Significance
Modern research on the origin of life started with Urey–Miller’s 1953 report on the spontaneous formation of amino acids upon application of electric discharge on a model of the pristine Earth atmosphere. Formamide provides a chemically sound starting material for the syntheses of prebiotic compounds; its role in prebiotics is becoming recognized. Kiloparsecs-wide clouds of formamide were observed in the interstellar space. The energy sources for the syntheses explored so far were largely thermal, and the catalysts used were mostly terrestrial. In the presence of meteorites and with high-energy protons, we observe the production of unprecedented panels of nucleobases, sugars, and, most notably, nucleosides. Carboxylic acids and amino acids complete the recipe. These findings extend prebiotic plausible scenarios well beyond our planet.
Keywords: origin of life, formamide, prebiotic syntheses, nucleosides, meteorites
Abstract
Liquid formamide has been irradiated by high-energy proton beams in the presence of powdered meteorites, and the products of the catalyzed resulting syntheses were analyzed by mass spectrometry. Relative to the controls (no radiation, or no formamide, or no catalyst), an extremely rich, variegate, and prebiotically relevant panel of compounds was observed. The meteorites tested were representative of the four major classes: iron, stony iron, chondrites, and achondrites. The products obtained were amino acids, carboxylic acids, nucleobases, sugars, and, most notably, four nucleosides: cytidine, uridine, adenosine, and thymidine. In accordance with theoretical studies, the detection of HCN oligomers suggests the occurrence of mechanisms based on the generation of radical cyanide species (CN·) for the synthesis of nucleobases. Given that many of the compounds obtained are key components of extant organisms, these observations contribute to outline plausible exogenous high-energy–based prebiotic scenarios and their possible boundary conditions, as discussed.
Hypothesizing formamide (FA) as parent molecule, we explored its potentiality in synthetic processes when exposed to proton irradiation. The purpose of this analysis is to verify a possible prebiotic scenario consisting of FA, considered here as starting one-carbon atom material, of proton beams mimicking solar energetic particles as energy source, and of meteorites as catalysts. The rationale of this approach is that the results could help in outlining exogenous prebiotic models and their boundaries.
FA (NH2CHO) is becoming one of the most intensively studied precursors for prebiotic syntheses of compounds potentially relevant for the origin of life (1–4). FA is a ubiquitous molecule in the universe. It has been detected in galactic centers (Sgr A and Sgr B), in star-forming regions of dense molecular clouds, in high-mass young stellar objects, in the interstellar medium and in comets and satellites (5–14).
With the appropriate mineral as catalyst, different ensembles of intermediates of genetic and metabolic apparatuses are simultaneously synthesized from FA under thermal conditions (i.e., by heating liquid FA between 333 and 453 K at room pressure). DNA and RNA components (15–21), amino acids (22, 23), sugars (24), and carboxylic acids (25, 26) have been obtained. Minerals tune the selectivity of these transformations (1, 2), the mechanistic pathways for the synthesis of nucleobases requiring pyrimidine (27–30) or imidazole intermediates (31, 32).
Within the solar system, ionizing cosmic radiation is generated by the Sun [solar cosmic rays (SCRs), primarily protons accelerated by flares and coronal mass ejections to energies typically of tens to hundreds megaelectronvolts] and is also formed by particles coming from the deep universe [galactic cosmic rays (GCRs)] (33). The SCRs and GCRs differ in their components and energy spectra, but their overwhelming component is protons.
As an alternative to heat, radiation is a plausible energy source for prebiotic processes (34–36). After irradiation, FA can reach singlet (S1 and S2) or triplet (T1) excited states by n(HOMO)/π*(LUMO) and π(HOMO-1)/π*(LUMO) electronic transitions, respectively (37). The energy profiles for the decomposition of excited states of FA have been studied at different theoretical levels, suggesting the formation of reactive nitrogen- and carbon-centered radical species (38–41). The synthesis of low–molecular-weight compounds (such as carbon oxides COx, HCN, isocyanide, and NH3) occurs by fast quenching or coupling reactions followed by dehydration, elimination, and dehydrogenation processes. This was shown by a variety of high-energy condensed and gas-phase processes, consisting on UV photodecomposition (42, 43), laser spark (44), synchrotron light (45), low-energy protons (200 keV) (46), and Lyman-α photons (47) irradiations. On the other hand, radiative conditions supposedly exist under which the radical species might react to yield complex and biologically relevant organic compounds. As an example, the synthesis of purine nucleobases by energetically favorable multistep addition of cyanide radicals (•CN) on FA has been predicted on the basis of the density function theory (B3LYP with the 6–311 G d.p basis set) (48). The CN• radical was discovered in interstellar space and in envelopes of giant stars (49). In addition, the energy profile of excited states of FA are profoundly modified after interaction with metal ions (50). The interaction of FA with Li+ and Na+ leads to a pronounced shift of the n-π* state to higher energies (while the π-π* state moves in opposite direction) with strong geometrical effects favoring the formation of •CN radicals. Thus, the interaction of FA with metal ions or minerals may generate conditions that are energetically favorable to the increase of the structural complexity of end products (51).
Meteorites might have played a major role in the chemical inventory of the early solar system for origin-of-life processes, behaving (52, 53) as carriers of organic molecules in connection with the Earth heavy-bombardment period (54, 55) and preceding the supposed earliest existence of living organisms (56). Evidence of the catalytic properties of meteorites in prebiotic chemistry was recently provided by thermal processes of condensed-phase FA at relatively high temperatures (333 and 413 K) (57, 58) or at the most extreme temperature conditions modeling impact events in Earth’s atmosphere or surface (3, 59). During their wandering, meteorites are exposed, under an extreme variety of temperature conditions, to ionizing radiation of cosmic origin (60) able to excite and dissociate FA (the ionization energy of the FA being ∼11–15 eV).
Thus, we asked the question: May protons and meteorites be the benign environment for the formation of biomolecules from FA? As a result, we observed an unprecedented one-pot synthesis of nucleosides, nucleobases, and other intermediates of the genetic and metabolic apparatuses by 170-MeV proton irradiation of FA. The formation of nucleosides is particularly noteworthy because of the known difficulty of obtaining these key components of nucleic acids in prebiotic conditions.
Results
FA was proton irradiated in the presence of meteorites. Detailed synthetic and analytical procedures are in Experimental Methods and SI Appendix. In outline, 11 meteorites classified into the iron (Canyon Diablo and Campo del Cielo), stony-iron (NWA 4482), chondrite (NWA 2828, Gold Basin, Dhofar 959, Orgueil, NWA 1465, and Chelyabinsk), and achondrite (NWA 5357 and Al Haggounia 001) families were used in the FA proton irradiation experiments. References, composition, historical and terrestrial provenience, and cosmo-origin data are available in SI Appendix, section S1.
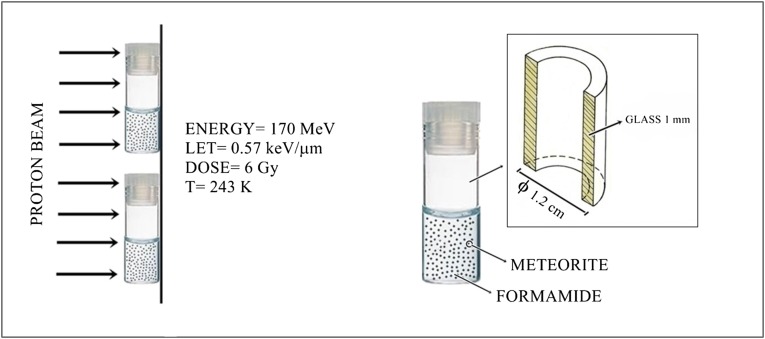
Neat FA (2.5 mL) mixed with meteorite powder (1.0% in weight, corresponding to 28 mg; similar grain size distribution of particles in the range of 0–125 μm) was irradiated at 243 K with 170-MeV protons for 3 min, as schematically shown in Fig. 1. The uniform proton field was bounded 10 × 10 cm2 by the collimator system. The averaged linear energy transfer (LET) was about 0.57 keV/μm, and the calculated absorbed dose was 6 Gy. The reactions were also performed with representative minerals that are known components of meteorites.
Fig. 1.
Neat FA mixed with meteorite powder was irradiated at 243 K with 170-MeV protons for 3 min. The uniform proton field was bounded 10 × 10 cm2 by the collimator system. The averaged linear energy transfer (LET) was 0.57 keV/μm, and the calculated absorbed dose was 6 Gy. Eleven meteorites classified into the iron (Canyon Diablo and Campo del Cielo), stony-iron (NWA 4482), chondrite (NWA 2828, Gold Basin, Dhofar 959, Orgueil, NWA 1465, and Chelyabinsk), and achondrite (NWA 5357 and Al Haggounia 001) families were used in the FA irradiations. The products were analyzed by gas chromatography–mass spectrometry (GC-MS) after formation of the corresponding trimethylsilyl ethers (TMS).
The presence of organics in the original sample [observed only in the parts-per-billion range (61, 62)] was prevented by extracting the powder with NaOH (0.1 M), CHCl3–CH3OH (2:1, vol/vol) and sulfuric acid followed by pyrolysis at 600 °C. After the treatment, the powders did not release organic substances. The products were analyzed by gas chromatography–mass spectrometry (GC-MS) after formation of the corresponding trimethylsilyl ethers (TMS). The analysis was limited to products ≥1 ng/mL, and the yield was calculated as milligrams (or micrograms) of product per milliliter of starting FA. Irradiation of FA without addition of meteorite powder was performed as reference. In this case, few products were detected in amounts lower than 0.05 μg/mL, including N,N-diethyl formamide, 3-hydroxy pyridine, pyruvic acid, and 2-propanol, besides unidentified peaks (Scheme, original chromatogram, and m/z fragmentation spectra of products are in SI Appendix, section S2. Previous study of irradiation of FA in ice at 20 K with low-energy protons (100 keV) only afforded CO, CO2, N2O, HNCO, and NH4+OCN− (63). Noteworthy, nucleosides, nucleobases (and their isomers and analogs), carboxylic acids, amino acids, and sugars were synthesized in the presence of meteorites. Intermediates for the formation of biomolecules were also detected, confirming previously proposed theoretical mechanisms (see below). Fig. 2 and Tables 1–5 show the most abundant products (m/z values and peak abundances are in SI Appendix, section S3; selected chromatograms and original m/z fragmentation spectra are in SI Appendix, sections S4 and S5, respectively). The treatment of FA with meteorites at 243 K in the absence of protons did not afford products in appreciable yield, even for prolonged reaction time (24 h).
Fig. 2.
Products of high-energy proton irradiation of FA in the presence of meteorites. (A1) Nucleobases, nucleobase analogs, and intermediates of the condensation. (A2) Sugars and sugar derivatives. (A3) Nucleosides. The yields of compounds in A1–A3 are in Table 1. (B) Carboxylic acids. The yields of compounds in B are in Table 3. (C) Amino acids. The yields of compounds in C are in Table 5.
Table 1.
Synthesis of nucleosides, nucleobases (and their analogs), and sugars: Products (in micrograms) grouped by meteorite type
| Product* | Canyon Diablo | Campo del Cielo | NWA 4482 | NWA 2828 | Gold Basin | Dhofar 959 | NWA 1465 | Chelyabinsk | Orgueil | NWA 5357 | Al Haggounia |
| Uracil (1) | — | — | 0.10 | 0.60 | 0.4 | — | 0.4 | 0.17 | 0.6 | 0.2 | 1.2 |
| Cytosine (2) | 0.3 | 0.1 | 0.23 | 0.32 | 0.28 | — | 0.35 | — | 0.47 | 0.5 | 0.7 |
| Thymine (3) | — | — | 0.05 | 0.05 | 0.08 | — | 0.1 | — | 0.4 | — | — |
| Adenine (4) | — | — | 0.20 | — | 0.04 | 0.13 | 1.3 | 0.05 | 1.2 | 0.6 | 0.54 |
| Guanine (5) | — | — | — | 0.2 | 0.05 | 1.10 | 1.4 | — | 0.8 | 0.08 | 0.57 |
| Purine (6) | — | — | — | 0.4 | 0.6 | — | — | — | — | — | — |
| Isocytosine (7) | 0.7 | 1.6 | 0.75 | 1.2 | 2.2 | — | 3.0 | — | 6.7 | 0.5 | 0.2 |
| Hypoxanthine (8) | 3.0 | 6.58 | — | — | 3.8 | 4.48 | 3.2 | 1.47 | 0.2 | — | 0.53 |
| 2,6-Diaminopurine (9) | — | — | — | 0.4 | 0.05 | — | 0.5 | — | 3.2 | — | 0.48 |
| Orotic acid (10) | — | — | — | — | — | — | 0.5 | — | — | — | 0.54 |
| 4,6-DHP (11) | 0.5 | 0.1 | — | — | — | — | — | — | 0.1 | 0.03 | 0.75 |
| 4(3H)pyrimidone (12) | — | — | — | 0.1 | — | — | 0.004 | — | 0.05 | 0.39 | — |
| 3(OH)pyridine (13) | — | — | 0.25 | — | — | — | — | — | 0.26 | — | — |
| AHMN (14) | — | — | — | — | 0.06 | — | — | — | 0.22 | 0.13 | |
| DAMN (15) | — | — | 0.05 | — | 0.12 | 0.35 | — | — | 0.93 | 0.47 | 0.03 |
| 4-AMI (16) | — | — | 0.19 | 0.15 | 0.2 | 0.05 | — | — | — | — | 0.52 |
| Ribose (17) | — | — | 1.01 | 0.16 | — | — | 0.30 | — | — | — | 0.10 |
| 2´-Deoxyribose (18) | — | — | 2.30 | 0.98 | 0.10 | 1.28 | 0.44 | 0.05 | 0.43 | — | 0.80 |
| Glucose (19) | — | — | — | 0.12 | — | — | — | — | 0.31 | — | — |
| 2´-Deoxyglucose (20) | — | — | 0.05 | 0.66 | 0.08 | 0.27 | 1.06 | — | 1.68 | — | — |
| Galactose (21) | — | — | 0.70 | — | — | 0.80 | — | 0.42 | — | ||
| Mannose (22) | — | — | — | — | 0.03 | — | 0.44 | — | — | — | — |
| Inositol (23) | — | — | 2.88 | — | — | — | — | — | — | — | — |
| Arabitol (24) | — | — | — | 0.67 | — | 0.39 | — | — | — | — | — |
| Uridine (25) | — | — | 0.54 | — | 0.03 | — | 0.96 | 0.01 | 1.0 | — | 0.45 |
| Cytidine (26) | — | — | — | — | — | — | 2.2 | — | 0.03 | — | — |
| Adenosine (27) | — | — | — | — | — | 0.58 | — | 0.76 | — | — | 0.49 |
| Thymidine (28) | — | — | — | — | — | — | — | — | — | — | 0.77 |
The data are the mean values of three experiments with SD less than 0.1%. Products are given in micrograms (per 1-mL FA reactions). See Nucleosides, Nucleobases, and Sugars.
Table 5.
Synthesis of amino acids in the presence of meteorites: Products (in micrograms) grouped by meteorite type
| Product* | Canyon Diablo | Campo del Cielo | NWA 4482 | NWA 2828 | Gold Basin | Orgueil | NWA 5357 | Al Haggounia |
| Glycine (51) | 0.11 | 0.20 | 0.86 | 0.13 | 0.05 | 0.05 | 0.11 | 0.12 |
| Formyl glycine (52) | 0.04 | 0.32 | 1.18 | 0.03 | 1.10 | 0.07 | 2.66 | 0.04 |
| Alanine (53) | 0.02 | 0.06 | 1.5 | 0.6 | — | 0.1 | 0.05 | — |
| Formyl alanine (54) | 0.1 | 0.03 | 0.56 | 0.23 | — | 0.03 | — | |
| 2(Me)alanine (55) | 0.13 | 0.82 | — | 1.0 | — | 1.0 | 1.14 | — |
| Proline (56) | — | — | 0.71 | 0.12 | — | 0.03 | — | — |
| Pyroglutamic acid (57) | — | — | — | 0.64 | 0.02 | — | — | 0.10 |
| β-AIBA (58) | — | — | — | — | — | — | 3.36 | — |
| 2-Pyrrolidone (59) | — | — | — | — | — | — | — | — |
| Urea (60) | — | — | — | — | 0.03 | 0.02 | — | 0.03 |
| Guanidine (61) | 0.05 | 0.12 | — | 0.62 | — | — | — | — |
The data are the mean values of three experiments with SD less than 0.1%. Products are given in micrograms (per 1-mL FA reactions). See Amino Acids.
Nucleosides, Nucleobases, and Sugars.
Nucleobases.
The complete set of nucleobases of RNA and DNA molecules [uracil (1), cytosine (2), thymine (3), adenine (4), and guanine (5)] was obtained in different yields and selectivities depending on the meteorite used in the irradiation (Table 1). As a general trend, stony-iron, chondrite (with the only exception of Chelyabinsk), and achondrite meteorites were more active than iron meteorites. Among chondrites, the total nucleobases abundances were higher with carbonaceous (Orgueil) and CV3 (NWA 1465) groups compared with the other types. A differential distribution of indigenous nucleobases in chondrites was also previously observed during extraction experiments (54). As for the selectivity of the transformation, Gold Basin, NWA 1465, and Orgueil produced the complete set of nucleobases. Other meteorites afforded at least four nucleobases: NWA 4482 (stony iron), which only lacked guanine, NWA 5357, and Al Haggounia (achondrites), which only lacked thymine. Nucleobases were isolated in the range of 0.1 μg/mL (Campo del Cielo) and 4.45 μg/mL (NWA 1465), respectively (total amount of nucleobases). Overall, pyrimidine and purine nucleobases were obtained in comparable amounts: pyrimidines in the range between 0.1 μg/mL (Campo del Cielo) and 1.9 μg/mL (Al Haggounia), and purines between 0.09 μg/mL (Gold Basin) and 2.7 μg/mL (NWA 1465), respectively. Along with nucleobases 1–5, purine (6) and several related analogs and isomers, including isocytosine (7), hypoxanthine (8), 2,6-diamino purine (9), uracil 6-carboxylic acid (orotic acid, 10), 4,6-dihydroxy pyrimidine (11), and 4(3H)-pyrimidone (12) were produced in appreciable yield. 3-Hydroxy pyridine (13) (detected in the reference irradiation) was observed in few cases (NWA 4482 and Orgueil). The biological relevance of compounds 7, 8, and 13 in the context of the origin of life and of chemiomimesis has been previously reviewed [i.e., for isocytosine (7) (64) and for hypoxanthine (8) (65–67)]. For pyrimidone derivatives, see ref. 68. 2,6-Diamino purine (9), considered as possible extraterrestrial marker because it is not typically found in terrestrial biochemistry (62), operates in some microorganisms as bioisoster of adenine efficiently pairing with thymine by three intramolecular hydrogen bonds, thus eliminating the major difference between the two types of Watson–Crick interactions (68). Orotic acid (10) is a key intermediate in the pyrimidine synthesis pathway (69), whereas 4,6-dihydroxypyrimidine (11) is one of the possible isomers of uracil.
2-Amino-2-hydroxymalonitrile (AHMN) (14), diaminomalonitrile (DAMN) (15), and 4-aminoimidazole (4-AMI) (16) were also detected in our experiments. These compounds are key intermediates in the highly exergonic synthesis of purine nucleobases through a multistep pathway requiring the addition of the cyanide radical (•CN) on FA. Minerals and meteorites are expected to tune the reactivity and selectivity of the condensation processes, via binding and concentrating, or quenching, the •CN radicals (70). Thus, the low reactivity observed for iron meteorites can be correlated to the known ability shown by iron ions in quenching radical species (71).
As for pyrimidine nucleobases, the synthesis of thymine (which is of notoriously difficult preparation under prebiotic conditions) requires formaldehyde (HCOH). Formaldehyde is generated “in situ” by the radical degradation of FA (72–75). Once formed, it can add on the C-5 electrophilic position of uracil to give 5-hydroxymethyl uracil as intermediate, which then rearranges to thymine (76).
Sugars.
The presence of HCOH is further confirmed by the detection of different monosaccharides, including pentoses [ribose (17) and 2′-deoxyribose (18)] and hexoses [glucose (19), 2′-deoxyglucose (20), galactose (21), and mannose (22)]. Inositol (23) and arabitol (24), produced by reduction of monosaccharides, were also observed. In GC-MS, almost all of the monosaccharides showed two peaks after TMS derivatization, due to α- and β-configuration of the anomeric OH (selected chromatograms are in SI Appendix, section S4) (77). Irrespective of the sugar, the mass spectra were characterized by m/z 204, 217, and 191 fragment ions, as specific markers (78). In the case of hexoses 19–22, the prevalence of the m/z 204 fragment ion confirmed the glucopiranose form, whereas that of m/z 217 for pentoses 17 and 18 suggested the glucofuranose structure (SI Appendix, sections S3 and S5) (79).
Monosaccharides and other sugar-like molecules are synthesized in prebiotic conditions by polymerization of HCOH, the so-called formose reaction, an aldolic-like condensation occurring under both thermal and radiation conditions (80–82). In our case, the prevalence of monosaccharides characterized by a vicinal cis-diol moiety suggests a possible participation of the surface of meteorites to condensation. In fact, monosaccharides should tend to grow with hydroxyl groups oriented on the same side of the molecule as a result of favorable interactions (complexation, electrostatic, or hydrogen bond interactions) with the defective sites and/or metals present on the surface of the minerals (83, 84). This effect is particularly evident in the case of minerals containing boron, which is able to form very stable complexes with vicinal hydroxyl groups (85–87).
Nucleosides.
The simultaneous presence of nucleobases and monosaccharides suggested the possibility of the formation of nucleosides. In this regard, the analysis was limited to the identification of natural ribonucleosides and 2′-deoxyribonucleosides, due to the high complexity of the reaction mixtures. Noteworthy, four nucleosides were detected in defined instances: uridine (25) (NWA 4482, NWA 1465, and Orgueil), cytidine (26) (NWA 1465), adenosine (27) (Dhofar 959), and thymidine (28) (Al Haggounia) (Table 1). As for nucleobases, stony iron, chondrites, and achondrites were the most efficient catalysts.
The structure of compounds (26–28) was unambiguously defined by comparison of the mass fragmentation peaks in the GC-MS analysis (as TMS derivatives) with original commercial samples and with data stored in the appropriate software [National Institute of Standards and Technology (NIST) bank of data]. The assignment was further confirmed by the addition method (comparison of the retention times). The nucleosides displayed the expected molecular ions (M) and specific fragment ions due to subsequent loss of trimethylsilyl (M-TMS) or trimethyl silanol (M-TMSOH) moieties (or part of them, e.g., methyl radicals M-15). Moreover, the presence of fragment ions characteristic of the sugar moiety (m/z 204, 217, and 191) and that of monosilylated nucleobases (produced after cleavage of the glycosidic bond) further confirmed the assigned structures (SI Appendix, sections S3 and S5) (88, 89).
Although the synthesis of nucleosides by irradiation of FA is less efficient than that of nucleobases, their presence is relevant because the formation of the glycosidic bond remains one of the most difficult process to be accomplished in prebiotic conditions. The direct condensation of preformed sugars and nucleobases does not, as judged on the basis of existing data, work efficiently in H2O either for kinetic and thermodynamic reasons (90). Examples of alternative reaction pathways are the multistep synthesis of β-ribocytidine-2′,3′-cyclic phosphate from glycolaldehyde and cyanamide (91) through pentose-oxazoline intermediate (92–95). The formation of acyclo nucleosides by thermal condensation of FA on TiO2 (in dark) is another example of synthesis of nucleoside analogs (19). The homochirality of 25–28 was not a concern of this study. Usually biomolecules are isolated as racemate from meteorites (96). Recently, the β-stereocontrolled β-glycosylation was achieved in homogeneous conditions by formation of noncovalent H-bonded complexes between ribose and nucleobase (97), and processes to amplify the natural chiral form of nucleosides, e.g., the solubility-based amplification, can occur under credible prebiotic conditions (98).
To analyze the role played by individual components of the inorganic matrix of meteorites on the reactivity of FA, the irradiation was repeated with minerals that were selected for being well-known components of meteorites. These included montmorillonite [phyllosilicate, (Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O)] and covellite (CuS) [detected in chondrites such as Orgueil and Yamato 691, respectively (99, 100)], chalcopyrite (CuFeS2) and magnetite (Fe3O4) (which were detected in Martian meteorites), and troilite (FeS) [detected in Martian meteorites and iron meteorites, such as Canyon Diablo (101, 102)]. As reported in Table 2, minerals are overall less efficient than meteorites in the synthesis of nucleobases. In particular, purine nucleobases prevailed on pyrimidines, adenine and guanine being isolated in the reactions with at least three of the minerals studied (montmorillonite, troilite, and magnetite; covellite was completely nonreactive). Cytosine was synthesized only in the presence of chalcopyrite and troilite, whereas none of the minerals led to the formation of uracil and thymine.
Table 2.
Synthesis of nucleobases and analogs by irradiation of FA with minerals
| Product* | Covellite | Chalcopyrite | Montmorillonite | Troilite | Magnetite |
| Uracil (1) | — | — | — | — | — |
| Cytosine (2) | — | 1.20 | — | 0.20 | — |
| Thymine (3) | — | — | — | — | — |
| Adenine (4) | — | — | — | — | 0.98 |
| Guanine (5) | — | — | 0.61 | 0.58 | 2.32 |
| Purine (6) | — | — | — | — | — |
| Isocytosine (7) | — | 2.18 | — | 0.13 | — |
| Hypoxanthine (8) | 0.07 | — | 1.13 | 0.72 | — |
| 2,6-Diaminopurine (9) | — | — | — | — | 1.67 |
| Orotic acid (10) | — | — | 0.40 | 1.09 | 0.40 |
| 4(3H)pyrimidone (12) | 0.63 | 3.0 | — | 0.41 | — |
| AHMN (14) | — | 0.11 | 0.08 | 0.12 | 2.31 |
| DAMN (15) | — | — | — | — | 0.36 |
| 4-AIM (16) | — | 0.13 | — | — | 2.31 |
The data are the mean values of three experiments with SD less than 0.1%. Products are given in micrograms (per 1-mL FA reactions). See Nucleosides, Nucleobases, and Sugars.
In the case of the analogs (and isomers) of nucleobases, hypoxanthine, isocytosine, and 2,6-diamino purine were obtained as major products, in addition to orotic acid, AHMN, DAMN, and 4-AMI. Nucleoside derivatives were not isolated. Only a few relationships could be defined in the reactivity of minerals versus that of meteorites (e.g., the similar selectivity in the synthesis of nucleobases shown by troilite and iron meteorites Canyon Diablo e Campo del Cielo; see Table 1 versus Table 2). As a general consideration, the presence of synergistic effects by multiple mineral components in meteorites is hinted to by the comparison of their performance with the low efficacy shown by single minerals.
Carboxylic Acids.
Carboxylic acids with increasing levels of structural complexity are key intermediates of numerous processes and metabolic cycles required in the cell for the production of energy and for the biosynthesis of primary and secondary metabolites. Different carboxylic acids have been identified in meteorites (103). FA irradiation afforded 21 carboxylic acids whose yield is orders-of-magnitude higher than that reported for indigenous organics in carbonaceous chondrites (Fig. 2 and Table 3) (61, 104). They include the following: oxalic (29), glycolic (30), pyruvic (31), lactic (32), malonic (34), succinic (35), oxalacetic (36), ketoglutaric (37), hexanoic (38), ketoisocaproic (39), citric (40), pimelic (41), octanoic (42), nonanoic (43), azelaic (44), lauric (45), myristic (46), palmitic (47), heptadecanoic (48), stearic (49), and arachidic (50) acids.
Table 3.
Synthesis of carboxylic acids in the presence of meteorites: Products (in micrograms) grouped by meteorite type
| C/molecule | Products* | Canyon Diablo | Campo del Cielo | NWA 4482 | NWA 2828 | Gold Basin | Dhofar 959 | NWA 1465 | Chelyabinsk | Orgueil | NWA 5357 | Al Haggounia |
| C2 | Oxalic (29) | 0.11 | 0.19 | 1.93 | 0.21 | — | 0.05 | 0.1 | 0.03 | 0.08 | 1.6 | 0.2 |
| Glycolic (30) | 0.1 | — | 0.51 | — | 0.11 | — | — | — | — | 0.1 | — | |
| C3 | Pyruvic (31) | 0.09 | 0.13 | 0.33 | 0.05 | 0.16 | 0.1 | 2.1 | 0.08 | — | 1.33 | 0.012 |
| Lactic (32) | 0.1 | — | 0.59 | 2.4 | 0.12 | 0.31 | 0.9 | 0.8 | 0.17 | 0.04 | 0.91 | |
| Malonic (33) | 0.01 | 0.01 | 3.23 | — | — | — | — | — | — | 0.36 | 0.2 | |
| Succinic (34) | — | — | 0.37 | 0.9 | 0.13 | 0.02 | 1.0 | 0.5 | 0.8 | — | 0.03 | |
| Oxaloacetic (35) | 0.02 | 0.04 | 1.54 | — | — | — | — | — | 0.52 | 1.95 | — | |
| C5 | Ketoglutaric (36) | — | — | 0.58 | 0.05 | — | — | 0.2 | — | 0.07 | — | — |
| C6 | Hexanoic (37) | 0.01 | 0.04 | — | — | — | — | — | — | — | 0.67 | — |
| Ketoisocaproic (38) | 0.03 | 0.022 | — | — | — | — | — | — | — | — | — | |
| Citric (39) | 0.01 | 0.01 | 0.01 | — | 0.1 | — | 0.3 | — | 0.05 | — | — | |
| C7 | Pimelic (40) | — | — | — | 0.03 | — | — | 0.1 | — | 0.01 | — | — |
| C8 | Octanoic (41) | 0.2 | 0.11 | — | — | — | — | 4.6 | — | — | 2.88 | — |
| C9 | Nonanoic (42) | — | — | 0.65 | — | — | — | — | — | — | 3.08 | — |
| Azelaic (43) | — | — | 0.58 | 1.1 | 0.29 | — | 4.3 | — | 0.24 | — | — | |
| C12 | Lauric (44) | — | — | 0.65 | 0.2 | — | — | 0.9 | — | 0.3 | — | 0.4 |
| C14 | Myristic (45) | — | — | 2.21 | — | — | — | 1.2 | — | 0.27 | — | — |
| C16 | Palmitic (46) | — | — | 0.64 | 2.5 | 1.6 | — | 2.0 | 0.35 | 2.05 | 0.19 | 0.42 |
| C17 | Heptadecanoic (47) | — | — | 0.37 | 0.1 | — | — | — | — | — | — | — |
| C18 | Stearic (48) | — | 3.2 | — | 2.6 | 2.5 | 1.8 | — | 0.49 | — | 0.12 | 0.38 |
| C20 | Arachidic (49) | — | — | — | — | 0.7 | — | — | — | — | 0.01 | 0.99 |
| Glycerol (50) | — | — | 1.21 | — | 0.10 | — | — | 1.45 | 0.46 | — | — |
The data are the mean values of three experiments with SD less than 0.1%. Products are given in micrograms (per 1-mL FA reactions). See Carboxylic Acids.
In terms of biological relevance, compounds 31, 34, 35, 36, and 39 [this latter isolated for the first time (to our knowledge) in FA condensation] are intermediates of the citric acid cycle [tricarboxylic acid cycle or Krebs cycle (KC)], one of the most ancient processes in cell metabolism (105). KC has been postulated to be (in its reversed version) a prebiotic device for the production of energy and organics in the carbon oxides rich primitive atmosphere (106). Acids 29 and 30 are involved in the glyoxilate cycle, an anabolic alternative pathway of the KC occurring in plants, bacteria, protists, and fungi (107), 32 in the Cori cycle and gluconeogenesis (108), whereas 38 is an intermediate in the metabolism of leucine (109), and 40 of lysine (110). 43 is involved in defense responses after infection in plants (111, 112), and the other long-chain carboxylic acids 44–49 are constituents of lipids (glycerol, the alcoholic component of lipids, was also detected in the reaction mixtures).
The chondrite NWA 1465 and the stony-iron meteorite NWA 4482 performed as the best catalysts (total amount of carboxylic acids, 17.7 and 14.19 mg/mL, respectively), followed by the other chondrites and achondrites, the iron meteorites being once more the least efficient systems. In terms of structural complexity (defined as the number of carbon atoms in the molecule, Cn), stony iron, chondrites, and achondrites catalyzed the synthesis of carboxylic acids with the largest variety of structural complexity (from C2 to C20 derivatives), whereas iron meteorites produced carboxylic acids containing at most eight carbon atoms (C8). The formation of acids 29–32 may be explained by the in situ generation of HCN followed by oligomerization to DAMN (actually detected in the reaction mixture), hydrolysis, and successive redox processes (112). Moreover, carboxylic acids have been also produced in low amount during UV photoprocessing of interstellar ice analogs (113), i.e., by slow deposition of simple mixtures of CO, NH3, and H2O, with formation, among others, of 30 and 32 (114). In this latter case, radical mechanisms involving nitrogen and carbon-centered species have been hypothesized (115). The addition of •CN to unsaturated hydrocarbons to give nitriles followed by reaction with ketene is another possible mechanism for the synthesis of meteoritic carboxylic acids. Notably, single minerals are more efficient in the synthesis of carboxylic acids than nucleobases and, irrespective of their elemental composition, produce a large variety of derivatives (Table 4). (The analysis was limited to derivatives with at least five carbon atoms.)
Table 4.
Synthesis of carboxylic acids in the presence of selected minerals: Products (in micrograms) grouped by meteorite type
| C/molecule | Products* | Covellite | Chalcopyrite | Montmorillonite | Troilite | Magnetite |
| C2 | Oxalic (29) | 0.05 | 1.78 | 0.67 | 1.24 | — |
| Glycolic (30) | 1.2 | 0.88 | — | — | — | |
| C3 | Pyruvic (31) | 0.6 | 0.55 | 0.63 | 0.61 | — |
| Lactic (32) | 0.07 | 1.33 | 0.84 | 1.82 | 0.43 | |
| Malonic (33) | — | 0.81 | 0.22 | — | — | |
| Succinic (34) | — | — | 1.13 | — | — | |
| Oxaloacetic (35) | 1.1 | 1.22 | — | — | 0.56 | |
| C5 | Ketoglutaric (36) | 0.08 | 0.16 | — | — | 0.80 |
| Citric (39) | 0.01 | 0.01 | — | — | — | |
| Lauric (44) | — | — | — | 0.12 | — | |
| Palmitic (46) | 0.1 | 1.15 | 9.5 | 3.43 | — | |
| Stearic (49) | 0.3 | 1.42 | 1.73 | 2.34 | — | |
| Glycerol (50) | — | 1.18 | — | — | — |
The data are the mean values of three experiments with SD less than 0.1%. Products are given in micrograms (per 1-mL FA reactions). See Carboxylic Acids.
These data suggest that the formation of carboxylic acids during proton irradiation of FA requires conditions less stringent, and therefore more easily accessible, relative to that of nucleosides and nucleobases. Therefore, the synergism between the various mineral components of meteorites appears to be, for carboxylic acids production, less necessary.
Amino Acids.
The analysis of amino acids was limited to a selected panel of meteorites. We observed, as abundant products, glycine (51), N-formylglycine (52), alanine (53), N-formylalanine (54), 2-methylalanine (55), proline (56), pyroglutamic acid (57), β-amino isobutyric acid (AIBA) (58), and 2-pyrrolidone (59) (Fig. 2 and Table 5). Glycine and alanine derivatives, the simplest amino acids, were in all experiments the most abundant products. In the case of chondrides, the averaged values for glycine and alanine (including the corresponding formyl derivatives) were 1.12 and 0.96 μg/mL, respectively. The molar ratio between the amount of these amino acids is in accordance with values previously reported for indigenous glycine and alanine found in chondrite meteorites (116).
Amino acids 51–55 and 59 have been previously synthesized during thermal condensation of FA. Formyl derivatives 52 and 54, which are simple models of the peptide bond, are produced from corresponding amino acids 51 and 53 by a formylation process that involves carbodiimide (not isolated in this case), urea (detected in the reaction mixtures), and an excess of FA (details in SI Appendix, section S6). Proline 56 was never previously detected in the condensation of FA and is a possible intermediate for the synthesis of 57 by oxidation occurring in the C2 or C5 position of the ring. 2-Pyrrolidone 59 is the lactame derivative of AIBA 58.
Amino acids are probably synthesized through a Strecker-like mechanism (Strecker-cyanohydrin) involving the formation of aminonitriles from hydrogen cyanide (HCN), ammonia, and carbonyl compounds such as aldehydes and ketones, leading to the final formation of amino acids (117–119). However, β- and γ-amino acids, as well as α,α-disubstituted derivatives (such as 55), cannot be generated by this reaction and require an alternative synthetic pathway, which again includes radical mechanisms (120).
In summary, we obtained three canonical α-amino acids: glycine, alanine, and proline. Glycine and alanine have been previously obtained by thermal condensation of FA in the presence of powder of meteorites (57). Glycine was also recently synthesized from FA in experimental conditions mimicking the impact of meteorites on the surface of our planet (3). The unprecedented production of a molecule as complex as proline is noteworthy.
Discussion
Building on the theoretical and experimental logics established by Oparin, Miller, and Oró, we have looked for conditions allowing the emergence of chemical complexity from FA. FA is rapidly becoming an accepted prebiotic precursor mostly due to its widespread presence in our galaxy (5) (and presumably elsewhere), to its physical–chemical properties, to the good compromise it offers between stability and reactivity, to its variegate synthetic behavior with large panels of terrestrial and meteorite catalysts, and thermal energy (as reviewed in refs. 1–4). High-energy synthesis of nucleobases from FA during the impact of an extraterrestrial body was recently reported (3), as inferred from simulation studies using laser-induced plasma dielectric breakdown. FA dissociation to CN and NH radicals resulted in the formation of nucleic bases.
The nature of the compounds detected is highly suggestive. A sizeable part of the components of the TCA and of other cycles is formed, and, with the exception of guanosine, little seems to be missing from a prebiotic kit ready to kick start the next level of complexity, closer to the roots of the extant terrestrial genetic materials. However, that of course would have required the right environment, a source of phosphates, alternating cycles, and the like: that is, a planet. Anyhow, these data show that the exogenous synthesis of the relevant starting components seems to be a nonfastidious process.
Are prebiotically plausible syntheses restricted to terrestrial ancient environments, and must the search for the origin of life be restricted to anoxic geothermal fields, alkaline vents, and black smokers? Without detracting at all from the possible terrestrial interest of each of these potential shrines, the findings reported here show that the energy source for bulk prebiotic syntheses might have been quite different from heat and that nonterrestrial minerals are plausible catalysts. The fact that proton irradiation induces FA to synthesize prebiotically relevant compounds widens the boundaries of possible prebiotic scenarios to regions other than planet Earth. In this perspective, one should at least schematically consider the following.
Intensity and Particle Energy.
Protons emanating from the Sun are divided into solar wind protons with energies of a few kiloelectronvolts, and solar flares and coronal mass ejection protons with energies of tens to hundred megaelectronvolts (121). Protons from GCRs have a range intensity of GeVs, tuned by the solar activity cycle. These particles usually do not react directly, but they can produce secondary protons that are able to activate chemical transformations (122). The nature of proton radiation used is a mimic for the solar wind, which, as discovered and measured by the Mechta probe (123) and by the Mariner 2 spacecraft (124), blows protons up to the limits of the solar system. Proton radiation is, in general, a universal and common source of energy. The irradiation conditions used here (170 MeV; dose, 6 Gy) are in the same range of intensity of the proton radiation of the solar wind and are of the same order of magnitude as that really experienced by samples during the abiotic phosphorylation of preformed nucleosides and peptide bond formation in conditions of space flight on the Biosputnik “BION-11” satellite (125, 126), and subsequently used in ground-based experiments to model chemical transformations on asteroid surfaces (127).
Temperature.
The 243 K used also corresponds to that previously experienced in prebiotic experiments performed in open-space satellite flights (125, 126). The average dose of 6 Gy, if applied in dark interstellar clouds to FA, should correspond to 2.8 × 10−6 eV per molecule, in agreement with the calculations by Moore (dosage of 30 eV per molecule in 108 y) (128). Thus, the sample irradiation that we performed would require about 9 y to occur in interstellar clouds.
In conclusion, we observed that FA reactivity is high enough to yield a manifold palette of products. Faster decay of reaction products is also expected, which is difficult to assess because of the complexity of the reaction mixture. Anyhow, the trade-off between synthesis/degradation is positive. Data on the intensity of proton radiation on early Earth were reported and discussed (129). The cosmic rays flux into Earth’s atmosphere was modeled (130) over the history of the planet based on the solar activity, on modulation of GCRs, and on estimations of the galactic star formation. These studies concluded that particle fluxes were at most twice their current intensity over the past 4.5 billion years and that Earth’s surface has been well shielded from cosmic rays throughout its history by a thick atmosphere (130). Under these conditions, biomolecules, such as amino acids, could have been synthesized in the atmosphere, as shown during proton irradiation of gas mixtures modeling the primordial Earth (131). However, due to the shielding atmosphere effect, high-energy particle processes on the surface of the planet have been most probably driven by in situ radioactivity (132).
Taken together, these results lead us to consider that relevant prebiotic processes yielding complex molecules as nucleosides might have occurred in an environment wider than the early Earth, possibly encompassing smaller wandering bodies of the solar system.
Experimental Methods
FA (Fluka; >99%) was used without further purification. Canyon Diablo and Campo del Cielo were obtained from Tim Güldenpfennig (Rottenburg, Germany); Dhofar 959 from Cometshopnew; NWA 1465, NWA 5357, and NWA 4482 from Sahara-nayzak; and NWA 2828, Gold Basin, Orgueil, and Al Haggounia from AZ Meteorites. Original sample of Chelyabinsk came from the collection of A.Y.R. GC-MS analyses were performed with a LC/GG MS Combo Agilent and Shimadzu GC-MS QP5050A with a Variant CP8944 column (WCOT fused silica; film thickness, 0.25 μm; stationary phase VF-5ms; Øί, 0.25 mm, length, 30 m).
Preparation of Meteorites Powder.
Dust samples of the appropriate meteorite (∼100 mg) were extracted by a two-step procedure to remove organics. The first consisted in the addition of 1.0 mL of 0.1 M NaOH and 3.0 mL of 2:1 chloroform–methanol, the second step in the addition of 1.0 mL of 0.1 M sulfuric acid and 3.0 mL of 2:1 chloroform–methanol. Between steps, the powder was recovered by centrifugation (4,430 × g, 10 min), and the supernatant phase was decanted. The supernatant contained organics that were soluble in both aqueous and organic solvents at high and low pH ranges, leaving behind the powder of the meteorite. In the case of chondrites, achondrites, and stony-iron samples, the powder was pyrolyzed at 600 °C to remove the insoluble organic component in the laboratory oven for 1 h.
Irradiation Experiments.
General procedure: Neat FA (1.0 mL) and catalytic amount of the meteorite powder (1.0% in weight; similar grain size distribution of particles in the range of 0–125 μm) were irradiated at 243 K with 170-MeV protons generated by Phasotron facility of the Joint International Nuclear Institute for 3 min. The uniform proton field was bounded 10 × 10 cm2 by the collimator system. The averaged LET was about 0.57 keV/μm, and the calculated absorbed dose was 6 Gy. The reactions were also performed with representative minerals (chalcopyrite, clay, troilite, magnetite, and covellite), which are known components of meteorites. At the end of the reaction, the mineral was recovered by centrifugation (4,430 × g, 10 min; Haereus Biofuge), and the excess NH2COH was removed by distillation (40 °C, 4 × 10−4 bar). The crude product was analyzed by GC-MS after treatment with N,N-bis-trimethylsilyl trifluoroacetamide in pyridine (620 μL) at 60 °C for 4 h in the presence of betulinol (CAS number 473-98-3) as internal standard (0.2 mg). Mass spectrometry was performed by the following program: injection temperature, 280 °C; detector temperature, 280 °C; gradient, 100 °C × 2 min, 10 °C/min for 60 min. To identify the structure of the products, two strategies were followed. First, the spectra were compared with commercially available electron mass spectrum libraries such as NIST (Fisons). Second, GC-MS analysis was repeated with standard compounds. All products have been recognized with a similarity index greater than 98% compared with the reference standards. To evaluate the possible role of inorganic constituents of the meteorite powder in the condensation of NH2COH, the reaction was repeated under similar experimental conditions with a panel of selected minerals and metal oxides, chalcopyrite, clay, troilite, magnetite, and covellite.
Supplementary Material
Acknowledgments
We thank Silvia Lopizzo for helpful contributions. This work was supported by Italian Space Agency Project “Esobiologia e Ambienti Estremi: Dalla Chimica delle Molecola alla Biologia degli Estremofili” Number 2014-026-R.0 (Codice Unico Progetto F 92I14000030005).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1422225112/-/DCSupplemental.
References
- 1.Saladino R, Crestini C, Pino S, Costanzo G, Di Mauro E. Formamide and the origin of life. Phys Life Rev. 2012;9(1):84–104. doi: 10.1016/j.plrev.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Saladino R, Botta G, Pino S, Costanzo G, Di Mauro E. Genetics first or metabolism first? The formamide clue. Chem Soc Rev. 2012;41(16):5526–5565. doi: 10.1039/c2cs35066a. [DOI] [PubMed] [Google Scholar]
- 3.Ferus M, et al. High-energy chemistry of formamide: A unified mechanism of nucleobase formation. Proc Natl Acad Sci USA. 2015;112(3):657–662. doi: 10.1073/pnas.1412072111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saitta AM, Saija F. Miller experiments in atomistic computer simulations. Proc Natl Acad Sci USA. 2014;111(38):13768–13773. doi: 10.1073/pnas.1402894111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adande G-R, Woolf N-J, Ziurys L-M. Observations of interstellar formamide: Availability of a prebiotic precursor in the galactic habitable zone. Astrobiology. 2013;13(5):439–453. doi: 10.1089/ast.2012.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halfen DT, Ilyushin V, Ziurys LM. Formation of peptide bonds in space: A comprehensive study of formamide and acetamide in SgrB2(N) Astrophys J. 2011;743:60–72. [Google Scholar]
- 7.Parnell J, Baron M, Lindgren P-J. Potential for irradiation of methane to form complex organic molecules in impact craters: Implications for Mars, Titan and Europa. Geochem Explor. 2006;89:322–325. [Google Scholar]
- 8.Hollis J-M, et al. Detection of acetamide (CH3CONH2): The largest interstellar molecule with a peptide bond. ApJ. 2006;643(1):L25–L28. [Google Scholar]
- 9.Gibb E-L, Whittet D-C-B, Boogert A-C-A, Tielens A. Interstellar ICE: The infrared space observatory legacy. Astrophys J Suppl Ser. 2004;151:35–73. [Google Scholar]
- 10.Despois D, Crovisier J, Bockelee-Morvan D, Biver N. 2002. Comets and prebiotic chemistry: The volatile component. From European Space Agency, [Special Publication] SP, SP-518. Proceeding of the Second European Workshop on Exo-Astrobiology, September 16–19 Graz, Austria, eds Lacoste H, Sawaya-Lacoste H (ESA Publications Division, Nordwijk, Netherlands), pp 123–127.
- 11.Gibb E-L, et al. An inventory of interstellar ices toward the embedded protostar W33A. Astrophys J. 2000;536:347–356. [Google Scholar]
- 12.Bockelee-Morvan D, et al. New molecules found in comet C/1995 O1 (Hale-Bopp). Investigating the link between cometary and interstellar material. Astron Astrophys. 2000;353:1101–1114. [Google Scholar]
- 13.Flygare WH, Benson RC, Tigelaar HL, Rubin RH, Swenson GW., Jr. 1973. Chemical and rotational state stability of interstellar formamide. Molecules in the Galactic Environment, eds Gordon MA, Snyder LE (John Wiley and Sons, New York), pp 173–179.
- 14.Gottlieb CA, Palmer P, Rickard LJ, Zuckerman B. Studies of interstellar formamide. Astrophys J. 1973;182:699–710. [Google Scholar]
- 15.Saladino R, Botta G, Pino S, Costanzo G, Di Mauro E. From the one-carbon amide formamide to RNA all the steps are prebiotically possible. Biochimie. 2012;94(7):1451–1456. doi: 10.1016/j.biochi.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Shanker U, Bhushan B, Bhattacharjee G, Kamaluddin G. Formation of nucleobases from formamide in the presence of iron oxides: Implication in chemical evolution and origin of life. Astrobiology. 2011;11(3):225–233. doi: 10.1089/ast.2010.0530. [DOI] [PubMed] [Google Scholar]
- 17.Barks HL, et al. Guanine, adenine, and hypoxanthine production in UV-irradiated formamide solutions: Relaxation of the requirements for prebiotic purine nucleobase formation. ChemBioChem. 2010;11(9):1240–1243. doi: 10.1002/cbic.201000074. [DOI] [PubMed] [Google Scholar]
- 18.Saladino R, et al. Synthesis and degradation of nucleobases and nucleic acids by formamide in the presence of montmorillonites. ChemBioChem. 2004;5(11):1558–1566. doi: 10.1002/cbic.200400119. [DOI] [PubMed] [Google Scholar]
- 19.Saladino R, et al. One-pot TiO2-catalyzed synthesis of nucleic bases and acyclonucleosides from formamide: Implications for the origin of life. ChemBioChem. 2003;4(6):514–521. doi: 10.1002/cbic.200300567. [DOI] [PubMed] [Google Scholar]
- 20.Costanzo G, Saladino R, Crestini C, Ciciriello F, Di Mauro E. Nucleoside phosphorylation by phosphate minerals. J Biol Chem. 2007;282(23):16729–16735. doi: 10.1074/jbc.M611346200. [DOI] [PubMed] [Google Scholar]
- 21.Costanzo G, et al. Generation of RNA molecules by a base-catalysed click-like reaction. ChemBioChem. 2012;13(7):999–1008. doi: 10.1002/cbic.201200068. [DOI] [PubMed] [Google Scholar]
- 22.Saladino R, et al. Origin of informational polymers: The concurrent roles of formamide and phosphates. ChemBioChem. 2006;7(11):1707–1714. doi: 10.1002/cbic.200600139. [DOI] [PubMed] [Google Scholar]
- 23.Saladino R, Barontini M, Cossetti C, Di Mauro E, Crestini C. The effects of borate minerals on the synthesis of nucleic acid bases, amino acids and biogenic carboxylic acids from formamide. Orig Life Evol Biosph. 2011;41(4):317–330. doi: 10.1007/s11084-011-9236-3. [DOI] [PubMed] [Google Scholar]
- 24.Saladino R, Neri V, Crestini C. Role of clays in the prebiotic synthesis of sugar derivatives from formamide. Philos Mag. 2010;90(17-18):2329–2337. [Google Scholar]
- 25.Saladino R, et al. The role of the formamide/zirconia system in the synthesis of nucleobases and biogenic carboxylic acid derivatives. J Mol Evol. 2010;71(2):100–110. doi: 10.1007/s00239-010-9366-7. [DOI] [PubMed] [Google Scholar]
- 26.Saladino R, et al. Photochemical synthesis of citric acid cycle intermediates based on titanium dioxide. Astrobiol. 2011;11(8):815–824. doi: 10.1089/ast.2011.0652. [DOI] [PubMed] [Google Scholar]
- 27.Yamada H, Hirobe M, Okamoto T. Formamide reaction 3. The reaction mechanism of purine ring formation and the reaction of formamide with hydrogen cyanide. Yakugaku Zasshi. 1980;100(5):489–492. [Google Scholar]
- 28.Yamada H, Hiroba M, Higashiyama K, Takahashi H, Suzuki K-T. Detection of carbon-13-nitrogen-15 coupled units in adenine derived from doubly labeled hydrogen cyanide or formamide. J Am Chem Soc. 1978;100(14):4617–4618. [Google Scholar]
- 29.Yamada H, Hirobe M, Higashiyama K, Takahashi H, Suzuki KT. Reaction mechanism for purine ring formation as studied by 13C-15N coupling. Tetrahedron Lett. 1978;19(42):4039–4042. [Google Scholar]
- 30.Ochiai M, Marumoto R, Kobayashi S, Shimazu H, Morita K. A facile one-step synthesis of adenine. Tetrahedron. 1968;24(17):5731–5737. [Google Scholar]
- 31.Wang J, Gu J, Nguyen M-T, Springsteen G, Leszczynski J. From formamide to purine: A self-catalyzed reaction pathway provides a feasible mechanism for the entire process. J Phys Chem B. 2013;117(32):9333–9342. doi: 10.1021/jp404540x. [DOI] [PubMed] [Google Scholar]
- 32.Roy D, Najafian K, von Ragué Schleyer P. Chemical evolution: The mechanism of the formation of adenine under prebiotic conditions. Proc Natl Acad Sci USA. 2007;104(44):17272–17277. doi: 10.1073/pnas.0708434104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalgarno A. The galactic cosmic ray ionization rate. Proc Natl Acad Sci USA. 2006;103(33):12269–12273. doi: 10.1073/pnas.0602117103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hudson JS, et al. A unified mechanism for abiotic adenine and purine synthesis in formamide. Angew Chem Int Ed Engl. 2012;51(21):5134–5137. doi: 10.1002/anie.201108907. [DOI] [PubMed] [Google Scholar]
- 35.Nuevo M, et al. Irradiation of pyrimidine in pure H2O ice with high-energy ultraviolet photons. Astrobiology. 2014;14(2):119–131. doi: 10.1089/ast.2013.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menor-Salván C, Marín-Yaseli MR. A new route for the prebiotic synthesis of nucleobases and hydantoins in water/ice solutions involving the photochemistry of acetylene. Chemistry. 2013;19(20):6488–6497. doi: 10.1002/chem.201204313. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen HT, Nguyen VS, Trung NT, Havenith RW, Nguyen MT. Decomposition pathways of the neutral and protonated formamide in some lower-lying excited states. J Phys Chem A. 2013;117(33):7904–7917. doi: 10.1021/jp405657y. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen VS, et al. Theoretical study of formamide decomposition pathways. J Phys Chem A. 2011;115(5):841–851. doi: 10.1021/jp109143j. [DOI] [PubMed] [Google Scholar]
- 39.Liu D, Fang WH, Fu XY. Ab initio molecular orbital study of the mechanism of photodissociation of formamide. Chem Phys Lett. 2000;318(4-5):291–297. [Google Scholar]
- 40.Antol I, Eckert-Maksić M, Barbatti M, Lischka H. Simulation of the photodeactivation of formamide in the nO-pi* and pi-pi* states: An ab initio on-the-fly surface-hopping dynamics study. J Chem Phys. 2007;127(23):234303–234306. doi: 10.1063/1.2804862. [DOI] [PubMed] [Google Scholar]
- 41.Duvernay F, et al. Matrix isolation Fourier transform infrared study of photodecomposition of formimidic acid. J Phys Chem A. 2005;109(49):11155–11162. doi: 10.1021/jp054903w. [DOI] [PubMed] [Google Scholar]
- 42.Maier G, Endres J. Isomerization of matrix-isolated formamide: IR-spectroscopic detection of formimidic acid. Eur J Org Chem. 2000;6:1061–1063. [Google Scholar]
- 43.Lundell J, Krajewska M, Rasanen M. Matrix isolation fourier transform infrared and ab initio studies of the 193-nm-induced photodecomposition of formamide. J Phys Chem A. 1998;102(33):6643–6650. [Google Scholar]
- 44.Ferus M, Kubelík P, Civiš S. Laser spark formamide decomposition studied by FT-IR spectroscopy. J Phys Chem A. 2011;115(44):12132–12141. doi: 10.1021/jp205413d. [DOI] [PubMed] [Google Scholar]
- 45.Leach S, Jochims H-W, Baumgärtel H. Photoionization mass spectrometric study of the prebiotic species formamide in the 10–20 eV photon energy range. J Phys Chem A. 2010;114(14):4847–4856. doi: 10.1021/jp9098182. [DOI] [PubMed] [Google Scholar]
- 46.Brucato JR, Strazzulla G, Baratta GA, Rotundi A, Colangeli L. Cryogenic synthesis of molecules of astrobiological interest: Catalytic role of cosmic dust analogues. Orig Life Evol Biosph. 2006;36(5-6):451–457. doi: 10.1007/s11084-006-9050-5. [DOI] [PubMed] [Google Scholar]
- 47.Dawley MM, Pirim C, Orlando TM. Radiation processing of formamide and formamide:water ices on silicate grain analogue. J Phys Chem A. 2014;118(7):1228–1236. doi: 10.1021/jp4042815. [DOI] [PubMed] [Google Scholar]
- 48.Jeilani YA, Nguyen HT, Newallo D, Dimandja JMD, Nguyen MT. Free radical routes for prebiotic formation of DNA nucleobases from formamide. Phys Chem Chem Phys. 2013;15(48):21084–21093. doi: 10.1039/c3cp53108b. [DOI] [PubMed] [Google Scholar]
- 49. Shimizu M (1973) Interstellar Dust and Related Topics. IAU Symposium, eds JM Greenberg, HC van de Hulst (Reidel, Dordrecht, The Netherlands), Vol 52, p 405.
- 50.Antol I, Barbatti M, Eckert-Maksić M, Lischka H. Quantum chemical calculations of electronically excited states: Formamide, its protonated form and alkali cation complexes as case studies. Monatsh Chem. 2008;139(4):319–328. [Google Scholar]
- 51.Adhikari DD, Thakuria T, Medhi C. MCSCF calculations on metal ion (Li+, Na+, Be2 +) affinities of a few carbonyl molecules in the ground and 1,3n π* excited states. Indian J Chem. 2000;39(8):792–801. [Google Scholar]
- 52.Fu X, Ouyang Z, Zou Y. A review of the search for life in our Solar System. Earth Sci Front. 2014;21(1):161–176. [Google Scholar]
- 53.Schmitt-Kopplin P, et al. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc Natl Acad Sci USA. 2010;107(7):2763–2768. doi: 10.1073/pnas.0912157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burton AS, Stern JC, Elsila JE, Glavin DP, Dworkin JP. Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem Soc Rev. 2012;41(16):5459–5472. doi: 10.1039/c2cs35109a. [DOI] [PubMed] [Google Scholar]
- 55.Chyba CF. The cometary contribution of the oceans of primitive Earth. Nature. 1987;330(6149):632–635. [Google Scholar]
- 56.Mojzsis SJ, et al. Evidence for life on Earth before 3,800 million years ago. Nature. 1996;384(6604):55–59. doi: 10.1038/384055a0. [DOI] [PubMed] [Google Scholar]
- 57.Saladino R, Botta G, Delfino M, Di Mauro E. Meteorites as catalysts for prebiotic chemistry. Chemistry. 2013;19(50):16916–16922. doi: 10.1002/chem.201303690. [DOI] [PubMed] [Google Scholar]
- 58.Saladino R, Crestini C, Cossetti C, Di Mauro E, Deamer D. Catalytic effects of Murchison material: Prebiotic synthesis and degradation of RNA precursors. Orig Life Evol Biosph. 2011;41(5):437–451. doi: 10.1007/s11084-011-9239-0. [DOI] [PubMed] [Google Scholar]
- 59.Ferus M, et al. High-energy chemistry of formamide: A simpler way for nucleobase formation. J Phys Chem A. 2014;118(4):719–736. doi: 10.1021/jp411415p. [DOI] [PubMed] [Google Scholar]
- 60.Tamburro A. Cosmic ray spectrum, composition, and anisotropy measured with IceCube. Nucl Instrum Methods Phys Res A. 2014;742:35–41. [Google Scholar]
- 61.Cooper G, Reed C, Nguyen D, Carter M, Wang Y. Detection and formation scenario of citric acid, pyruvic acid, and other possible metabolism precursors in carbonaceous meteorites. Proc Natl Acad Sci USA. 2011;108(34):14015–14020. doi: 10.1073/pnas.1105715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Callahan M-P, et al. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc Natl Acad Sci USA. 2011;108(34):13995–13998. doi: 10.1073/pnas.1106493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brucato JR, Baratta GA, Strazzulla G. An infrared study of pure and ion irradiated frozen formamide. Astron Astrophys. 2006;455:395–399. [Google Scholar]
- 64.Saladino R, et al. Synthesis and degradation of nucleic acid components by formamide and iron sulfur minerals. J Am Chem Soc. 2008;130(46):15512–15518. doi: 10.1021/ja804782e. [DOI] [PubMed] [Google Scholar]
- 65.Saladino R, Botta G, Pino S, Costanzo G, Di Mauro E. Materials for the onset. A story of necessity and chance. Front Biosci (Landmark Ed) 2013;18(4):1275–1289. doi: 10.2741/4179. [DOI] [PubMed] [Google Scholar]
- 66.Bean HD, et al. Formation of a β-pyrimidine nucleoside by a free pyrimidine base and ribose in a plausible prebiotic reaction. J Am Chem Soc. 2007;129(31):9556–9557. doi: 10.1021/ja072781a. [DOI] [PubMed] [Google Scholar]
- 67.Hirao I, Kimoto M, Yamashige R. Natural versus artificial creation of base pairs in DNA: Origin of nucleobases from the perspectives of unnatural base pair studies. Acc Chem Res. 2012;45(12):2055–2065. doi: 10.1021/ar200257x. [DOI] [PubMed] [Google Scholar]
- 68.Kirnos MD, Khudyakov IY, Alexandrushkina NI, Vanyushin BF. 2-Aminoadenine is an adenine substituting for a base in S-2L cyanophage DNA. Nature. 1977;270(5635):369–370. doi: 10.1038/270369a0. [DOI] [PubMed] [Google Scholar]
- 69.Visek WJ, Shoemaker JD. Orotic acid, arginine, and hepatotoxicity. J Am Coll Nutr. 1986;5(2):153–166. doi: 10.1080/07315724.1986.10720122. [DOI] [PubMed] [Google Scholar]
- 70.Ferus M, et al. On the road from formamide ices to nucleobases: IR-spectroscopic observation of a direct reaction between cyano radicals and formamide in a high-energy impact event. J Am Chem Soc. 2012;134(51):20788–20796. doi: 10.1021/ja310421z. [DOI] [PubMed] [Google Scholar]
- 71.Li L, et al. Iron-chelating agents never suppress Fenton reaction but participate in quenching spin-trapped radicals. Anal Chim Acta. 2007;599(2):315–319. doi: 10.1016/j.aca.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 72.Sharkey WH, Mochel W-E. Mechanism of the photoöxidation of amides. J Am Chem Soc. 1959;81(12):3000–3005. [Google Scholar]
- 73.Friesen DA, Haedely JV, Langford CH. The photooxidative degradation of of JV-methylpyrrolidone in the presence of Cs3PW12O40 and TiO2 colloid photocatalysts. Environ Sci Technol. 1999;33:3193–3198. [Google Scholar]
- 74.Arnaud R, Fanton E, Gardette JL. Photochemical behaviour of semi-aromatic polyamides. Polym Degrad Stabil. 1994;45:361–369. [Google Scholar]
- 75.Barker RH. Additives in fibers and fabrics. Environ Health Perspect. 1975;11:41–45. doi: 10.1289/ehp.751141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cline RE, Fink RM, Fink K. Synthesis of 5-substituted pyrimidines via formaldehyde addition. J Am Chem Soc. 1959;81(10):2521–2527. [Google Scholar]
- 77.Sparkman OD, Penton ZE, Kitson FG. 2011. Gas Chromatography and Mass Spectrometry (Elsevier, Oxford), 2nd Ed, pp 407–410.
- 78.De Jongh DC, et al. Analysis of trimethylsilyl derivatives of carbohydrates by gas-chromatography and mass-spectrometry. J Am Chem Soc. 1969;91(7):1728–1740. [Google Scholar]
- 79.Medeiros PM, Simoneit BRT. Analysis of sugars in environmental samples by gas-chromatography mass-spectrometry. J Chrom A. 2007;114(2):271–278. doi: 10.1016/j.chroma.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 80.Gollihar J, Levy M, Ellington AD. Biochemistry. Many paths to the origin of life. Science. 2014;343(6168):259–260. doi: 10.1126/science.1246704. [DOI] [PubMed] [Google Scholar]
- 81.Harman CE, Kasting JF, Wolf ET. Atmospheric production of glycolaldehyde under hazy prebiotic conditions. Orig Life Evol Biosph. 2013;43(2):77–98. doi: 10.1007/s11084-013-9332-7. [DOI] [PubMed] [Google Scholar]
- 82.Appayee C, Breslow R. Deuterium studies reveal a new mechanism for the formose reaction involving hydride shifts. J Am Chem Soc. 2014;136(10):3720–3723. doi: 10.1021/ja410886c. [DOI] [PubMed] [Google Scholar]
- 83.Kim HJ, et al. Synthesis of carbohydrates in mineral-guided prebiotic cycles. J Am Chem Soc. 2011;133(24):9457–9468. doi: 10.1021/ja201769f. [DOI] [PubMed] [Google Scholar]
- 84.Hashizume H, Theng BKG. Adenine, adenosine, ribose and 5′-AMP adsorption to allophone. Clays Clay Miner. 2007;55(6):599–605. [Google Scholar]
- 85.Scorei R, Cimpoiaşu VM. Boron enhances the thermostability of carbohydrates. Orig Life Evol Biosph. 2006;36(1):1–11. doi: 10.1007/s11084-005-0562-1. [DOI] [PubMed] [Google Scholar]
- 86.Ricardo A, Carrigan MA, Olcott AN, Benner SA. Borate minerals stabilize ribose. Science. 2004;303(5655):196. doi: 10.1126/science.1092464. [DOI] [PubMed] [Google Scholar]
- 87.Prieur BF. Etude de l’activité prébiotique potentielle de l’acide borique. CR Acad Sci Chim/Chem. 2001;4:667–670. [Google Scholar]
- 88.Swinton D, et al. Purification and characterization of the unusual deoxynucleoside, α-N-(9-β-D-2′-deoxyribofuranosylpurin-6-yl)glycinamide, specified by the phage Mu modification function. Proc Natl Acad Sci USA. 1983;80(24):7400–7404. doi: 10.1073/pnas.80.24.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sparkman OD, Penton ZE, Kitson FG. 2011. Gas Chromatography and Mass Spectrometry (Elsevier, Oxford), 2nd Ed, pp 369–371.
- 90.Orgel L-E. Prebiotic chemistry and the origin of the RNA world. Crit Rev Biochem Mol Biol. 2004;39(2):99–123. doi: 10.1080/10409230490460765. [DOI] [PubMed] [Google Scholar]
- 91.Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459(7244):239–242. doi: 10.1038/nature08013. [DOI] [PubMed] [Google Scholar]
- 92.Holy A. 2-Deoxy-L-uridine: Total synthesis of a uracil 2-deoxynucleoside from a sugar 2-aminooxazoline through a 2,2-anhydronucleoside intermediate. Nucl Acid Chem. 1978;1:347–353. [Google Scholar]
- 93.Stankevich EI, Drizina I, Liepins E, Snikeris D. Synthesis of nucleosides from aminooxazolines of pentafuranoses. Novosti Khimii Nukleozidovi Nukleotidov. 1978;1:63–64. [Google Scholar]
- 94.Wierenga W, Loughman BE, Gibbons AJ, Renis HE. beta-D-Arabinofuran[1′,2′:4,5]oxazolo-1,3,5-triazine-5-N-methyl-4,6-dione and analogues, unusually specific immunosuppressive agents. J Med Chem. 1978;21(6):558–562. doi: 10.1021/jm00204a011. [DOI] [PubMed] [Google Scholar]
- 95.Abushanab E, Pragnacharyulu Palle VP. 1998. Preparation of pyrimidine nucleosides AZT and d4T analogs PCT. Int Appl WO 9806729 A1 19980219.
- 96.Breslow R, Levine MS. Amplification of enantiomeric concentrations under credible prebiotic conditions. Proc Natl Acad Sci USA. 2006;103(35):12979–12980. doi: 10.1073/pnas.0605863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh P, Singh A, Kaura J, Holzerb W. 2014. H-bond activated glycosylation of nucleobases: Implications for prebiotic nucleoside synthesis RSC Adv 4:3158–3161.
- 98.Breslow R, Cheng ZL. On the origin of terrestrial homochirality for nucleosides and amino acids. Proc Natl Acad Sci USA. 2009;106(23):9144–9146. doi: 10.1073/pnas.0904350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manuel N. Bass Montmorillonite and serpentine in Orgueil meteorite. Geochim Cosmochim Acta. 1971;35(2):139–147. [Google Scholar]
- 100.Friedmann EI, Wierzchos J, Ascaso C, Winklhofer M. Chains of magnetite crystals in the meteorite ALH84001: Evidence of biological origin. Proc Natl Acad Sci USA. 2001;98(5):2176–2181. doi: 10.1073/pnas.051514698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Andrews JE, Brimblecombe P, Jickells TD, Liss PS, Reid BJ (2004) An Introduction to Environmental Chemistry (Wiley-Blackwell, Oxford), 2nd Ed.
- 102.Yu Y, Gee J. Spinel in Martian meteorite SaU 008: Implications for Martian magnetism. Earth Planet Sci Lett. 2005;232(3–4):287. [Google Scholar]
- 103.Pizzarello S, Cooper GW, Flynn GJ. Meteorites and the Early Solar System II. Univ Arizona Press; Tucson, AZ: 2006. pp. 625–651. [Google Scholar]
- 104.Sephton MA. Treatise on Geochemistry II. Imperial College London; London: 2013. pp. 1–31. [Google Scholar]
- 105.Nelson DL, Cox MM. 2000. Lehninger Principles of Biochemistry (Worth Publishers, New York), 3rd Ed, Vol 16, pp 567–597.
- 106.Smith E, Morowitz HJ. Universality in intermediary metabolism. Proc Natl Acad Sci USA. 2004;101(36):13168–13173. doi: 10.1073/pnas.0404922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Storrey K, editor. Functional Metabolism: Regulation and Adaption. Wiley; Hoboken, NJ: 2004. pp. 221–223. [Google Scholar]
- 108.McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, Nutrition, and Human Performance. Wolters Kluwer/Lippincott Williams & Wilkins Health; Philadelphia: 2010. [Google Scholar]
- 109.Mero AA, et al. Effects of alfa-hydroxy-isocaproic acid on body composition, DOMS and performance in athletes. J Int Soc Sports Nutr. 2010;7:1. doi: 10.1186/1550-2783-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Snyder HR, Brooks LA, Shapiro SH (1943) Pimelic acid from cyclohexanone. Org Synth Coll 2:531–535.
- 111.Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT. Priming in systemic plant immunity. Science. 2009;324(5923):89–91. doi: 10.1126/science.1170025. [DOI] [PubMed] [Google Scholar]
- 112.Eschenmoser A. On a hypothetical generational relationship between HCN and constituents of the reductive citric acid cycle. Chem Biodivers. 2007;4(4):554–573. doi: 10.1002/cbdv.200790050. [DOI] [PubMed] [Google Scholar]
- 113.Muñoz Caro GM, Schutte WA. UV-photoprocessing of interstellar ice analogs: New infrared spectroscopic results. Astron Astrophys. 2003;412(1):121–132. [Google Scholar]
- 114.Agarwal VK, et al. Photochemical reactions in interstellar grains photolysis of CO, NH3, and H2O. Orig Life Evol Biosph. 1985;16(1):21–40. doi: 10.1007/BF01808047. [DOI] [PubMed] [Google Scholar]
- 115.Meinert C, et al. Photochirogenesis: Photochemical models on the origin of biomolecular homochirality. Symmetry. 2010;2(2):1055–1080. [Google Scholar]
- 116.Parker ET, et al. Primordial synthesis of amines and amino acids in a 1958 Miller H2S-rich spark discharge experiment. Proc Natl Acad Sci USA. 2011;108(14):5526–5531. doi: 10.1073/pnas.1019191108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peltzer ET, Bada JL, Schlesinger G, Miller SL. The chemical conditions on the parent body of the Murchison meteorite: Some conclusions based on amino, hydroxy and dicarboxylic acids. Adv Space Res. 1984;4(12):69–74. doi: 10.1016/0273-1177(84)90546-5. [DOI] [PubMed] [Google Scholar]
- 118.Cronin JR, Cooper GW, Pizzarello S. Characteristics and formation of amino acids and hydroxy acids of the Murchison meteorite. Adv Space Res. 1995;15(3):91–97. doi: 10.1016/s0273-1177(99)80068-4. [DOI] [PubMed] [Google Scholar]
- 119.Botta O, Glavin DP, Kminek G, Bada JL. Relative amino acid concentrations as a signature for parent body processes of carbonaceous chondrites. Orig Life Evol Biosph. 2002;32(2):143–163. doi: 10.1023/a:1016019425995. [DOI] [PubMed] [Google Scholar]
- 120.Elsila JE, Dworkin JP, Bernstein MP, Martin MP, Sandford SA. Mechanisms of aminoacids formation in interstellar ice analogs. Astrophys J. 2007;660:911–918. [Google Scholar]
- 121.Ferrari F, Szuszkiewicz E. Cosmic rays: A review for astrobiologists. Astrobiology. 2009;9(4):413–436. doi: 10.1089/ast.2007.0205. [DOI] [PubMed] [Google Scholar]
- 122.Klapdor-Kleingrothaus HV, Zuber K. 2000. Cosmic radiation. Particle Astrophysics, eds Falcke H, Hehl FW (Institute of Physics Publishing, Bristol, UK), pp 223–247.
- 123. NASA National Space Science Data Center, Luna 1 (1959). Available at nssdc.gsfc.nasa.gov/nmc/masterCatalog.do?sc=1959-012A. Accessed August 4, 2007.
- 124.Neugebauer M, Snyder CW. Solar plasma experiment. Science. 1962;138(3545):1095–1097. doi: 10.1126/science.138.3545.1095-a. [DOI] [PubMed] [Google Scholar]
- 125.Kuzicheva EA, Simakov MB. Abiogenic synthesis of nucleotides in conditions of space flight of the Biosputnik “BION-11.”. Adv Space Res. 1999;23(2):387–391. [Google Scholar]
- 126.Kuzicheva EA, Gontareva NB. The possibility of nucleotide abiogenic synthesis in conditions of “Kosmos-2044” satellite space flight. Adv Space Res. 1999;23(2):393–396. [Google Scholar]
- 127.Simakov MB. Asteroids and origin of life-two steps of chemical evolution on the surface of these objects. Earth Planets Space. 2008;60:75–82. [Google Scholar]
- 128.Moore MH. The physics and chemistry of ice in the interstellar medium. In: d’Hendecourt L, Joblin C, Jones A, editors. Solid Interstellar Matter: The ISO Revolution. Springer; Berlin: 1999. pp. 199–218. [Google Scholar]
- 129.Dartnell LR. Ionizing radiation and life. Astrobiology. 2011;11(6):551–582. doi: 10.1089/ast.2010.0528. [DOI] [PubMed] [Google Scholar]
- 130.Svensmark H. Cosmic rays and the biosphere over 4 billion years. Astron Nachr. 2006;327:871–875. [Google Scholar]
- 131.Kobayashi K, Tsuji T. Abiotic synthesis of uracil from carbon monoxide, nitrogen and water by proton irradiation. Chem Lett. 1997;26:903–904. [Google Scholar]
- 132.Draganic I. Radiolysis of water: A look at its origin and occurrence in the nature. Radiat Phys Chem. 2005;72:181–186. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.