aperture. This would be similar to a human ability to zoom in on a visual scene. The study was performed in a natural situation with wild bats. To perform the study, we developed tools to record and analyze bats’ beams and faces simultaneously. We show that bats rapidly and dramatically alter their biosonar field of view to functionally adjust sensory acquisition. They do so by changing their mouth gape. Hence, we show that bats change the shape of their emitter for active sensing.
Keywords: bats, beamforming, echolocation, active sensing, sensory perception
Abstract
Active sensing, where sensory acquisition is actively modulated, is an inherent component of almost all sensory systems. Echolocating bats are a prime example of active sensing. They can rapidly adjust many of their biosonar parameters to optimize sensory acquisition. They dynamically adjust pulse design, pulse duration, and pulse rate within dozens of milliseconds according to the sensory information that is required for the task that they are performing. The least studied and least understood degree of freedom in echolocation is emission beamforming—the ability to change the shape of the sonar sound beam in a functional way. Such an ability could have a great impact on the bat’s control over its sensory perception. On the one hand, the bat could direct more energy into a narrow sector to zoom its biosonar field of view, and on the other hand, it could widen the beam to increase the space that it senses. We show that freely behaving bats constantly control their biosonar field of view in natural situations by rapidly adjusting their emitter aperture—the mouth gape. The bats dramatically narrowed the beam when entering a confined space, and they dramatically widened it within dozens of milliseconds when flying toward open space. Hence, mouth-emitting bats dynamically adjust their mouth gape to optimize the area that they sense with their echolocation system.
The ability to actively adjust sensory acquisition is a key feature of almost all sensory systems. A capability to selectively control the sensory “field of view” could have a major impact on sensory perception. It would allow an animal to adjust the amount of acquired information in a task-dependent manner, zooming in on an object of interest and zooming out when a wider sector should be sensed. Many animals can shift their sensory attention (e.g., by changing gaze) or their focal plane (e.g., human vision), but there are no animals that are known to constantly adjust their sensory field of view under natural conditions. Echolocating bats perceive their environment acoustically by emitting ultrasonic pulses and analyzing the received echoes (1). The volume of space that is covered by the sound pulse and therefore, sensed by the bat depends on the emitted beamform—the spatial shape of the emission (2–9). Bats could potentially benefit greatly if they could change the form of their emitted beam in a functional manner, a property usually referred to in engineering as beamforming (10).
Jakobsen and coworkers (11) recently summarized some of the reasons why a bat might narrow its biosonar beam. These reasons include (i) focusing sound into a narrower sector to improve the localization of objects, (ii) eliminating undesired echoes from the back or the sides of the bat, and (iii) increasing the sensing range by directing more energy forward. All of these come with a cost of reducing the volume of space that is scanned by the bat. It is, therefore, reasonable to expect that a bat would widen its beam under certain conditions, such as when scanning its surroundings during orientation or navigation.
Most echolocating bats emit sound through the mouth (12). The biosonar beam of these bats can be modeled using the “piston model,” which represents a piston-shaped emitter in an infinite baffle (13). According to the piston model (and other emission models as well), a bat can adjust its beam by altering one of two parameters. First, it can change the spectral content of the sound pulse. Increasing the frequency would result in a narrower beam. Several bats that use frequency-modulated pulses seem to use this strategy at the terminal part of an attack on prey (3). Second, the bat can potentially change the aperture of its emitter. By opening its mouth wider, it can narrow the beam and vice versa. However, there is currently no direct evidence that bats change the emitter aperture for beamforming in this way.
We studied beamforming in mouth-emitting Bodenheimer's pipistrelle bats (Hypsugo bodenheimeri) under natural field conditions as well as in a controlled experimental setup. We started by recording and photographing bats as they came to drink at a small desert pond using an array of 12 ultrasonic microphones and a multiflash photography setup. Drinking on the wing requires fine maneuvering skills, which could benefit from active sensory adjustments (14, 15). When descending toward the pond and then ascending from it, the bats had to enter a confined space and then leave it, rapidly changing the degree of clutter around them—the density of nearby objects creating undesired echoes. To deal with these sensory challenges, we predicted that bats will alter their beamform while descending into the confined space or later, ascending out of it using one (or both) of the two mechanisms mentioned above. We used the audio recordings to reconstruct the bats’ emitted beams, and we measured their corresponding mouth gape in the images so that we could assess if and how bats control the beamform. To validate that our results were not a consequence of the drinking per se, we performed a second controlled experiment in which bats flew through a narrow (0.5 × 0.5-m2 cross-section) 1.5-m-long tunnel and emerged from it into an open space environment (with less background echoes). We photographed the bats in flight to analyze their mouth gape and simultaneously recorded their echolocation pulses.
We found that bats actively adjusted their beam by changing their mouth gape (i.e., the size of the emitter). Bats widened their mouth when entering a more confined cluttered environment, thus dramatically narrowing their beam width, and they narrowed the gape when flying toward the open, thus dramatically widening their beam. Bats that flew through a confined tunnel exhibited the same behavior—widening their mouth gape inside the tunnel and narrowing it when emerging into open space. We argue that this behavior aimed to functionally control the volume of the environment sensed by the bat to improve sensing—decreasing the scanned volume when entering a confined space and increasing it when flying into open space.
Results
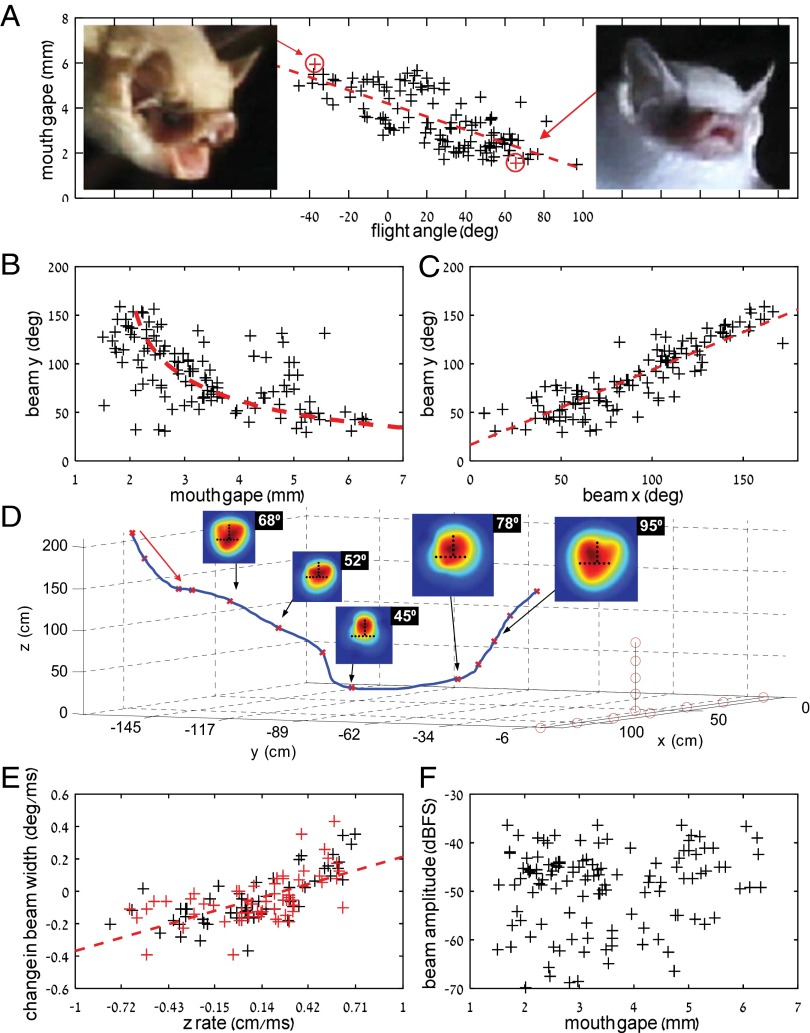
Bats widened their mouth gape when descending toward the pond to drink, and they narrowed their mouth gape when ascending (Materials and Methods and Fig. 1A) (beam width was defined as the double-sided −6-dB amplitude drop). Mouth gape changed dramatically—by more than fourfold (1.5–6.5 mm). The bats’ sonar beam width changed accordingly; when the bats widened their mouth, the echolocation beam narrowed and vice versa (Fig. 1B). Like the mouth gape, the beam width changed dramatically by more than fourfold (35–160°), following the predictions of the piston model (red line in Fig. 1B). Using ensonification, we confirmed that, when descending toward the pond, the bats were entering a highly cluttered (echoic) environment, whereas when ascending, the clutter was reduced (Fig. S1).
Fig. 1.
H. bodenheimeri narrowed mouth gape to widen its echolocation beam when ascending and vice versa. The data depicted are pooled across all recorded approaches. (A) Correlation of mouth gape with flight angle (i.e., the angle between the direction of flight and the pond) reveals that bats narrow their gape when ascending and vice versa [n = 122 samples; Pearson correlation, R = 0.77, P = 0.0001 (R = 0.73, P = 0.0001)]. The numbers in parentheses represent the statistics when eliminating one-half of the data points, thus reducing the risk of using nonindependent data points (Materials and Methods). Images show the mouth gape for two typical flight angles (marked in red)—extreme descending and extreme ascending. Note that beams were directed toward the array, even in high-ascent angles (Fig. S3). The red lines depict the best linear fit using the total least squares regression. (B) Beam width (y axis) as a function of mouth gape shows that the beam widened when the bats narrowed their gape and vice versa [n = 122 samples; R2 = 0.52 (R = 0.72)]. The red dashed line shows the prediction of the piston model for the same mouth gape at a frequency of 59 kHz. (C) Beam width (y axis) vs. beam width [x axis; n = 122 samples; Pearson correlation, R = 0.87, P = 0.0001 (R = 0.88, P = 0.0001)]. (D) An example of a bat’s full 3D flight path, including five emitted beams. Black dots on each beam depict the positions of the microphones relative to the beam. This particular trial did not end with drinking—notice the fast ascending and fast widening of the beam (the width of each beam is given in degrees in the top-right corner). The array microphones are shown as red circles. Note that the last beam analyzed (95°) was still directed toward the array (Fig. S3). (E) The beam widening/narrowing rate as a function of the ascending/descending rate reveals that bats widen the beam faster when ascending faster and vice versa [n = 122 samples; Pearson correlation, R = 0.64, P = 0.0001 (R = 0.72, P = 0.0001)]; 20 flight trajectories were used for this analysis: 10 on the first night with the pond covered by a black wooden board that prevented the bats from drinking and 10 on the second night without the board. The changes in altitude and beam were calculated for consecutive pulses. (F) Pulse amplitude vs. mouth gape [n = 122 samples; Pearson correlation, R = 0.08, P = 0.30 (R = 0.09, P = 0.55)]. Decibel full scale (dBFS) represents the amplitude normalized by the maximum possible recording level of the system in a 20log10 scale. These amplitudes were estimated at 1 m from the bat’s mouth by compensating for the geometric spreading and the atmospheric attenuation.
The frequency of the echolocation calls varied little along the part of the flight that we analyzed (the most intense frequency was 56.9 ± 0.24 kHz) (Fig. S2). The observed shift was only enough to account for a change of 3° in the beam width according to the piston model. Hence, the changes that we observed in beam width could not result from frequency changes. The bats’ beams were symmetric, and their width increased or decreased simultaneously along both axes (Fig. 1C), as was previously suggested (9). Fig. 1D presents an example of one full trial, in which a bat descended—widening its gape and narrowing the beam—and then, ascended—narrowing its gape and thus, widening the beam.
Interestingly, there was a significant correlation between the rate of ascending or descending and the rate of changing the beam width. This was the case both when the bats could drink (black “+” in Fig. 1E) and when they could not drink, because we temporarily covered the pond by a black wooden board (Materials and Methods and Fig. 1E, red “+”). Regression analysis revealed no difference between the two treatments (Materials and Methods). This means that, when a bat changed its height more rapidly (upward or downward), it altered its beam width more rapidly, suggesting a functional sensory behavior (Discussion). We did not find any correlation between the mouth gape and the absolute emission intensity (Fig. 1F). Hence, the bats did not open their mouth simply to increase pulse intensity.
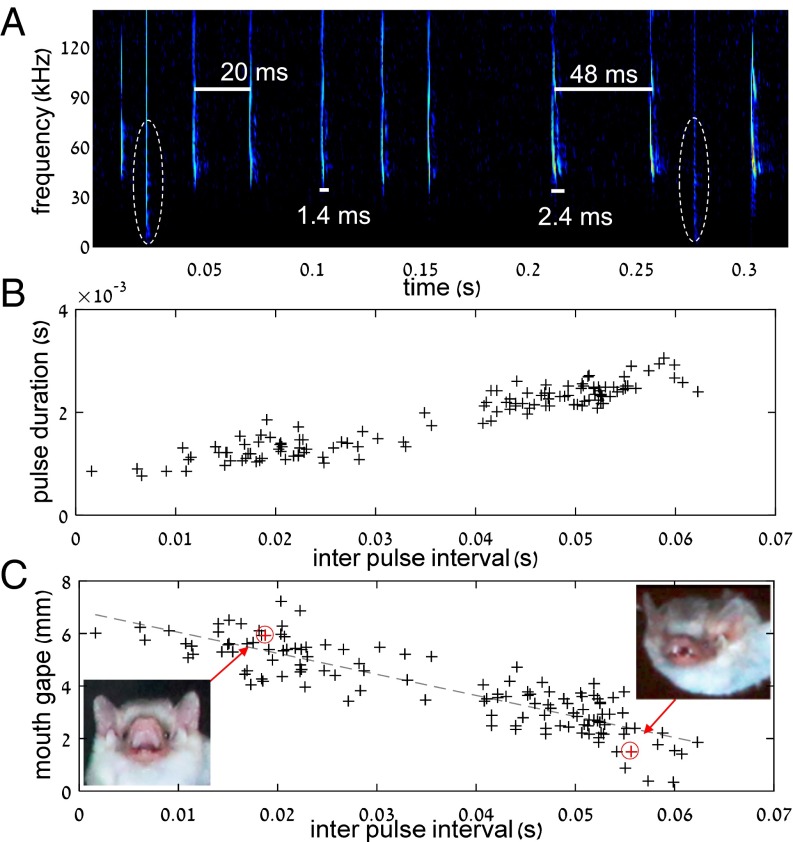
To validate that the observed changes in gape were not merely a result of the drinking behavior, we performed a second experiment, in which we flew six bats (H. bodenheimeri) under different levels of clutter in a controlled setup. We released bats inside a narrow (0.5 × 0.5 m2) tunnel and recorded their mouth gape and echolocation pulses as they flew through it and emerged toward an open (much less echoic) environment (Materials and Methods). The results confirmed the field experiments. The bats widened their mouth gape in the cluttered environment (the tunnel), while narrowing the mouth gape when emerging into the open (Fig. 2). The average mouth gape inside the cluttered tunnel was almost two times larger than that in the open environment (n = 6, Wilcoxon signed-rank test, P < 0.001). Moreover, there was a strong significant correlation between the echolocation behavior inside the tunnel and the mouth gape: the mouth gape increased as the interpulse intervals and the duration of pulses shortened and vice versa, suggesting that the deeper the bat was in the tunnel, the wider its gape (Fig. 2C). The overall observed change in mouth gape in the controlled tunnel experiment was similar to that observed in the drinking behavior (approximately fourfold). The bats’ pulse frequency did not significantly differ inside and outside the tunnel (56.8 ± 0.16 and 56.7 ± 0.18 kHz, respectively; Wilcoxon signed-rank test, n = 6 bats, P = 0.4), proving that the beam was narrowed in the confined tunnel and widened when the bat flew into the more open space.
Fig. 2.
Widening and narrowing the mouth gape play a functional sensory role. (A) A spectrogram showing part of a tunnel trial. The moment of exiting the tunnel can be clearly recognized according to the increase in interpulse interval (from ∼25 to ∼50 ms) and the increase in pulse duration (from ∼1.5 to ∼2.5 ms). Dashed ellipses depict two flashes representing two images that were analyzed (one inside and one outside the tunnel). (B) Pulse duration vs. interpulse interval reveals two clear clusters of echolocation behavior representing echolocation inside and outside the tunnel. (C) Mouth gape as a function of interpulse interval. There was a strong negative correlation between the two, suggesting that bats opened their mouth inside the tunnel (n = 127 samples; Pearson correlation, R = 0.87, P < 10−5). Two images showing wide and narrow gapes are presented.
Discussion
We show that bats actively control their biosonar beam width by changing the size of their emitter (the mouth gape). We argue that this change in mouth gape is a functional sensory behavior, aiming to increase the biosonar field of view when entering an open space and to decrease it in more confined situations. This sensory adjustment might serve the function of reducing undesired background echoes or focusing more energy on the target in a highly cluttered confined environment. Flying through a narrow tunnel (which our bats did) is an extremely challenging sensory task, in which a bat must deal with loud echoes returning shortly after each emission. In such situations, a bat could greatly benefit from narrowing its beam width, thus eliminating undesired echoes from the walls of the tunnel and focusing energy into a narrow sector at the center of the tunnel. When emerging from the tunnel (or ascending from the pond), the bats would gain from widening their beam width to increase the volume that they scanned in a newly entered open environment. We only measured the relative emitted sound levels, but they indicate that on-axis intensity is independent of gape size, which would suggest that the changes that we observed in beam width of bats ascending from the pond account for a dramatic fourfold change in the area sensed by the bat (the beam’s cross-section).
In the drinking experiments, we found a significant correlation between the rate of ascending/descending in flight and the rate of altering the beam width. This means that, when a bat ascended more rapidly, it also closed its mouth more rapidly, suggesting that closing the mouth is not simply an automatic response performed after drinking. There is no reason for a bat that is ascending more quickly to close its mouth more rapidly unless narrowing the mouth gape has some other function—such as a sensory function. Importantly, this correlation between the rate of ascending and the rate of widening the beam was also observed in trials in which the bats did not drink (see Materials and Methods), again strongly implying that this behavior has a sensory function. This argumentation is especially true for the ascent, because one could argue that bats were descending thinking they are about to drink but then ascending without water in their mouth. We, therefore, hypothesize that, during the ascent, bats functionally widened their beams to adjust their biosonar field of view after it had been dramatically narrowed while they were descending.
Controlling the beam through changes in mouth gape might be advantageous over changing pulse frequency, because the bat can continue using the frequency that it is most adapted to in terms of both emission and perception. Moreover, by changing the emitter’s aperture and not the frequency, the bat does not need to trade off other physical characteristics of sound waves, such as frequency-dependent attenuation. For example, if a bat narrows its beam by increasing the frequency of the emitted pulse, it will have to pay a price in the sensing range, because higher frequencies attenuate more rapidly. Finally, because an emitter typically has an optimal resonance region, the frequency at which a bat can emit loudest may be restricted to a specific frequency range.
Surlykke et al. (5) showed that bats in the field emit narrower beams than in the laboratory. We believe that our results do not contradict this finding. One explanation for the apparent discrepancy between the two studies is that Surlykke et al. (5) studied foraging bats, which would benefit from narrowing their beam to increase the sensing range or focus on a particular target. In contrast, we studied bats that were rapidly moving between environments with very different degrees of clutter. Our bats were not foraging and would have benefited from widening their beam to scan the new space that they were entering. As explained in the Introduction, adjusting the beam might have several different functions, and therefore, it is not surprising that bats sometimes widen and sometimes narrow their beams in the field depending on the task that they are performing. Our study shows that bats can dynamically and rapidly adjust their beam according to their sensory requirements within dozens of milliseconds. Beam control by changing mouth gape, thus, provides bats with another degree of freedom in their ability to actively shape their sensory perception of the world.
Materials and Methods
Experimental Setup and Recordings at the Pond.
Recordings and experiments were performed according to permits from the Israeli National Park Authority (no. 2014/40579) and the Institutional Animal Care and Use Committee (no. L-11-054). Drinking recordings were made during five full nights (two in November of 2013 and three in July of 2014) at a small natural pond in the Arava Desert in Israel (exact coordinates are 30°95′ N, 35°36′ E). The pond was ∼3 × 2 m2, with high dense reeds on two of its banks and steep muddy banks on the other two sides. Hence, the approach toward the water surface required fine maneuvering by the bats. We blocked part of the pond with reeds so that a 2 × 1.5-m2 open-water surface remained. The array was placed at one end of this surface—at the opposite side from where access was the easiest. Most bats, thus, flew toward the array when drinking. The experimental setup included an array of 12 ultrasonic wide-band microphones (CM16; connected to an Hm1216 AD Converter; Avisoft), two digital single-lens reflex (dSLR) cameras [one Canon EOS 5D MkIII and one Canon EOS 5D Mk I with either a 100-mm Macro lens (f = 2.8) or a 50-mm lens (f = 1.8)], and a system of three synchronized Canon 580 EX II Flashes (Fig. 3A). The two cameras were synchronized by the flashes, but they were pointed in slightly different directions and focused on different planes (∼50 cm apart) to allow better coverage of the flight trajectory. Image analysis was performed on each image separately (see below). To control for the effect of drinking on the opening of the mouth in one of the nights, we placed a smooth and highly reflective black Formica-covered wooden plate (2 × 1 m2) covering most of the pond. On this night, bats descended and tried to drink from the plate, and thus, they performed a full drinking approach without reaching the water and without filling their mouth with water (14). On the other nights, we removed the wooden plate and allowed the bats to drink normally. However, we could not be sure if the bats drank in all of the trials.
Fig. 3.
The experimental setup and methods. (A) The microphone array, three synchronized flashes, two cameras, and a trigger line. The array blocked the entire width of the pond. (B) A typical spectrogram showing two flashes (dashed ellipses around 10–20 kHz) and the closest two pulses (solid ellipses). (C) Head feature markers used by the neural network. (D) Performance of the neural network in measuring the gapes from data from the same bats that were used for training (red dots) and the gapes of new validation data points from the fourth bat (black ×s). Notice that 15 images were taken for each gape, but the data points are overlaid on each other. (E) Tail to head vector (white arrow) and flight angle (θ). The red dashed line represents the pond’s horizontal surface. The cameras were mounted horizontally. (F) Flight angle (estimated vs. real values for different yaws).
A red-light photoelectric switch (Omron E3JM-R4M4-G) was positioned 166 cm in front of the microphone array and 40 cm above the water. Its beam was directed parallel to the array. Bats descending to drink crossed the beam and hence, simultaneously triggered three flashes set to strobe function (each with four consecutive flashes at intervals of 80/120 ms depending on the night). The change in flash rate was introduced to cover a larger part of the approach. The flashes were set to 1/64 full-power output equal to ∼1/30,000-s exposure time. The flash series, thus, lasted 320/480 ms, covering a substantial part of the drinking approach, including the descent, the surface approach, and the ascent. The two dSLR cameras were set at f = 11 and operated in bulb or B mode. A sequence of three to four images of the bat was usually recorded on each frame. The shutters were opened manually using a cable release.
Simultaneously, to image acquisition, audio was recorded with 12 ultrasonic microphones placed 20 cm apart in two perpendicular lines. The horizontal line covered 1.2 m (seven microphones), and the vertical one covered 0.9 m (five microphones and one microphone on the horizontal) (Fig. 3A). The array was placed at the narrow end of the pond, thus spanning its entire width. Bats typically approached to drink from the other end (because of vegetation), thus heading toward the array. Sequences of 4 s were recorded at a 375-kHz sampling rate and a dynamic range of 16 bit. Audio triggering was performed manually whenever the flash system was triggered by a bat (with a 2-s pretrigger setup).
Bat activity at the pond was high, with several species (mainly H. bodenheimeri and Asellia tridens) arriving to drink dozens of times throughout each night. We recorded 312 approaches of H. bodenheimeri, the species used in this work. We could not determine how many different individuals were recorded, but most likely, it was many, because activity continued throughout each night. Because of different exclusion criteria (see below), we used 122 images of H. bodenheimeri from 78 approaches (therefore, less than two images per flight on average). We pooled the data across all individuals for the different analyses. The use of data from many individuals increased variability and therefore, reduced the risk of using nondependent data points. We performed additional manipulations to increase our confidence even further (Materials and Methods, Statistical Analysis).
Image to Audio Synchronization.
Images and audio were synchronized by detecting the flashes’ sound in the audio recordings (Fig. 3B). For all audio analysis, we could, therefore, take the bat pulse that was closest (in time) to the specific image of the bat (the flash). Because of audio and photo exclusion criteria (see below), we finally had 78 flights for which we had synchronized audio and images. This data allowed us to analyze a total of 122 beams that had corresponding images. The time interval between an image and its closest echolocation pulse was 8.5 ± 5.5 ms (Fig. 3B). This interval was much shorter than the average interpulse interval, which was 50 ± 7 ms. Audio and image analysis was performed with Matlab.
Tunnel Experiments.
These trials were performed on the last night of the recordings. Six bats (H. bodenheimeri) were used in the experiment. A large flight tent (4 × 3 × 2.5 m3) was built near the pond where the previous recordings were made. The walls of the tent were made of fine mesh and thus, hardly reflected any echoes (target strength of −36 dB). A 0.5 × 0.5-m2-wide and 1.5-m-long cardboard tunnel was placed on a table 1.2 m above the ground at the center of the tunnel. Two cameras and three flashes were used (see above), but one camera was directed into the tunnel, whereas the other was placed distally and captured the bat when it was outside the tunnel. A synchronized Avisoft Microphone (CM16 with an Hm116 A/D converter) was positioned in front of the tunnel (but a bit below it to avoid echoes). Bats were released at one end of the tunnel. They flew through it and emerged into the open flying into the mesh, which they probably did not perceive (because it hardly reflected echoes). For each such flight, four flashes were fired (250 ms apart), usually resulting in four images of the bat—two inside the tunnel from one camera and two outside the tunnel from the other camera. A synchronized audio file was recorded for each trial. We could determine if the bat was in or out of the tunnel based on the different views of the two cameras as well as the echolocation parameters, which were dramatically different. Both interpulse intervals and pulse duration were much shorter inside the tunnel (as expected from biosonar theory), thus allowing us to determine the position of the bat (Fig. 2 A and B). We flew each bat ∼15 times and ended up using six recorded flights for each bat.
Image Analysis.
Gape estimation.
Gape measurements imposed a challenge, because we only had a 2D image of the 3D space (bats at different angles and distances). In addition, pixel units of the digital images had to be converted to a metric system. A machine-learning artificial neural network approach was chosen to estimate the mouth gape, solving both the angle and size invariance problems. A two-layer, feed-forward, 10 hidden neurons network was trained with the Levenberg–Marquardt backpropagation algorithm to determine the mouth gape from a given set of bat features. The network received as input seven points that were manually marked on a bat’s head: the two ears, the two eyes, the forehead (between the two ears), and the two lips (Fig. 3C). It then provided as output the distance between the lips (the gape) in millimeters. Because a human marked the points of interest (e.g., the eyes), there was no difficulty of dealing with problems, such as changing illumination. We first trained the network using four stuffed bats (two male and two female H. bodenheimeri; The Steinhardt National Natural History Museum, Tel Aviv, Israel). The mouths of these bats were opened in six predefined known gapes (1–6 mm), and their images were taken from multiple angles and distances representing the variation in the real data. We trained the network with images of three bats; 84 images of these bats were randomly divided into 70% training set, 15% validation set, and 15% testing set. We then took 28 images from a fourth bat that was not used for training and applied the network on images of this bat. The maximum error of estimation on the new bat was 0.3 mm (Fig. 3D). It is important to note that the network’s output could be any value—not only integers and not limited to the range on which it was trained.
It should not be surprising that a neural network can learn this task. The algorithm only needs to compensate for the two angles of the bat (pitch and yaw) and its distance. For example, for a bat with a size that is known, it would be enough to measure one horizontal line on its face (e.g., the distance between the eyes) and divide it by the known distance between the eyes to determine the yaw angle, and the same can be done for pitch (with a vertical line). The seven features that were used (and the distance relations between them) provide a lot of information about these two angles, and the data are normalized so that the scale was invariant. This normalization allowed the algorithm to overcome the lack of knowledge on the bat’s size. The size variance among bats (e.g., the distance between the eyes) was much smaller than the variance of the measured phenomenon—changes in mouth gape.
After training the network, for analyzing the images of the actual bats in the field, each bat image was cropped and marked, and the marked pixel coordinates were fed into the neural network for gape estimation. Only images with estimated gapes between 1.5 and 6.5 mm were used in additional analysis. This procedure excluded ∼10% of the images, which were probably beyond the parameter space on which the model was trained—for instance, flight angles that were beyond what we presented to the neural network.
Flight angle estimation.
The flight angle (i.e., the angle between the direction of flight and the pond) was also calculated from the bat’s image. To this end, along with the features that were marked on the head to estimate gape, we marked the bat’s tail and estimated the tail to head axis (Fig. 3E), which was then used to trigonometrically calculate the flight angle relative to the horizontal pond (the cameras were horizontal). Two steps were taken to compensate for changes in the bat’s yaw (azimuth angle relative to the camera). First, the yaw was estimated by rotating a cylinder image until it was aligned with the bat (Fig. S4). Second, a function that mapped 2D to 3D angles (given the yaw) was used to estimate flight angle. This mapping function was calculated using a computer simulation (Fig. S4). We tested this procedure of estimating the flight angle with a cylindrical toy model. This model was placed in different known yaw and flight angles covering the range of the angles that were observed in the experiment. The model’s flight angle was then estimated from the images, and the correcting function was applied. Results prove that our method was able to estimate flight angle reliably with an error of up to 6° (Fig. 3F). Negative flight angles denote descending bats, and positive values denote ascending bats. Thanks to the fact that bats approached drinking in a rather stereotypical trajectory (heading toward the array), the yaw angle was limited. Moreover, the flight trajectory analyzed by us was always less than 500 ms, and thus, the bats did not change this angle much.
Audio Analysis.
Pulse detection.
Raw audio signal was initially filtered with a Butterworth band-pass filter of order 10 and cutoff frequencies of 40 and 140 kHz to remove noise and focus on the bat’s emitted spectral range. Calls were automatically detected at a threshold of 20 dB above noise floor level and a minimum of a 0.3-ms duration. They were then manually scrutinized before additional analysis.
Trajectory reconstruction.
Time difference of arrival analysis was used for 12 microphone recordings to reconstruct the bats’ positions at each pulse (16) and later, the full flight path. The time of arrival at each microphone was calculated by finding a point of maximum cross-correlation between the different channels, and the 3D position of the bat was then calculated with the multilateration technique. The reconstructed trajectory path was postprocessed by a 3D Kalman filter to remove outlying points that originated from noisy time estimations.
Beam reconstruction.
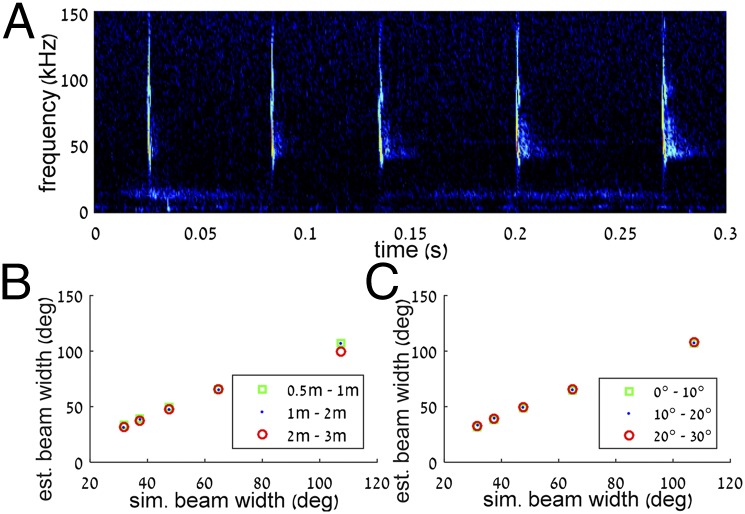
We only analyzed beams that were directed within the borders of the array. Before the estimation of the beam, the y-axis (vertical) recordings and the x-axis (horizontal) recordings were tested for being unimodal and convex (comparing the middle microphone with the side ones). This procedure assured us that the beam was directed within the borders of the array (i.e., within a 1.2 × 0.9-m2 rectangle). Beams that did not meet this criterion were removed from additional analysis. This rejection method was validated using simulations (see below). Next, for the beams that were included in the analysis, the envelope of each microphone’s recording was estimated, and the peak was extracted. Because the peak frequency varied little in our data (Fig. S2), this procedure is equivalent to estimating the beam at the peak frequency (∼56.8 kHz). The measurements were corrected according to the different sensitivities of the microphones as well as for spherical loss and atmospheric attenuation; thus, the intensity was calculated at 1 m from the bat’s mouth. This correction was done using the estimated position of the bat and according to the ambient temperature and humidity (22 °C and 70%, respectively). Microphones that recorded outlier values (much weaker than their neighbors) were replaced with corrected values—linearly interpolated according to their neighbors. The effect of microphone directionality was compensated for using the angle between the bat and the microphone. The beam itself was then interpolated using a bicubic spline interpolation. Audio recordings had high signal to noise levels and did not suffer from strong echoes easing beam reconstruction (Fig. 4A). Moreover, we did not analyze beams emitted by bats that were more than 3 m from the array to decrease the variability of possible distances and angles. It is noteworthy that the results described in this paper are comparative. Thus, the differences that we found between the beams of ascending and descending bats would still be valid even if we used a slightly different beam estimation approach (e.g., different interpolation). In total, we analyzed 101 beams. The x and y beam widths were estimated at −6-dB amplitude from the peak on a 2D reconstructed beam projection. Then, given the bat’s position, the angular beam widths could be calculated. We performed two control simulations to validate our method and confirm that the bat’s distance from the array and its flight angle relative to it did not bias the results. We used the full piston model (Eq. 1) to simulate beams of bats positioned at different distances and angles:
Fig. 4.
Audio analysis and beam reconstruction. (A) A typical recording (spectrogram) including five calls of an approaching bat. (B) Simulations of beam reconstruction for bats at different distances from the array. This range of distances represented the range of distances that we analyzed in the real data. (C) Simulation of beam reconstruction for different angles toward the array. This range of angles represented the range of angles that we analyzed in the real data.
| [1] |
We then sampled these beams as they would have been sampled by the array and reconstructed them using the method described above. Results showed that, for the range of distances (0–3 m) and flight angles (0°–30°) observed in the real data, we could reliably reconstruct the beams with an error of up to 5° (Fig. 4 B and C).
Where S is the sound amplitude level in decibels, is the Bessel function of first kind, is the wavenumber, is the aperture radius, is the sound absorption, is the angle relative to beam peak, and is the distance from source element.
We also used these simulations to validate our method for rejecting beams that were not directed toward the array (i.e., beams with peaks that were not centered within the array’s borders; see above). To this end, we simulated 10 beams that were directed outside of the array’s border (up to 30° from its borders) and 10 beams that were directed within its borders. We then ran the beam rejection process described above. All beams that were directed within the array were detected as such and reconstructed, and all beams that were directed more than 5° from the array boundary (9 of 10) were rejected by our method.
Statistical Analysis.
Pearson linear correlations were computed with Matlab. Because we pooled data across many bats and had a maximum of four data points per trial (usually less), data points were nondependent. However, to make sure that the high observed correlations do not result from interdependencies, we ran the same analysis without using consecutive beams within a trajectory. Correlation values for this manipulation (along with the new P values) are presented in parentheses beside the values for all data points in Fig. 1. This manipulation did not alter the correlation significance.
Pond Ensonification.
Signals were generated using an ultrasonic speaker (vifa; Avisoft) that emitted a 3-ms frequency-modulated chirp around the relevant bat frequencies (70–30 kHz). The echoes were recorded with a CM16 Microphone (Avisoft) sampled by an Hm116 A/D converter with a sampling rate of 275 kHz. The speaker was positioned at six different locations along common trajectories used by the bat and pointed at different directions (Fig. S1). The echoes’ envelope was estimated to analyze the echoes’ intensity.
Supplementary Material
Acknowledgments
We thank K. Koselj for helpful comments on the manuscript. We also thank Philip Bamberger, Bethany Rielly, and Jack Wright for help with data collection.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.A.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1422843112/-/DCSupplemental.
References
- 1.Griffin DR. Listening in the Dark; the Acoustic Orientation of Bats and Men. Yale Univ Press; New Haven, CT: 1958. [Google Scholar]
- 2.Jakobsen L, Ratcliffe JM, Surlykke A. Convergent acoustic field of view in echolocating bats. Nature. 2013;493(7430):93–96. doi: 10.1038/nature11664. [DOI] [PubMed] [Google Scholar]
- 3.Jakobsen L, Surlykke A. Vespertilionid bats control the width of their biosonar sound beam dynamically during prey pursuit. Proc Natl Acad Sci USA. 2010;107(31):13930–13935. doi: 10.1073/pnas.1006630107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yovel Y, Falk B, Moss CF, Ulanovsky N. Active control of acoustic field-of-view in a biosonar system. PLoS Biol. 2011;9(9):e1001150. doi: 10.1371/journal.pbio.1001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surlykke A, Boel Pedersen S, Jakobsen L. Echolocating bats emit a highly directional sonar sound beam in the field. Proc Biol Sci. 2009;276(1658):853–860. doi: 10.1098/rspb.2008.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuta N, et al. Adaptive beam-width control of echolocation sounds by CF-FM bats, Rhinolophus ferrumequinum nippon, during prey-capture flight. J Exp Biol. 2013;216(Pt 7):1210–1218. doi: 10.1242/jeb.081398. [DOI] [PubMed] [Google Scholar]
- 7.Hartley DJ, Suthers RA. The sound emission pattern of the echolocating bat, Eptesicus fuscus. J Acoust Soc Am. 1989;85(3):1348. doi: 10.1121/1.395684. [DOI] [PubMed] [Google Scholar]
- 8.Henze D, O’Neill WE. The emission pattern of vocalizations and directionality of the sonar system in the echolocating bat, Pteronotus parnelli. J Acoust Soc Am. 1991;89(5):2430–2434. doi: 10.1121/1.400975. [DOI] [PubMed] [Google Scholar]
- 9.Mogensen F, Mohl B. Sound radiation patterns in the frequency domain of cries from a vespertilionid bat. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1979;134(2):165–171. [Google Scholar]
- 10.Skolnik M. Radar Handbook. 3rd Ed McGraw-Hill; New York: 2008. [Google Scholar]
- 11.Surlykke A, Jakobsen L, Kalko EKV, Page RA. Echolocation intensity and directionality of perching and flying fringe-lipped bats, Trachops cirrhosus (Phyllostomidae) Front Physiol. 2013;4:143. doi: 10.3389/fphys.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons NB. Mammal Species of the World : A Taxonomic and Geographic Reference, 2-Volume Set. Johns Hopkins Univ Press; Baltimore: 2005. [Google Scholar]
- 13.Strother GK, Mogus M. Acoustical beam patterns for bats: Some theoretical considerations. J Acoust Soc Am. 1970;48(6 Suppl 2) doi: 10.1121/1.1912304. [DOI] [PubMed] [Google Scholar]
- 14.Greif S, Siemers BM. Innate recognition of water bodies in echolocating bats. Nat Commun. 2010;1:107. doi: 10.1038/ncomms1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siemers BM, Stilz P, Schnitzler HU. The acoustic advantage of hunting at low heights above water: Behavioural experiments on the European ‘trawling’ bats Myotis capaccinii, M. dasycneme and M. daubentonii. J Exp Biol. 2001;204(Pt 22):3843–3854. doi: 10.1242/jeb.204.22.3843. [DOI] [PubMed] [Google Scholar]
- 16.Holderied MW, von Helversen O. Echolocation range and wingbeat period match in aerial-hawking bats. Proc Biol Sci. 2003;270(1530):2293–2299. doi: 10.1098/rspb.2003.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.