Significance
Coffinite, USiO4, is an important alteration mineral of uraninite. Its somewhat unexpected formation and persistence in a large variety of natural and contaminated low-temperature aqueous settings must be governed by its thermodynamic properties, which, at present, are poorly constrained. We report direct calorimetric measurements of the enthalpy of formation of coffinite. The calorimetric data confirm the thermodynamic metastability of coffinite with respect to uraninite plus quartz but show that it can form from silica-rich aqueous solutions in contact with dissolved uranium species in a reducing environment. These constraints on thermodynamic properties support that coffinitization in uranium deposits and spent nuclear fuel occurs through dissolution of UO2 (often forming hexavalent uranium intermediates) followed by reaction with silica-rich fluids.
Keywords: uranium, coffinite, USiO4, U(IV) minerals, calorimetry
Abstract
Coffinite, USiO4, is an important U(IV) mineral, but its thermodynamic properties are not well-constrained. In this work, two different coffinite samples were synthesized under hydrothermal conditions and purified from a mixture of products. The enthalpy of formation was obtained by high-temperature oxide melt solution calorimetry. Coffinite is energetically metastable with respect to a mixture of UO2 (uraninite) and SiO2 (quartz) by 25.6 ± 3.9 kJ/mol. Its standard enthalpy of formation from the elements at 25 °C is −1,970.0 ± 4.2 kJ/mol. Decomposition of the two samples was characterized by X-ray diffraction and by thermogravimetry and differential scanning calorimetry coupled with mass spectrometric analysis of evolved gases. Coffinite slowly decomposes to U3O8 and SiO2 starting around 450 °C in air and thus has poor thermal stability in the ambient environment. The energetic metastability explains why coffinite cannot be synthesized directly from uraninite and quartz but can be made by low-temperature precipitation in aqueous and hydrothermal environments. These thermochemical constraints are in accord with observations of the occurrence of coffinite in nature and are relevant to spent nuclear fuel corrosion.
In many countries with nuclear energy programs, spent nuclear fuel (SNF) and/or vitrified high-level radioactive waste will be disposed in an underground geological repository. Demonstrating the long-term (106–109 y) safety of such a repository system is a major challenge. The potential release of radionuclides into the environment strongly depends on the availability of water and the subsequent corrosion of the waste form as well as the formation of secondary phases, which control the radionuclide solubility. Coffinite (1), USiO4, is expected to be an important alteration product of SNF in contact with silica-enriched groundwater under reducing conditions (2–8). It is also found, accompanied by thorium orthosilicate and uranothorite, in igneous and metamorphic rocks and ore minerals from uranium and thorium sedimentary deposits (2, 4, 5, 8–16). Under reducing conditions in the repository system, the uranium solubility (very low) in aqueous solutions is typically derived from the solubility product of UO2. Stable U(IV) minerals, which could form as secondary phases, would impart lower uranium solubility to such systems. Thus, knowledge of coffinite thermodynamics is needed to constrain the solubility of U(IV) in natural environments and would be useful in repository assessment.
In natural uranium deposits such as Oklo (Gabon) (4, 7, 11, 12, 14, 17, 18) and Cigar Lake (Canada) (5, 13, 15), coffinite has been suggested to coexist with uraninite, based on electron probe microanalysis (EPMA) (4, 5, 7, 11, 13, 17, 19, 20) and transmission electron microscopy (TEM) (8, 15). However, it is not clear whether such apparent replacement of uraninite by a coffinite-like phase is a direct solid-state process or occurs mediated by dissolution and reprecipitation.
The precipitation of USiO4 as a secondary phase should be favored in contact with silica-rich groundwater (21) [silica concentration >10−4 mol/L (22, 23)]. Natural coffinite samples are often fine-grained (4, 5, 8, 11, 13, 15, 24), due to the long exposure to alpha-decay event irradiation (4, 6, 25, 26) and are associated with other minerals and organic matter (6, 8, 12, 18, 27, 28). Hence the determination of accurate thermodynamic data from natural samples is not straightforward. However, the synthesis of pure coffinite also has challenges. It appears not to form by reacting the oxides under dry high-temperature conditions (24, 29). Synthesis from aqueous solutions usually produces UO2 and amorphous SiO2 impurities, with coffinite sometimes being only a minor phase (24, 30–35). It is not clear whether these difficulties arise from kinetic factors (slow reaction rates) or reflect intrinsic thermodynamic instability (33). Thus, there are only a few reported estimates of thermodynamic properties of coffinite (22, 36–40) and some of them are inconsistent. To resolve these uncertainties, we directly investigated the energetics of synthetic coffinite by high-temperature oxide melt solution calorimetry to obtain a reliable enthalpy of formation and explored its thermal decomposition.
Results and Discussion
We used two independently prepared coffinite samples. A phase-pure coffinite sample prepared at Institut de Chimie Séparative de Marcoule (ICSM), France is labeled “coffinite-F” and a less pure material prepared at Forschungszentrum Jülich, Germany is labeled “coffinite-G.” In both cases, considerable purification after initial synthesis was done to separate impurities (SI Appendix). The purpose of measuring and comparing both samples was to test whether consistent thermochemical data could be obtained on materials prepared and purified independently in different laboratories.
Before calorimetric measurements, samples were characterized by TEM, infrared spectroscopy (IR), powder X-ray diffraction (XRD), simultaneous differential scanning calorimetry and thermogravimetric analysis (DSC-TG) coupled with mass spectrometric analysis of evolved gases (MS-EGA), and by EPMA. The details are given in Experimental Methods and in SI Appendix.
The XRD patterns (SI Appendix, Fig. S1) show the reflections expected for a zircon-type structure (space group I41/amd). Lattice parameters are a = b = 6.983(3) Å and c = 6.263(4) Å for coffinite-G, and a = b = 6.990(1) Å and c = 6.261(1) Å for coffinite-F. Crystallite size was estimated from X-ray peak broadening (65 nm for coffinite-G and 85 nm for coffinite-F). This observation is consistent with previous reports (8, 24, 26, 34, 40). Although the particle size may affect the thermodynamic properties, we did not investigate this further. Even though coarse-grained but impure coffinite (>10 μm) has been documented from the Grants uranium region, New Mexico (8, 41), Colorado (8, 10), and hydrothermal deposits in Czech Republic (42), natural coffinite and materials produced in SNF alteration are usually fine-grained, having similar particle size as our synthetic samples. So, the data obtained here are applicable to most “real world” situations.
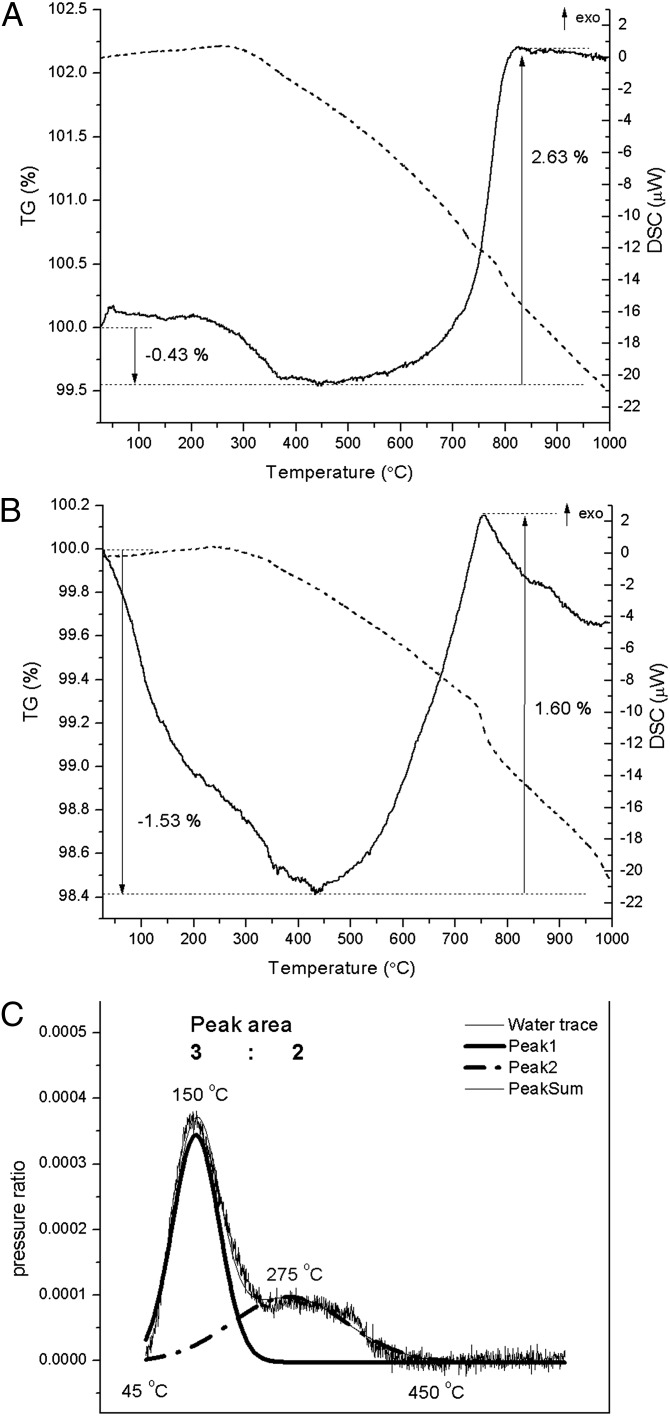
TG (Fig. 1) indicates a negligible amount of water in sample coffinite-F, not quantifiable from the MS trace. This observation agrees with Raman spectra on the same sample which show no signal associated with water (43). The water content of coffinite-G quantified by MS-EGA is 0.37 mol H2O per mole of USiO4 (slightly less than that estimated from TG analysis: 0.43 mol H2O per mole of USiO4). The water signal from MS suggests that there may be two water bonding sites, one with release near 150 °C and the other around 275 °C. The second peak (Fig. 1C) has a high-temperature tail up to 450 °C, suggesting some water molecules are strongly interacting with the sample.
Fig. 1.
DSC-TG of coffinite-F [DSC trace in bold solid, and TG trace in bold dash (A)] and coffinite-G (B) with corresponding H2O+ (m/z +18) trace from MS-EGA (C).
Above 450 °C, both samples oxidize and slowly decompose in air, as indicated by weight increase on the TG trace due to oxidation of U(IV). This is consistent with previous reports (24, 30). After heating to 1,000 °C in air, no trace of USiO4 is detected in either sample. Coffinite-F decomposes to U3O8 and amorphous SiO2 (SI Appendix, Fig. S2), and coffinite-G decomposes to a mixture of crystalline and amorphous SiO2 phases and a uranium-rich phase (possibly containing some silica and whose XRD pattern resembles that of U3O8).
EPMA of coffinite-F (SI Appendix, Table S1) confirms USiO4 stoichiometry. The X-ray map (SI Appendix, Fig. S3) and backscattered electron image show that coffinite-F is pure and homogeneous with no detectable secondary phases. As for coffinite-G, the Si/U ratio of this sample is 4.19 and its chemical composition is USiO4·3.19SiO2·0.37H2O, obtained by combining EPMA result and water content from MS, described in detail in SI Appendix. This bulk composition is used to interpret the calorimetric data.
Through thermochemical cycles (SI Appendix, Tables S2 and S3), the enthalpy of formation (ΔHf,ox) of coffinite-F from binary oxides (uraninite plus quartz) is 24.6 ± 3.1 kJ/mol, obtained from calorimetry in sodium molybdate (3Na2O·4MoO3); and 26.7 ± 4.7 kJ/mol, derived from calorimetry in lead borate (2PbO·B2O3) (Table 1). Using independent measurements in two different solvents confirms consistency and indicates that there were no unanticipated problems in calorimetric procedures. Details of calorimetry are given in SI Appendix.
Table 1.
Measured enthalpies of drop solution and enthalpies of formation from oxides
| Source | ΔHds, kJ/mol | ΔHf,ox, kJ/mol |
| Coffinite-F | −121.43 ± 1.54* | 24.6 ± 3.1 |
| −102.01 ± 3.10† | 26.7 ± 4.7 | |
| Coffinite-G | 44.14 ± 0.94* | (−5.5 ± 3.5) |
| 44.14 ± 0.94* | 24.1 ± 3.5‡ | |
| 44.39 ± 0.94* | 23.9 ± 4.0§ |
Measured in 3Na2O·4MoO3 solvent.
Measured in 2PbO·B2O3 solvent.
This value obtained from a correction considering the water was strongly bonded and its interaction enthalpy was assumed to be −80 kJ/mol.
This value obtained from a correction considering metaschoepite as an impurity phase containing all of the water.
Due to limited sample amount, calorimetry of coffinite-G was performed only in 3Na2O·4MoO3 solvent. Correction for excess silica and water was required to derive ΔHf,ox of coffinite from this sample. The excess Si is present as amorphous to poorly crystalline silica (SI Appendix, Fig. S4), and was corrected by using the drop solution enthalpy of silica glass. The correction for excess water is less simple. If the water is “free water” and thus weakly bonded to the sample, the corrected ΔHf,ox of coffinite is −5.5 ± 3.5 kJ/mol (SI Appendix, Table S4). However, it is unlikely the H2O is free water, as IR shows water signal for the sample dried at 200 °C for 48 h (SI Appendix, Fig. S6), and MS shows that the water is removed gradually up to 450 °C, which suggests that at least part of the H2O in this sample is strongly bonded. Assuming water in coffinite is adsorbed on the surface with an integral adsorption enthalpy of −80 kJ/mol per mole of H2O, similar to the values observed for water adsorption on alumina and titania (44–46) and, through proper thermochemical cycles (SI Appendix, Table S4), a value of ΔHf,ox of coffinite (24.1 ± 3.5 kJ/mol) is obtained, in agreement with the results for coffinite-F (Table 1).
The tightly bound water could originate from metaschoepite (UO3·2H2O), which could be formed from partial oxidation of U(IV) (47) that originally was not incorporated in the coffinite structure, but rather was embedded in the gelatinous layer of excess amorphous silica (33). This phase may be hard to detect by XRD, especially if it is fine-grained or poorly crystalline. If all of the water is in metaschoepite, the composition of coffinite-G can be written as 0.815(USiO4)·3.379SiO2·0.185(UO3·2H2O). Making this correction through an appropriate thermochemical cycle (SI Appendix, Table S5) gives the corrected ΔHf,ox of USiO4 as 23.9 ± 4.0 kJ/mol, again in agreement with the value for coffinite-F.
Thus, our measurements indicate that coffinite is energetically metastable with respect to uraninite plus quartz by about 25 kJ/mol. Coffinite is also energetically metastable with respect to mixture of UO2 and amorphous SiO2, as the enthalpy difference between quartz and glass is 9.1 kJ/mol (48). For the Gibbs free energy of coffinite formation from uraninite plus quartz to be negative at 25 °C, the entropy of formation must be strongly positive and probably unreasonably large for a solid-state reaction. Thus, we conclude that coffinite is thermodynamically metastable relative to uraninite plus quartz under ambient conditions. This conclusion is in good agreement with the solubility experiments and calculations of Szenknect et al. (40) using reference entropy data (22, 40) and obtaining ΔHf,ox = 10 ± 32 kJ/mol (Table 2). The present values disagree with ΔHf,ox = -5.8 kJ/mol estimated by Langmuir (22) or ΔHf,ox = 4.3 ± 5.6 kJ/mol collected in the book edited by Grenthe (37) and suggest that the estimation by Szenknect et al. (40) is more reliable than those values (Table 2).
Table 2.
Comparison of thermodynamics of formation of coffinite at 25 °C obtained in different studies
| Source | ΔHf,ox, kJ/mol* | ΔGf,ox, kJ/mol* | ΔSf,ox, J mol−1⋅K−1† | ΔH°f, kJ/mol | ΔG°f, kJ/mol |
| Langmuir (22) | −5.7‡ | 5.6 | −37.9 | −2,001.3 | −1,882.4 |
| Grenthe et al. (37) | 4.3 ± 5.6‡ | 4.4 ± 4.2 | −0.4 ± 12.8 | −1,991.3 ± 5.4 | −1,883.6 ± 4.0 |
| Hemingway (36) | 2 ± 6 | −1,886 ± 6 | |||
| Szenknect (40) | −105 ± 32‡ | 16 ± 6 | −407 ± 56 | −2,101 ± 32 | −1,872 ± 6 |
| 10 ± 32‡,§ | −19.1 ± 12.8§ | ||||
| Fleche (39) | 82 | −1,913 | |||
| Coffinite-F | 24.6 ± 3.1 | −1,971.0 ± 3.4‡ | |||
| 26.7 ± 4.7 | −1,968.9 ± 4.9‡ | ||||
| Coffinite-G | (-5.5 ± 3.5) | −2,001.1 ± 3.8‡ | |||
| 24.1 ± 3.5 | −1,971.5 ± 3.8‡ | ||||
| 23.9 ± 4.0 | −1,971.7 ± 4.2‡ |
The substantially positive ΔHf,ox also explains why coffinite cannot be formed directly from UO2 and SiO2 and agrees with the observation that coffinite decomposes upon heating to a moderate temperature as seen from DSC-TG experiments.
Coffinite metastability was also inferred by Costin et al. (33) in hydrothermal synthesis. They noted that the dissolution–reprecipitation process slows toward the coffinite end of the Th1−xUxSiO4 series, forming a correspondingly increasing amount of a Th–U dioxide phase. As a result, the formation of a uranothorite phase with x > 0.26 (coffinite included) is suggested to be thermodynamically unfavorable (40). In addition, a Th–U dioxide phase and amorphous silica were inevitably present in these products (24, 30).
Because coffinite is metastable with respect to uraninite plus quartz, why can it form and persist widely in uranium ore deposits (2, 12, 19)? First, one should realize that coffinite can be stable with respect to aqueous species over a wide range of concentrations. Langmuir (22) assumed the average silica concentration to be 10−3 M in the solution where the aqueous equilibrium between coffinite and uraninite is established (5): USiO4(s) + 2H2O(l) ⇄ UO2(s) + Si(OH)4(aq). A calculation based on Gibbs free energy obtained from solubility experiments done by Szenknect et al. (40) and auxiliary data (37) gives the Gibbs free energy of this reaction to be 5.7 ± 5.8 kJ/mol, which is essentially zero. Thus, their analysis suggests that coffinite can form from aqueous U(IV) in contact with silica-rich aqueous solutions, even though it is metastable with respect to crystalline UO2 plus SiO2. Our enthalpy data support this general conclusion. Therefore, coffinite can be an alteration product of UO2 under repository, hydrothermal, metamorphic, or even igneous conditions as long as its formation can proceed through precipitation from aqueous solution.
The presence of water may play an additional significant role in stabilizing the coffinite phase or favoring the coffinitization process (4). Deditius et al. (8) studied the composition of natural coffinite and found it can crystallize with variable amounts of H2O apparently incorporated in the material. However, whether this water is truly in the coffinite structure (24, 49), is associated with the excess silica (24, 43), or represents a fine intergrowth of coffinite and some other phase, e.g., metaschoepite, is not clear.
Although it is plausible that coffinite forms as an alteration product from interaction of uraninite with Si-rich fluids, there may also be an alternative explanation for its formation. Consider underground nuclear waste repositories, ore deposits, or natural reactors where the high alpha dose (6, 25, 50) triggers radiolysis of water to form H2O2 (25, 51–53); the localized oxidative conditions would help dissolve uraninite into more soluble uranyl (UO22+) species (47). Organic matter in the natural system (6, 8, 12, 18, 28) could then play an important reducing role to precipitate coffinite as summarized by Deditius et al. (8). A sequence of UO2 oxidation by peroxide, transport of U(VI) species in aqueous solution, and reduction by organic matter in the presence of a silica source could produce coffinite at locations distant from the initial UO2 phase. Under such conditions, USiO4 could form if its crystallization were kinetically favored over that of UO2. Thus, the process of coffinitization may involve a sequence of reactions: UO2 dissolution under locally oxidizing conditions, transport of the dissolved U(VI) species into more reducing environments containing dissolved silica, followed by coffinite precipitation.
Experimental Methods
Coffinite-F and -G samples were prepared by hydrothermal synthesis routes, described elsewhere (33, 35). Because these syntheses routes inevitably have UO2 and amorphous silica as byproducts, further purification is needed. HNO3 and KOH solution were used to wash the samples as described in the purification protocol proposed by Clavier et al. (54). More synthesis and purification details are shown in SI Appendix.
XRD measurements were performed at room temperature using a Bruker D8 diffractometer with Bragg–Brentano geometry (Cu Κα radiation, 40 kV, and 40 mA), using a step size of 0.016–0.028 °. Lattice parameters were refined by the Le Bail method (55).
DSC-TG measurements were performed with a Setaram LabSys instrument coupled with a quadrupole mass spectrometer (MKS Cirrus2) for evolved gas analysis. Coffinite samples (∼10 mg) were placed in Pt crucibles without lids and heated in airflow (40 mL/min) to 1,000 °C at 10 °C/min. For quantitative analysis of evolved gases MS traces for H2O+ (m/z = 18) and CO2 (m/z = 44) were calibrated by decomposition of calcium oxalate in the same experimental conditions.
Chemical composition and homogeneity were determined using a Cameca SX-100 electron microprobe with wavelength dispersive spectroscopy (15-kV accelerating voltage, 10-nA beam current, and a spot size of 1 μm). Samples were pelletized, polished, and carbon coated before analysis. The decomposition products of coffinite-G were recovered after DSC-TG and the ratio of Si/U was further analyzed.
High-temperature oxide melt solution calorimetry was conducted using a custom-built Tian-Calvet twin microcalorimeter (52–54). Powdered samples were hand pressed into small pellets (∼5 mg) and were dropped from room temperature into either 30 g of molten 2PbO·B2O3 solvent at 802 °C, or 20 g of molten 3Na2O·4MoO3 solvent presaturated with 100 mg of SiO2 (56) at 703 °C, each held in Pt crucibles. O2 gas was continuously bubbled through the melt at 5 mL/min to ensure an oxidizing environment and facilitate dissolution of samples to prevent local saturation (57). Flushing O2 gas at ∼50 mL/min through the calorimeter chamber assisted in maintaining a constant gas environment above the solvent and removing any evolved gases (57). The calorimeter was calibrated using the heat content of ∼5 mg α-Al2O3 pellets (58, 59). Upon rapid and complete dissolution of the sample, the enthalpy of drop solution ΔHds was obtained. Finally, using appropriate thermochemical cycles, enthalpies of formation from the oxides ΔHf,ox were calculated.
Previous studies show that silica has a low solubility in 3Na2O·4MoO3 but silica-containing samples dissolve, precipitating silica as cristobalite (56). A test of this solvent on the dissolution of zircon structure materials was done by dropping ZrSiO4 and HfSiO4 in this setup and yielded consistent results with experiments done in molten lead 2PbO·B2O3 solvent at 802 °C.
Supplementary Material
Acknowledgments
The authors acknowledge H. P. Brau and X. Le Goff from ICSM for the TEM images of coffinite-F. Preparation, characterization, and purification of sample coffinite-F were done at ICSM. Funding for these experiments was supported by the Nucléaire, Energie, Environnement, Déchets, et Société Resources program of the CNRS. Preparation, purification of the coffinite-G sample, and part of the characterization were done at Forschungszentrum Jülich. The calorimetric and characterization work at University of California, Davis, was supported as part of the Materials Science of Actinides, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Award DESC0001089. We also gratefully acknowledge the support of the US Department of Energy through the Los Alamos National Laboratory/Laboratory Directed Research & Development Program and the G. T. Seaborg Institute for this work.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507441112/-/DCSupplemental.
References
- 1.Stieff LR, Stern TW, Sherwood AM. Preliminary description of coffinite—a new uranium mineral. Science. 1955;121(3147):608–609. [Google Scholar]
- 2.Goldhaber MB, Hemingway BS, Mohagheghi A, Reynolds RL, Northrop HR. Origin of coffinite in sedimentary-rocks by a sequential adsorption-reduction mechanism. Bull Mineral (Paris) 1987;110(2-3):131–144. [Google Scholar]
- 3.Smits G. (U, Th)-bearing silicates in reefs of the Witwatersrand, South-Africa. Can Mineral. 1989;27(4):643–656. [Google Scholar]
- 4.Janeczek J, Ewing RC. Coffinitization - a mechanism for the alteration of UO2 under reducing conditions. Proc MRS. 1991;257:487–504. [Google Scholar]
- 5.Janeczek J, Ewing RC. Dissolution and alteration of uraninite under reducing conditions. J Nucl Mater. 1992;190(2):157–173. [Google Scholar]
- 6.Savary V, Pagel M. The effects of water radiolysis on local redox conditions in the Oklo, Gabon, natural fission reactors 10 and 16. Geochim Cosmochim Acta. 1997;61(21):4479–4494. [Google Scholar]
- 7.Bros R, Hidaka H, Kamei G, Ohnuki T. Mobilization and mechanisms of retardation in the Oklo natural reactor zone 2 (Gabon) - inferences from U, REE, Zr, Mo and Se isotopes. Appl Geochem. 2003;18(12):1807–1824. [Google Scholar]
- 8.Deditius AP, Utsunomiya S, Ewing RC. The chemical stability of coffinite, USiO4·nH2O; 0 < n < 2, associated with organic matter: A case study from Grants uranium region, New Mexico, USA. Chem Geol. 2008;251(1-4):33–49. [Google Scholar]
- 9.Speer JA. The actinide orthosilicates. Rev Mineral. 1980;5(1):113–135. [Google Scholar]
- 10.Hansley PL, Fitzpatrick JJ. Compositional and crystallographic data on REE-bearing coffinite from the Grants Uranium region, northwestern New-Mexico. Am Mineral. 1989;74(1-2):263–270. [Google Scholar]
- 11.Janeczek J, Ewing RC. Mechanisms of lead release from uraninite in the natural fission reactors in Gabon. Geochim Cosmochim Acta. 1995;59(10):1917–1931. [Google Scholar]
- 12.Gauthier Lafaye F, Holliger P, Blanc PL. Natural fission reactors in the Franceville basin, Gabon: A review of the conditions and results of a ''critical event'' in a geologic system. Geochim Cosmochim Acta. 1996;60(23):4831–4852. [Google Scholar]
- 13.Fayek M, Janeczek J, Ewing RC. Mineral chemistry and oxygen isotopic analyses of uraninite, pitchblende and uranium alteration minerals from the Cigar Lake deposit, Saskatchewan, Canada. Appl Geochem. 1997;12(5):549–565. [Google Scholar]
- 14.Jensen KA, Ewing RC. The Okelobondo natural fission reactor, southeast Gabon: Geology, mineralogy, and retardation of nuclear-reaction products. Geol Soc Am Bull. 2001;113(1):32–62. [Google Scholar]
- 15.Fayek M, Harrison TM, Ewing RC, Grove M, Coath CD. O and Pb isotopic analyses of uranium minerals by ion microprobe and U-Pb ages from the Cigar Lake deposit. Chem Geol. 2002;185(3-4):205–225. [Google Scholar]
- 16.Forster HJ. Composition and origin of intermediate solid solutions in the system thorite-xenotime-zircon-coffinite. Lithos. 2006;88(1-4):35–55. [Google Scholar]
- 17.Janeczek J, Ewing RC. Phosphatian coffinite with rare earth elements and Ce-rich francoisite-(Nd) from sandstone beneath a natural fission reactor at Bangombe, Gabon. Mineral Mag. 1996;60(401):665–669. [Google Scholar]
- 18.Janeczek J. Mineralogy and geochemistry of natural fission reactors in Gabon. Rev Mineral Geochem. 1999;38(1):321–392. [Google Scholar]
- 19.Janeczek J, Ewing RC, Oversby VM, Werme LO. Uraninite and UO2 in spent nuclear fuel: A comparison. J Nucl Mater. 1996;238(1):121–130. [Google Scholar]
- 20.Fayek M, Kyser TK. Characterization of multiple fluid-flow events and rare-earth-element mobility associated with formation of unconformity-type uranium deposits in the Athabasca Basin, Saskatchewan. Can Mineral. 1997;35(3):627–658. [Google Scholar]
- 21.Amme M, Wiss T, Thiele H, Boulet P, Lang H. Uranium secondary phase formation during anoxic hydrothermal leaching processes of UO2 nuclear fuel. J Nucl Mater. 2005;341(2-3):209–223. [Google Scholar]
- 22.Langmuir D. Uranium solution-mineral equilibria at low-temperatures with applications to sedimentary ore-deposits. Geochim Cosmochim Acta. 1978;42(6):547–569. [Google Scholar]
- 23.Gaucher EC, et al. A robust model for pore-water chemistry of clayrock. Geochim Cosmochim Acta. 2009;73(21):6470–6487. [Google Scholar]
- 24.Fuchs LH, Hoekstra HR. The preparation and properties of uranium(IV) silicate. Am Mineral. 1959;44(9-10):1057–1063. [Google Scholar]
- 25.Sattonnay G, et al. Alpha-radiolysis effects on UO2 alteration in water. J Nucl Mater. 2001;288(1):11–19. [Google Scholar]
- 26.Lian J, et al. Response of synthetic coffinite to energetic ion beam irradiation. J Nucl Mater. 2009;393(3):481–486. [Google Scholar]
- 27.Nord GI. 1977. Characteristics or fine-grained black uranium ores by transmission electron microscopy. Short Papers of the US Geological Survey, Uranium-Thorium Symp, US Geological Survey Circular 753 (US Dept. of the Interior, Geological Survey, Golden, CO), pp 29–31.
- 28.Nagy B, et al. Organic-matter and containment of uranium and fissiogenic isotopes at the Oklo natural reactors. Nature. 1991;354(6353):472–475. [Google Scholar]
- 29.Robit V. 2005. Etude des phases neoformees lors de la dissolution du combustible nucleaire en condition de stockage geologique: Influence des ions silicate. PhD Thesis (Universite Paris-Sud-11, Paris)
- 30.Hoekstra HR, Fuchs LH. Synthesis of coffinite-USiO4. Science. 1956;123(3186):105. [Google Scholar]
- 31.Mulak J. Crystal-field parameters in USiO4 from temperature-dependence of paramagnetic-susceptibility. J Solid State Chem. 1977;21(2):117–126. [Google Scholar]
- 32.Pointeau V, et al. Synthesis and characterization of coffinite. J Nucl Mater. 2009;393(3):449–458. [Google Scholar]
- 33.Costin DT, et al. How to explain the difficulties in the coffinite synthesis from the study of uranothorite? Inorg Chem. 2011;50(21):11117–11126. doi: 10.1021/ic2016758. [DOI] [PubMed] [Google Scholar]
- 34.Costin DT, et al. Preparation and characterization of synthetic Th0.5U0.5SiO4 uranothorite. Prog Nucl Energy. 2012;57:155–160. [Google Scholar]
- 35.Labs S, et al. Synthesis of Coffinite, USiO4, and structural investigations of UxTh(1-x)SiO4 solid solutions. Environ Sci Technol. 2014;48(1):854–860. doi: 10.1021/es403995b. [DOI] [PubMed] [Google Scholar]
- 36.Hemingway BS. 1982. Thermodynamic properties of selected uranium compounds and aqueous species at 298.15 K and 1 bar and at higher temperatures; preliminary models for the origin of coffinite deposits. US Geological Survey Open-File Report 82-619, p 89. Available at pubs.er.usgs.gov/publication/ofr82619. Accessed February 3, 2015.
- 37.Grenthe I, et al. Chemical Thermodynamics of Uranium. Elsevier; Amsterdam: 1992. pp. 31, 64, 72, 334–336. [Google Scholar]
- 38.Guillaumont R, et al. 2004. Update on the Chemical Thermodynamics of Uranium, Neptunium, Plutonium, Americium and Technetium (North Holland Elsevier Science, Amsterdam) pp 59, 549.
- 39.Fleche JL. Thermodynamical functions for crystals with large unit cells such as zircon, coffinite, fluorapatite, and iodoapatite from ab initio calculations. Phys Rev B. 2002;65(24):245116. [Google Scholar]
- 40.Szenknect S, et al. From uranothorites to coffinite: A solid solution route to the thermodynamic properties of USiO4. Inorg Chem. 2013;52(12):6957–6968. doi: 10.1021/ic400272s. [DOI] [PubMed] [Google Scholar]
- 41.Rhett DW. Mechanism of uranium retention in intractable uranium ores from northwestern New-Mexico. Jom-J Min Met Mat S. 1979;31(10):45–50. [Google Scholar]
- 42.Ramdohr P. The Ore Minerals and Their Intergrowths. 2nd Ed Pergamon; New York: 1980. [Google Scholar]
- 43.Clavier N, et al. From thorite to coffinite: A spectroscopic study of Th(1-x)U(x)SiO4 solid solutions. Spectrochim Acta A Mol Biomol Spectrosc. 2014;118:302–307. doi: 10.1016/j.saa.2013.08.093. [DOI] [PubMed] [Google Scholar]
- 44.Ushakov SV, Navrotsky A. Direct measurements of water adsorption enthalpy on hafnia and zirconia. Appl Phys Lett. 2005;87(16):164103. [Google Scholar]
- 45.Levchenko AA, Li GS, Boerio-Goates J, Woodfield BF, Navrotsky A. TiO2 stability landscape: Polymorphism, surface energy, and bound water energetics. Chem Mater. 2006;18(26):6324–6332. [Google Scholar]
- 46.Tavakoli AH, et al. Amorphous alumina nanoparticles: Structure, surface energy, and thermodynamic phase stability. J Phys Chem C. 2013;117(33):17123–17130. [Google Scholar]
- 47.Guo X, et al. Energetics of metastudtite and implications for nuclear waste alteration. Proc Natl Acad Sci USA. 2014;111(50):17737–17742. doi: 10.1073/pnas.1421144111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robie RA, Hemingway BS. Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar pressure and at higher temperatures. US Geol Surv Bull. 1995;2131:16. [Google Scholar]
- 49.Stieff LR, Stern TW, Sherwood AM. Coffinite, a uranous silicate with hydroxyl substitution - a new mineral. Am Mineral. 1956;41(9-10):675–688. [Google Scholar]
- 50.Bodansky D. Nuclear Energy: Principles, Practices, and Prospects. American Institute of Physics; Woodbury, NY: 1996. pp. 246–273. [Google Scholar]
- 51.Amme M. Contrary effects of the water radiolysis product H2O2 upon the dissolution of nuclear fuel in natural ground water and deionized water. Radiochim Acta. 2002;90(7):399–406. [Google Scholar]
- 52.McNamara B, Buck E, Hanson B. Observation of studtite and metastudtite on spent fuel. Mater Res Soc Symp P. 2003;757:401–406. [Google Scholar]
- 53.Kubatko K-AH, Helean KB, Navrotsky A, Burns PC. Stability of peroxide-containing uranyl minerals. Science. 2003;302(5648):1191–1193. doi: 10.1126/science.1090259. [DOI] [PubMed] [Google Scholar]
- 54.Clavier N, et al. Purification of uranothorite solid solutions from polyphase systems. J Nucl Mater. 2013;441(1-3):73–83. [Google Scholar]
- 55.Le Bail A. Whole powder pattern decomposition methods and applications: A retrospection. Powder Diffr. 2005;20(04):316–326. [Google Scholar]
- 56.Gorman-Lewis D, et al. Thermodynamic properties of soddyite from solubility and calorimetry measurements. J Chem Thermodyn. 2007;39(4):568–575. [Google Scholar]
- 57.Navrotsky A, et al. The behavior of H2O and CO2 in high temperature lead borate solution calorimetry of volatile-bearing phases. Am Mineral. 1994;79(11-12):1099–1109. [Google Scholar]
- 58.Navrotsky A. Progress and new directions in high temperature calorimetry. Phys Chem Miner. 1977;2(1-2):89–104. [Google Scholar]
- 59.Navrotsky A. Progress and new directions in high temperature calorimetry revisited. Phys Chem Miner. 1997;24(3):222–241. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.