Significance
African trypanosomes cause human and animal diseases and evade the host immune systems by periodically switching which variant surface glycoprotein (VSG) they express. The mechanisms that result in expression of one of the numerous VSG genes at a time and the switching of expression between different VSG genes are poorly understood. We show that specific steps in the inositol phosphate (IP) pathway control both monoallelic VSG gene transcription and the switching of VSG gene expression. The data indicate that the cellular amounts, locations, and molecular interactions of specific IP pathway enzymes and their metabolites control both processes. The results offer new drug targets and insights into the epigenetic control of gene expression by this biologically ubiquitous pathway.
Keywords: transcription, trypanosomes, antigenic variation, telomere silencing, inositol phosphates
Abstract
African trypanosomes evade clearance by host antibodies by periodically changing their variant surface glycoprotein (VSG) coat. They transcribe only one VSG gene at a time from 1 of about 20 telomeric expression sites (ESs). They undergo antigenic variation by switching transcription between telomeric ESs or by recombination of the VSG gene expressed. We show that the inositol phosphate (IP) pathway controls transcription of telomeric ESs and VSG antigenic switching in Trypanosoma brucei. Conditional knockdown of phosphatidylinositol 5-kinase (TbPIP5K) or phosphatidylinositol 5-phosphatase (TbPIP5Pase) or overexpression of phospholipase C (TbPLC) derepresses numerous silent ESs in T. brucei bloodstream forms. The derepression is specific to telomeric ESs, and it coincides with an increase in the number of colocalizing telomeric and RNA polymerase I foci in the nucleus. Monoallelic VSG transcription resumes after reexpression of TbPIP5K; however, most of the resultant cells switched the VSG gene expressed. TbPIP5K, TbPLC, their substrates, and products localize to the plasma membrane, whereas TbPIP5Pase localizes to the nucleus proximal to telomeres. TbPIP5Pase associates with repressor/activator protein 1 (TbRAP1), and their telomeric silencing function is altered by TbPIP5K knockdown. These results show that specific steps in the IP pathway control ES transcription and antigenic switching in T. brucei by epigenetic regulation of telomere silencing.
Only one of the ∼20 telomeric expression sites (ESs) is transcribed at a time in Trypanosoma brucei in the mammalian infectious stage bloodstream (BF) or metacyclic forms (MFs), whereas no ES is transcribed in the insect stage procyclic forms (PFs) (1). Each ES contains 1 telomeric variant surface glycoprotein (VSG) gene, whose expression confers a distinct cellular antigenic type and up to 12 expression site-associated genes (ESAGs) whose functions are incompletely understood (Fig. 1A) (2–4). The parasites evade immune clearance by periodically changing antigenic type by switching transcription between ESs or by ES recombination with the ∼2,500 non-ES VSG genes and pseudogenes (5, 6). ESs are transcribed by RNA polymerase I (Pol I), which initiates at the single promoter at all ESs but terminates within a few kilobases except at one fully transcribed ES (7, 8).
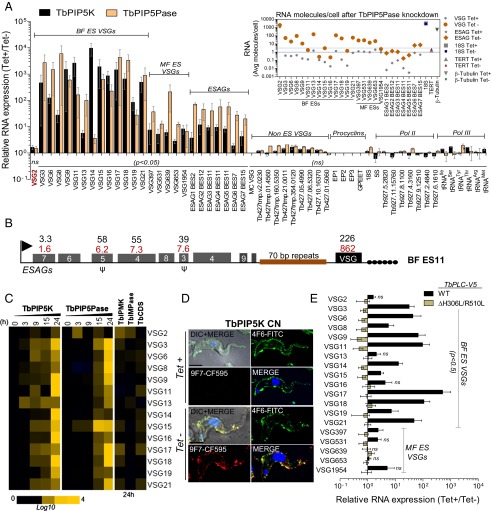
Fig. 1.
IP pathway genes that affect ES transcription in BF T. brucei. (A) Diagram of a T. brucei BF ES. The promoter (flag) that is 3′ to 50-bp repeats drives Pol I transcription of ESAGs and the downstream VSG gene, which is flanked by 70 bp and TTAGGG telomeric repeats (closed circles). Ψ indicates pseudogenes. Fifteen BF and 5 MF ESs have been sequenced in the strain Lister 427 (47, 48). MFs ESs lack ESAG genes. (B) Part of the predicted IP metabolic pathway in T. brucei. Enzyme names are colored to correspond to C, and those whose genes were not manipulated are in gray. See Tables S1 and S2 for enzyme and metabolite abbreviations and gene IDs. (C) In vitro growth of CN BF cells (Left) and qPCR analysis (Right) of gene expression 24 h after conditional knockdown of IP pathway genes; tet+ = 0.5 µg/mL. (D) In vitro growth (Left) of BF cells that overexpress (OX) WT or mutant (H306L/R510L) PLC and Western analysis (Right) of the cells at 24 h. The membrane was probed with anti-V5 Mabs, stripped, and reprobed with anti-HSP70 Mab; tet+ = 1 µg/mL. Growth data in C and D are the means (and SEM) of five and three experiments, respectively. See Fig. S2 for cell viability.
RNAi knockdown of expression of the nuclear protein TbRAP1 that interacts with the TTAGGG-binding factor (TbTRF) or of the nuclear lamina protein 1 (TbNUP1) or deletion of the histone-lysine N-methyltransferase DOT1B (TbDOT1B) alleles each results in transcription of silent ESs (9–11). Similarly, RNAi knockdown of the transcription facilitating histone chaperone suppressor of ty 16 (TbSPT16) or the SWI2/SNF2-related chromatin-remodeling protein TbISWI or deletion of histone deacetylase sirtuin 2-related protein 1 (TbSIR2RP1) each increases transcription near the promoter but not the VSG gene of silent ESs (12–14). These results indicate that the control of ES transcription involves telomeric silencing (15), which entails the functions and interactions of multiple proteins and chromatin remodeling (16, 17), but importantly, what regulates these processes is unknown.
The inositol phosphate (IP) pathway regulates multiple cellular processes in eukaryotes including chromatin remodeling and gene expression (18–21). Soluble IPs or lipid-conjugated phosphatidylinositols (PIs) occur in various cellular compartments, where they act as cofactors in the regulation of the functions or interactions of proteins (22, 23). Knowing this, we explored the role of the IP pathway in the control of telomeric ESs transcription and VSG switching (see Tables S1 and S2 for gene IDs). We found that conditional knockdown of TbPIP5K or TbPIP5Pase or overexpression of TbPLC resulted in transcription of all known telomeric VSG and ESAG genes but no change in transcription of procyclin or genes that are transcribed by Pol II or Pol III. Reexpression of TbPIP5K after a temporary knockdown restored monoallelic VSG gene transcription and was accompanied by a high frequency of VSG switching. The IP enzymes and metabolites are primarily on the plasma membrane, but TbPIP5Pase is in the nucleus, proximal to telomeres, and is associated with TbRAP1, and their silencing function is altered by TbPIP5K knockdown. Thus, the IP pathway controls ES transcription and antigenic switching by regulation of telomere silencing.
Results
Specific Steps of the IP Pathway Affect Transcription of Subtelomeric ESs.
The T. brucei genome encodes ∼26 genes that predict enzymes for the IP pathway including those for IP and PI kinases and phosphatases, a PLC, and enzymes for the synthesis or recycling of inositol and PIs (24), although the specificities of all these enzymes have not yet been directly determined. A portion of the IP pathway that is relevant to this paper is shown in Fig. 1B. We made conditional null (CN) BF cell lines of IP pathway genes by replacing the endogenous alleles of the selected genes with drug resistance markers in cells in which we inserted a copy of the gene that has its transcription dependent on the presence of tetracycline (tet). Tet-induced expression of the target gene in CNs resulted in mRNA levels similar to the endogenous levels in the parental cell line SM427 (Fig. 1C). Removal of tet resulted in 95–99% reduction in the target gene mRNA levels and in some cases resulted in inhibition of cell growth after 48 h. We also made a BF cell line that conditionally overexpresses V5-tagged TbPLC in the presence of tet by inserting into the rRNA intergenic region an additional copy of the gene that is transcribed by a tet-dependent promoter. Addition of tet resulted in a 3.7- and 3.3-fold increase, respectively, of WT or catalytically inactive mutated (H306L/R510L) (25) TbPLC mRNA as measured by quantitative PCR (qPCR) and additional V5-tagged TbPLC protein as seen by Western analysis, but had no effect on cell growth (Fig. 1D).
Conditional knockdown of TbPIP5K or TbPIP5Pase resulted in the expression, i.e., derepression, of all known silent BF and MF ESs (Fig. 2A). The abundances of the VSG mRNAs were increased by up to 10,000-fold relative to cells that express these genes (i.e., cells growing in tet). ESAG mRNAs are highly conserved among BF ESs, but the abundances of those that could be distinguished between ESs were increased by up to two orders of magnitude (Fig. 2 A and B and Fig. S1). The differences in the relative increases among the VSG and ESAG mRNAs are likely due to their differential posttranscriptional regulation (7). Calculation of the cellular amounts of the VSG and ESAG mRNAs indicated that the absolute levels of these mRNAs per cell were increased after 18 h of TbPIP5Pase knockdown (Fig. 2A, Inset and Tables S3 and S4). The knockdowns did not affect expression of non-ES VSG genes and pseudogenes. The marginal increases in non-ES VSG gene expression may be due to periodic recombination between the ESs and VSG genes, which occurs within the cell populations. The knockdowns did not affect expression of procyclins or rRNAs, both of which, like the ES, are transcribed by Pol I, or the expression of genes that are transcribed by Pol II or Pol III (Fig. 2A and Table S5). VSG derepression was detected as early as 3 h after the knockdowns, and immunofluorescence (IF) analysis with monoclonal antibodies (Mabs) for two different VSGs (6) showed that individual cells express both VSGs on their surface following TbPIP5K knockdown (Fig. 2 C and D). Overexpression of V5-tagged TbPLC also resulted in derepression of VSG genes in BF and MF ESs and mutation of the TbPLC catalytic site (H306L/R510L) ablated this effect (Fig. 2E). Thus, multiple ESs are derepressed upon perturbation of expression of IP enzymes, the cells express multiple VSGs, and the derepression is specific to ESs.
Fig. 2.
IP pathway gene functions affect subtelomeric ESs silencing. (A) Relative mRNA levels by qRT-PCR analysis after knockdown of TbPIP5K or TbPIP5Pase genes for 24 or 18 h, respectively, by withdrawal of tet from CN cell lines; tet+, 0.5 µg/mL. CN cell lines express only the VSG2 gene in tet+. (Inset) Average RNA molecules per cell 18 h after knockdown of TbPIP5Pase (Tables S3 and S4). The gray line indicates one RNA molecule per cell, and the blue line indicates the average RNA molecules per cell of several Pol II transcribed genes. See Table S5 for additional expression analysis of Pol II transcribed genes and Dataset S1 for primers. (B) Fold increase of mRNAs from ES11 24 and 18 h after conditional knockdown, respectively, of TbPIP5K (red) and TbPIP5Pase (black). See Fig. S1 for other ESs. The ES gene organization shown is from strain 427 (47) and may differ in CNs cell lines. (C) Time course of VSG gene expression after knockdown of TbPIP5K or TbPIP5Pase and after 24-h knockdown of TbIPMK, TbCDS, or TbIMPase in CN cell lines. (D) IF analysis following knockdown of TbPIP5K for VSG2 using FITC-conjugated anti-VSG 4F610–1 Mab (green) and for a VSG that is recognized by 9F715–1 Mab using CF-594–conjugated antibody (red). DNA is stained with DAPI (blue). (Scale bar, 2.5 µm.) (E) Relative levels of ES VSG mRNAs 24 h after conditional overexpression (OX) of V5-tagged WT or mutant PLC. The qPCR data are the means (SEM) of two to six experiments, normalized relative to telomerase reverse transcriptase (TERT) and β-tubulin and significant at P < 0.05, except as indicated as not significant (ns).
Conditional knockdown of other IP pathway genes including inositol polyphosphate multikinase (TbIPMK), inositol (1, 4) monophosphatase (TbIMPase), and cytidine diphosphate-diacylglycerol synthase (TbCDS) (Fig. 1B) for 24 h had no effect on the relative abundances of ES mRNAs (Fig. 2C). The conditional knockdowns of TbPIP5K, TbPIP5Pase, TbIPMK, and TbCDS resulted in growth inhibition of T. brucei BFs, with most parasites dying by 72 h, but null mutants of TbIMPase are viable (Fig. 1C). Lethality that ultimately occurs by 72 h is likely due to multiple factors including the loss of essential IP metabolites, e.g., which function in protein anchoring, and the disruption of their “second messenger” functions. Nevertheless, the effects on ES transcription of TbPIP5K and TbPIP5Pase knockdown or TbPLC overexpression are not due to cell lethality. Overexpression of V5-tagged TbPLC did not affect cell growth (Fig. 1D). In addition, over the 24-h cell growth period after knockdown, TbPIP5K and TbPIP5Pase CN cell morphology and cell cycle were unaffected, and flow cytometry analysis of live/dead cells showed that 89–91% of the cells were alive (Fig. S2). As detailed below, ∼90% of the cells were also viable when tet was added back 24 h after knockdowns in CN cells. Thus, steps in the IP pathway that affect phosphorylation at inositol position 5 specifically impact the control of ES transcription.
Temporary Knockdown of TbPIP5K Increases the Frequency of VSG Switching.
The release from 24-h conditional knockdown by adding tet back to the parasite culture restored ES monoalellic expression; however, most cells switched to expression of a different VSG gene. Expression of TbPIP5K or TbIPMK was temporarily knocked down by withdrawal of tet for 24 h, and then tet was added back, and numerous clones were made by limiting dilution. Five serial 10-fold dilutions resulted in clones in about one of three wells, as is typical, and only about 4% of these clones failed to grow, indicating a high level of viability after 24 h in the absence of tet. The qPCR analysis of 104 randomly isolated clones following the temporary knockdown of TbPIP5K revealed that 45 clones (43%) expressed the same VSG as before the knockdown, i.e.,VSG2. Analysis of 44 clones that did not express VSG2 for the expression of the 19 VSGs that are in the BF or MF ESs in the strain 427 (Fig. 2) showed that 17 clones (16% of the total) transcribed VSG13, 2 (2%) VSG8, 2 (2%) VSG21, and 1 (1%) transcribed VSG19 (Fig. S3). None of the clones analyzed expressed VSGs that were in the MF ESs. The other 22 clones (21%) did not express any of the original VSGs in the ESs, and they likely expressed a VSG gene that arose by recombination involving the numerous non-ES VSG genes in the genome. Analysis of 50 clones derived from TbPIP5K CN cells growing in tet (no knockdown) showed no switch from the original VSG2 expression. In addition, none of the 82 clones obtained from TbIPMK CN switched from VSG2 expression whether they were derived from temporary knockdown cells (44 clones) or not (38 clones), indicating that the switch was not due to nonspecific effects of knockdown. The switching rate following TbPIP5K reexpression was 1.6 × 10−2 cells per cell cycle, which is ∼103-fold higher than reported for in vitro switching (26). Overall, following the temporary knockdown of TbPIP5K, about two-thirds of the clones expressed the original VSG or VSGs that were in known BF ESs. The other clones did not express either of these types of VSGs and presumably expressed VSGs that resulted from recombination involving the numerous non ES VSG genes. Thus, release from the temporary knockdown of TbPIP5K restored VSG monoalellic expression and revealed a high switching frequency of the VSG gene expressed.
Derepression of VSG Genes Is Associated with an Increase in the Number of Telomeric and Pol I Foci.
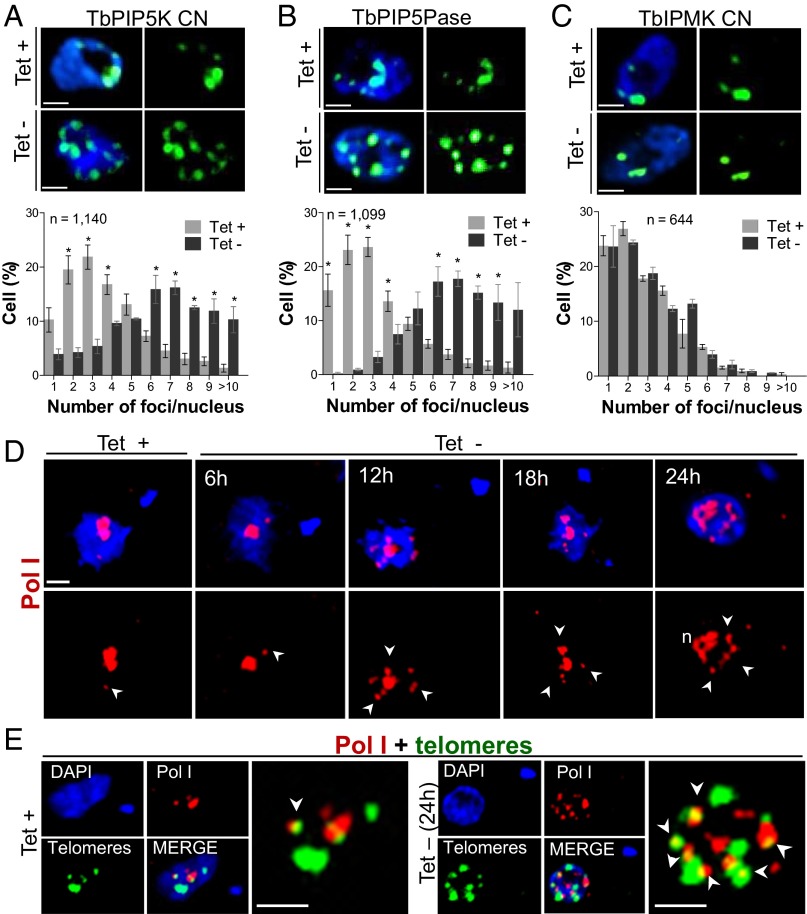
FISH analysis using a telomeric repeat probe and 3D deconvolution microscopy showed that the nuclei of ∼80% of BF parental or CN cells that express IP pathway genes (i.e., tet+) had a few prominent telomeric foci and generally less than five foci (Fig. 3 A–C and Fig. S4). These foci likely represent clusters of telomeres because T. brucei has ∼200 telomeres [from ∼100 minichromosomes and 11 large chromosomes (24)]. Knockdown of TbPIP5K or TbPIP5Pase for 24 h resulted in ∼80% of the cells having 6 to >10 telomeric foci per nucleus, with an apparently broadened intranuclear distribution. Knockdown of TbIPMK, which does not result in ES derepression, did not affect the number of telomeric foci, which was similar to that in WT BF or PF cells (Fig. S4). The TbPIP5K and TbPIP5Pase knockdowns did not affect parasite growth, cell cycle and morphology or cell viability over this 24-h period (see above), suggesting that these effects are not due to nonspecific factors. Similarly, knockdown of TbPIP5Pase also resulted in multiple extranucleolar Pol I foci. These foci became evident 12 h after knockdown, which correlates with the early ES derepression and some of the Pol I foci partially colocalized with telomeric foci (Fig. 3 D and E). Because these cells transcribe multiple ESs, the multiple Pol I foci likely correspond to different sites of ES transcription by Pol I. Longer periods of TbPIP5Pase knockdown resulted in less coherent nucleolar Pol I fluorescence (Fig. 3D at 24 h), perhaps due to Pol I depletion from the nucleolus. Thus, expression of multiple ESs correlated with an increase in the number of telomeric and Pol I foci, and their distribution implies their repositioning within the nucleus at sites where transcription occurs.
Fig. 3.
Effect of TbPIP5K and TbPIP5Pase knockdown on the number of telomeric and Pol I foci. (A–C) FISH analysis with the PNA-(CCCTAA)3-FITC probe for telomeres after 24 h knockdown of TbPIP5K, TbPIP5Pase or TbIPMK expression; tet +, 0.5 µg/mL. Quantification of telomeric foci per nucleus are shown below the images. See also Fig. S4. (D) IF analysis of Pol I foci after conditional knockdown of TbPIP5Pase. (E) Pol I and telomeric foci 24 h after knockdown of TbPIP5Pase. Pol I was detected with Mab 16B1a (8) followed by anti–IgG-AlexaFluor 594 (red), telomeres with a FITC-conjugated PNA-(CCCTAA)3 probe (green), and DNA stained with DAPI (blue). Arrowheads indicate regions of Pol I foci (D) or Pol I and telomeric foci colocalization (E). Data in A–C are the means (SEM) of three experiments. *Significance at P < 0.05. (Scale bar, 1 µm.)
Subcellular Distribution of IP Pathway Enzymes and Metabolites.
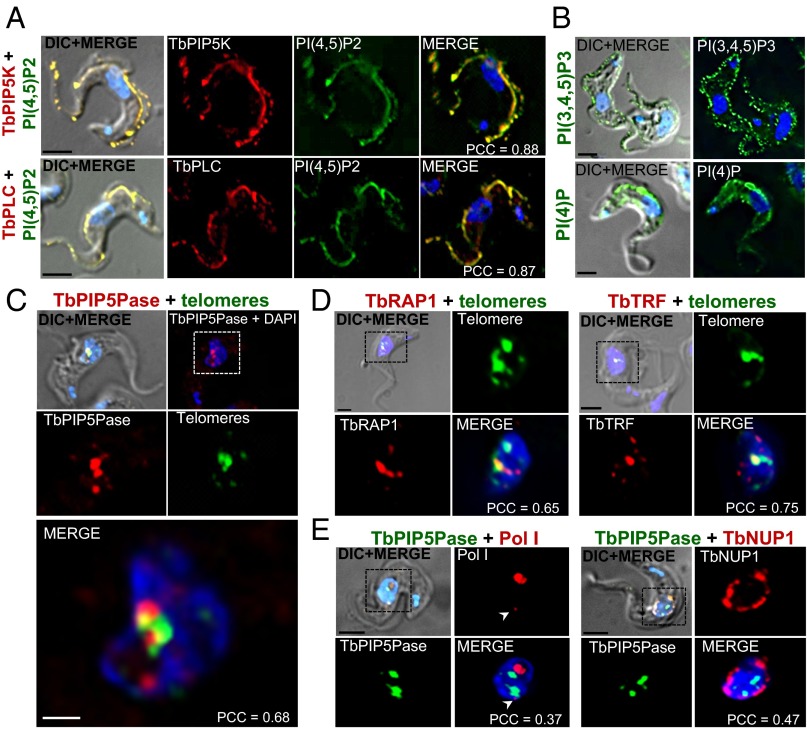
We generated tet-inducible V5-tagged cell lines of IP pathway enzymes in BF T. brucei. IF analysis of V5-tagged TbPIP5K revealed it to be predominantly located at the inner face of the plasma membrane and to be distributed over much of the cell and flagellar membranes and to colocalize with its product PI(4,5)P2 (Fig. 4A). Similarly, V5-tagged TbPLC was predominantly located at the inner face of the plasma and flagellar membranes and colocalized with its substrate PI(4,5)P2. PI(4,5)P2 was detected in permeabilized cells with anti-PI(4,5)P2 monoclonal antibodies, showing its location at the inner face of the plasma membrane (Fig. S5), and expression of a GFP-tagged PH domain probe that recognizes PI(4,5)P2 confirmed its location. In addition, IF analysis with specific monoclonal antibodies showed that PI(4)P and PI(3,4,5)P3 were also predominately at the plasma membrane (Fig. 4B). TbPIP5K knockdown resulted in reduced PI(4,5)P2 and PI(3,4,5)P3 signal from the plasma membrane, indicating that these metabolites result from TbPIP5K activity (Fig. S5). In contrast, V5-tagged TbPIP5Pase was primarily located in the nucleus and was adjacent to and partially colocalized with telomeres (Fig. 4C). A similar pattern of colocalization with telomeres was found for hemagglutinin (HA)-tagged TbRAP1 and TbTRF, which are proteins known to localize at the telomeres (Fig. 4D). The location of most TbPIP5Pase-V5 was distinct from that of the nuclear envelope protein TbNUP1-HA and from the nucleolar Pol I (Fig. 4E). Overall, TbPIP5K and TbPLC and the metabolites PI(4)P, PI(4,5)P2, and PI(3,4,5)P3 are primarily located at the inner face plasma membrane, whereas TbPIP5Pase is primarily a nuclear protein that is located near telomeres.
Fig. 4.
Subcellular locations of IP pathway proteins and metabolites. (A) IF analysis of (Upper) V5-tagged TbPIP5K (red) and its product PI(4,5)P2 (green) and (Lower) V5-tagged TbPLC (red) and its substrate PI(4,5)P2 (green). (B) PI(4)P and PI(3,4,5)P3 metabolites. (C) V5-tagged TbPIP5Pase (red) and telomeres (green). (D) (Left) HA-tagged TbRAP1 (red) and telomeres (green) and (Right) HA-tagged TbTRF (red) and telomeres (green). (E) (Left) V5-tagged TbPIP5Pase (green) and Pol I (red) and (Right) V5-tagged TbPIP5Pase (green) and HA-tagged TbNUP1 (red). Arrowheads indicates extranucleolar Pol I. Metabolites were detected with FITC-conjugated Mabs that are specific to each metabolite. A FITC-conjugated PNA-(CCCTAA)3 probe was used to detect telomeres by FISH, and DNA was stained with DAPI (blue). V5-tagged and HA-tagged proteins were detected with anti-V5 and anti-HA antibodies (see SI Materials and Methods for details). The mean of the Pearson coefficient of correlation (PCC) from multiple cells are indicated for colocalization. See Fig. S5 for the effects of TbPIP5K knockdown on metabolites and staining of nonpermeabilized cells. (Scale bars: A, B, D, and E, 2.5 µm; C, 1 µm.)
TbPIP5Pase Is a PI 5 Phosphatase with Preference for Substrates with a 4 Phosphate.
The TbPIP5ase is conserved in kinetoplastids (∼30–50% amino acid identity) but shares less than 12% overall sequence identity with the human and yeast 5-phosphatase enzymes. Nevertheless, it conserves the K292 and G293 residues that are required for recognition of the inositol ring and 1-phosphate, (green box, Fig. 5A), which is characteristic of all phosphoinositides and the H312, D360, and N362 residues that form the 5-phosphate binding pocket in human 5-phosphatases (red boxes) (27). Native TAP tagged TbPIP5Pase was purified from T. brucei BF by affinity chromatography with IgG-Sepharose via the protein-A domain, and the activity of the TEV-eluate was assayed with several IP and PI substrates (Fig. 5 B and C). The purified enzyme had negligible activity with several soluble IPs but preferential activity with PI(4,5)P2 and PI(3,4,5)P3. There was some activity with PI(5)P and PI(3,5)P2 but essentially no activity with potential PI substrates that lack a 5-phosphate. The presence of a 4-phosphate in PI substrates that contain a 5-phosphate enhanced the enzyme activity. These results indicate that TbPIP5Pase dephosphorylates 5-phosphate substrates with preference for those that also contain a 4-phosphate (Fig. 5D). Kinetic analysis showed higher activity with PI(4,5)P2 compared with PI(3,4,5)P3 and only low activity with I(1,4,5)P3 and primarily at exceptionally high substrate concentrations (Fig. 5E). Enzymologic analysis revealed that the Km and the Vmax was slightly different for PI(4,5)P2 and PI(3,4,5)P3, but the enzyme efficiency was similar to both substrates (Fig. 5 D and F). This enzyme has been annotated as an I(1,4,5)P3 5-phosphatase in GeneDB based on sequence homology, but these analyses indicate that it is a PI 5-phosphatase with specificity toward PI(4,5)P2 and PI(3,4,5)P3.
Fig. 5.
Enzymatic analysis of TbPIP5Pase. (A) Alignment of TbPIP5Pase sequence with the human and yeast 5 phosphatases showing conservation of key residues. The green box indicates residues that are conserved in the 5 phosphate pocket and the red boxes those that recognize the 1 phosphate and inositol ring. (B) SDS/PAGE (4–20%) imperial stained TEV-eluate of TAP-tagged enzyme (asterisk) purified from T. brucei BF (Left) and Western analysis with Mab anti-CBP (calmodulin-binding peptide) (Right). (C) Activity of TEV-elute of the native enzyme with various IP and PI substrates (Materials and Methods). (D) Diagram showing conversion of the preferred substrates and enzyme efficiency (Kcat/Km) in [(mol/liter)-1/S−1] obtained from data in F. (E) Kinetic analyses of TEV-eluted TbPIP5Pase. (F) Enzymologic analyses of TEV-eluted TbPIP5Pase comparing velocities with PI(4,5)P2 and PI(3,4,5)P3. Data in C, E, and F are the mean (SEM) of three experiments in triplicate; significance is *P < 0.05 or ***P < 0.0001.
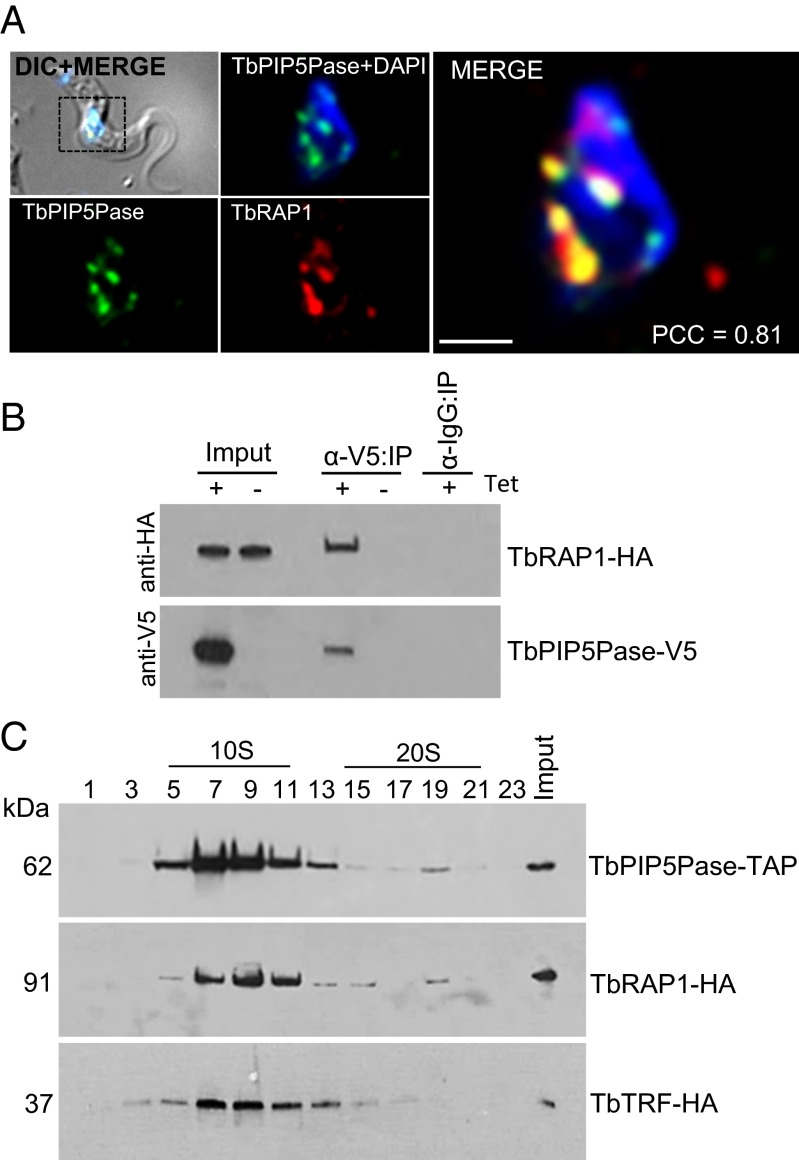
TbPIP5Pase Interacts with the Telomeric Protein TbRAP1.
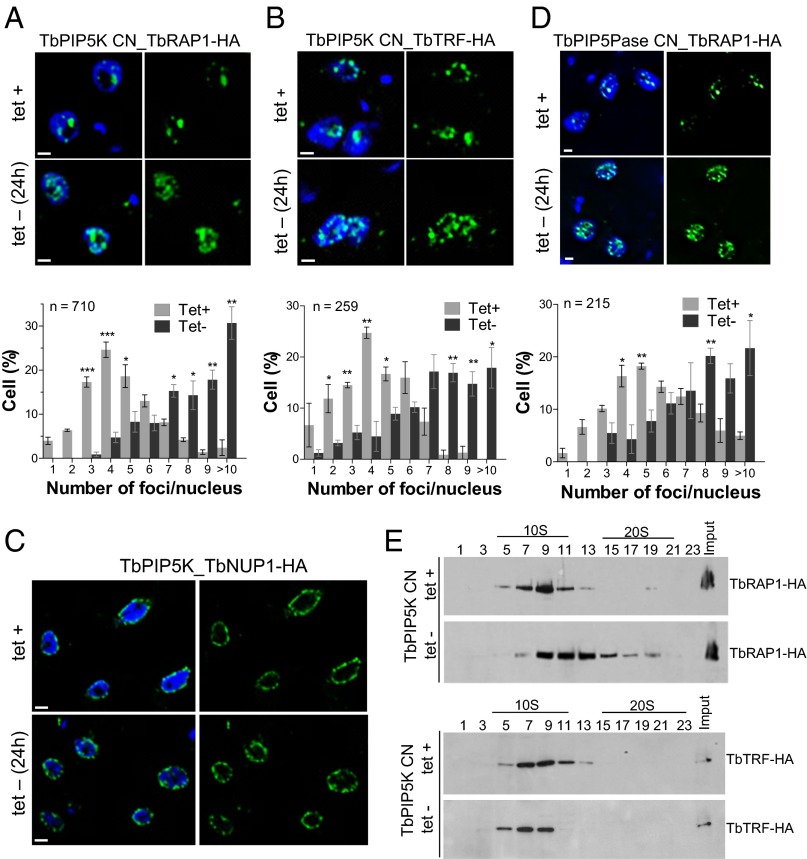
Due to the nuclear location of TbPIP5Pase near telomeres, we analyzed whether it associates with known telomeric proteins. TbRAP1 is a telomeric protein that associates with the TbTRF, and TbRAP1 knockdown has been shown to result in ES derepression (9). We replaced an endogenous allele of TbRAP1 with an HA-tagged allele in a cell line that expresses V5-tagged TbPIP5Pase in a tet-dependent fashion. IF analysis with Mabs that are specific for the tags showed that TbPIP5Pase-V5 and TbRAP1-HA colocalize (Fig. 6A). TbRAP1-HA was coimmunoprecipitated with TbPIP5Pase-V5 that had been pulled down from total BF lysate with anti-V5 Mabs, as revealed by Western analysis using anti-HA Mabs (Fig. 6B). In addition, TbPIP5Pase-TAP and TbRAP1-HA predominantly sedimented at ∼10S as shown by Western analysis of fractions collected from 10% to 30% (vol/vol) glycerol gradients (Fig. 6C). However, a discrete peak was also consistently detected at ∼20S for both proteins. Analysis of TbTRF-HA also showed that it sediments predominantly at ∼10S, but we did not detect an ∼20S peak. These results indicate that TbPIP5Pase, TbRAP1, and TbTRF interact, directly or indirectly. TbRAP1 and TbPIP5Pase may be in two complexes that may include TbTRF and differ in their abundance and sedimentation coefficient, possibly due to their associations with additional proteins.
Fig. 6.
TbPIP5Pase associates with telomeric proteins. (A) IF showing colocalization of V5-tagged TbPIP5Pase (green) and HA-tagged TbRAP1 (red). (Scale bar, 1 µm.) (B) Western analysis with anti-V5 and anti-HA Mabs of V5-tagged TbPIP5Pase immunoprecipitated with Mab anti-V5 from total lysate of cells that express tet-inducible TbPIP5Pase-V5 and constitutively express HA-tagged TbRAP1. (C) Western analysis of 10–30% (vol/vol) glycerol gradient fractions of lysates from T. brucei BFs that express TAP tagged TbPIP5Pase (Top), HA-tagged TbRAP1 (Middle), or HA-tagged TbTRF (Bottom) using the rPAP reagent and anti-HA Mab, respectively.
IP Pathway Controls TbRAP1 Interactions and Telomeric Silencing.
To assess the effect of manipulating the IP pathway genes on these telomeric proteins, we replaced an endogenous allele of TbRAP1, TbTRF, or TbNUP1 with HA-tagged versions in a TbPIP5K CN cell line and examined the effects of knockdown of TbPIP5K by IF (Fig. 7 A–C). TbPIP5K knockdown for 24 h resulted in increased numbers of TbRAP1-HA and TbTRF-HA foci but had no obvious effect on numbers or apparent locations of the nuclear envelope protein TbNUP1-HA. Similarly, knockdown of TbPIP5Pase in a cell line that has an HA-tagged TbRAP1 allele also resulted in increased numbers of TbRAP1-HA foci (Fig. 7D). These results indicate that positioning of TbRAP1 and TbTRF is affected in a TbPIP5K- or TbPIP5Pase-dependent fashion, similarly to the effect on telomeres as analyzed by FISH (Fig. 3). Because no obvious effect was detected on TbNUP1-HA after TbPIP5K knockdown, it indicates that the IP pathway metabolites affect specifically telomeres and telomeric proteins.
Fig. 7.
Effects of TbPIP5K knockdown on telomeric proteins. (A–D) IF analysis with anti-HA Mab of HA-tagged TbRAP1, TbTRF, or TbNUP1 before and after TbPIP5K or TbPIP5Pase knockdown for 24 h. Quantification of the foci is shown below the images except in C and are the means (SEM) of three experiments each (*P < 0.05 and **P < 0.001). (E) Western analysis with anti-HA Mab of 10–30% (vol/vol) glycerol gradient fractions of cellular lysates from CN TbPIP5K cells that express a TbRAP1-HA (Upper) or TbTRF-HA (Lower) tagged gene. Knockdown was for 18 h by withdrawal of the 0.5 µg/mL tet. (Scale bars, 2.5 µm.)
To analyze whether the IP pathway controls the associations of the telomeric proteins, we analyzed TbRAP1 and TbTRF sedimentation after knockdown of TbPIP5K. TbPIP5K knockdown for 18 h shifted TbRAP1-HA to a higher sedimentation coefficient in 10–30% (vol/vol) glycerol gradients (Fig. 7E). In contrast, TbPIP5K knockdown shifted TbTRF-HA to a slightly lower sedimentation coefficient. These results imply that TbRAP1 and TbTRF interactions and their effect on telomeric silencing function in a TbPIP5K-dependent fashion, which may be due to the effect of specific 5-phosphate metabolites, e.g., PI(4,5)P2, on their interactions and function. In addition, the increase in the coefficient of sedimentation of TbRAP1-HA (Fig. 7E) correlates with simultaneous transcription of multiple ESs, which suggests that the ∼20S TbRAP1-HA peak (Fig. 6C) may be related to transcriptionally active ESs. Overall, specific IP pathway enzymes and presumably their metabolites control ES transcription by affecting the interactions and functions of proteins involved in telomeric silencing.
Discussion
We found that specific steps in the IP pathway function in the control of ES transcription and antigenic switching in T. brucei. Conditional knockdown of TbPIP5K or TbPIP5Pase or overexpression of TbPLC resulted in transcription of multiple ESs and cells that expressed more than one VSG protein on their surface. This transcription was specific to telomeric ESs and did not affect transcription of procyclin genes or genes that are transcribed by Pol II or Pol III. The derepression of multiple ESs was accompanied by an increase in the number of telomeric foci and the appearance of multiple extranucleolar Pol I foci. Reexpression of TbPIP5K after temporary knockdown restored monoalellic VSG transcription, and the majority of resultant cells changed which VSG was expressed. TbPIP5K, TbPLC, and their metabolites are primarily located at the plasma membrane. However, TbPIP5Pase is in the nucleus near telomeres. TbPIP5Pase is in a complex with TbRAP1 and TbTRF, and knockdown of TbPIP5K altered their association and their distribution within the nucleus. The results indicate that specific steps in the IP pathway control monoallelic ES transcription via telomeric silencing and VSG switching and hence antigenic variation in T. brucei.
The IP pathway transcriptional control reported here is specific to telomeric ESs. The specificity is likely due to a combination of recognition of the ES promoters by Pol I and their subtelomeric locations, which provides for the process of telomeric silencing (15, 28). The sequences of the ES promoters are highly conserved (∼90% nucleotide identity), and they are functionally related to promoters of the rRNA and procyclin genes, which are also transcribed by Pol I but are not at telomeric sites (2, 28). The specificity of the ES transcriptional control is maintained through the life cycle of the parasite, during which no ESs are transcribed in procyclic forms and ESs that lack ESAGs are selectively transcribed in MFs (29, 30). The silencing of all ESs in PFs and the selective silencing of all but one ES in MF and BF life cycle stages likely entails responses of the regulatory system to stage-specific differences in parasite metabolism, physiology, and environment, i.e., 28 °C vs. 37 °C and different nutrients. It would not be surprising if the IP pathway contributed to the stage-specific regulation of ES transcription.
The process by which all but one of the ESs are silenced involves multiple proteins that include TbRAP1 and TbTRF (9, 31). TbPIP5Pase associates, directly or indirectly, with TbRAP1 and TbTRF as shown here by the results of our IF colocalization, pull down, and sedimentation experiments (Figs. 6 and 7). In addition, we found that knockdown of TbPIP5K shifts the sedimentation of TbRAP1 and TbTRF, which implies that specific IP metabolite levels affect the associations among these proteins. The proteins TbDOT1B and TbNUP1, which function in histone H3 methylation and heterochromatin organization, respectively, also participate in ES regulation (10, 11). Hence, remodeling of the chromatin at telomeric regions and associations among telomeric proteins contribute to ES silencing, and this process appears to be controlled, at least in part, by the IP pathway.
The overall control of transcription of a single ES may also involve the relocation of ESs within the nucleus. The appearance of increased numbers of telomeric, TbRAP1, TbTRF, and Pol I foci in the nucleus when multiple ESs are transcribed, as shown here, suggests that the control of ES transcription may entail repositioning of telomeres in the nucleus. The fewer telomeric foci when a single ES is expressed also suggests that the silent telomeres may be clustered, a process that has been associated with telomeric position effect silencing in other organisms (16, 32). Telomere clustering in yeast is accompanied by the association of the telomeres with the nuclear envelope and the formation of heterochromatin (33). The single active ES forms a discrete extranucleolar Pol I focus that is detectable by IF, named the expression site body (ESB) (8). The appearance of several Pol I foci when multiple ESs are fully transcribed suggests that these are multiple sites of complete ES transcription by Pol I, which have more Pol I associated with them than do ESs where transcription is abortive. Thus, there can be multiple subnuclear sites where ESs can be transcribed, and the single ESB that is seen in normal cells is likely due to its substantial content of associated Pol I.
Enzymologic analysis of the TbPIP5Pase shows that it is a 5-phosphatase that is specific for PI(4,5)P2 and PI(3,4,5)P3, which are the metabolic products of TbPIP5K, and PI(4,5)P2 is also the substrate of TbPLC (Fig. 1B). TbPIP5K and TbPLC, respectively, generate the 5 position phosphorylated lipid-associated and soluble metabolites that correlate with the control of ES transcription. Our IF experiments show that these enzymes and their lipid-associated metabolites are located at the plasma membrane. However, the PI and the soluble IP metabolites (for which we have no antibodies) may be present in the nucleus but at lower levels, perhaps as a result of their flux (34). In yeast and mammalian cells, the IP pathway enzymes and metabolites are found at the plasma membrane and also in intracellular organelles, vesicles, and the nucleus, where they exert different regulatory roles (23, 35). The nuclear and telomeric location of TbPIP5Pase and its association with TbRAP1 and TbTRF suggests that TbPIP5Pase functions via physical association in a fashion that is responsive to the 5 phosphate metabolites PI(4,5)P2 or PI(3,4,5)P3, and it may exert a local control of these metabolite levels. TbPIP5Pase, TbRAP1, and TbTRF all primarily sediment at ∼10S in glycerol gradients, but another less abundant peak is consistently seen at ∼20S. TbPIP5K knockdown resulted in the shift of TbRAP1 to a higher sedimentation coefficient but had minor effects on TbTRF sedimentation. The higher TbRAP1 sedimentation correlates with the derepression of multiple ESs and may result from TbRAP1 association with additional proteins or modifications that affect the conformation of TbRAP1 or other chromatin-associated proteins or DNA. The data imply that the IP metabolites may directly affect TbRAP1 interaction with TbTRF and perhaps other telomere-associated proteins. Specific IP metabolites may repress ES transcription by controlling the association of proteins that function in telomeric silencing, e.g., TbRAP1. Perturbation of these metabolite levels may alter the associations of these telomeric proteins and thus result in the transcription of numerous ESs.
Reexpression of TbPIP5K after temporary knockdown restored monoallelic VSG expression. Analysis of the cloned cell lines recovered after the knockdown revealed that most cells switched the VSG gene expressed at a frequency that is three orders of magnitude higher than the normal in vitro rate (26). There was a range of frequencies for the VSG expressed with the VSG2, which was expressed before knockdown, being the most frequent followed by VSG13 and others at lower frequency (Fig. S3). About 21% of the recovered cell lines did not express any of the VSG genes that were originally in the telomeric ESs, and thus they likely expressed a VSG gene that arose by recombination. This range of frequencies following temporary knockdown is reminiscent of the preferential order of expression that occurs during antigenic variation following infection (36). The differences in ES sequences and/or epigenetic chromatin marks may contribute to their probability of not being silenced and hence the order of expression during antigenic variation. None of the five VSG genes that are in the MF ESs were expressed following the temporary knockdown, which may be due to a low probability of them being selected for expression or it may reflect a mechanism that selects them for preferential expression in the metacyclic stage as indicated above. The frequent occurrence of switching to expression of VSGs that appear to have arisen by recombination implies that the conditions that enabled transcription of the ESs may have increased the probability of recombination because the temporary perturbation only spanned four to five cell divisions. Active transcription is known to favor homologous recombination (37). RNAi knockdown of the telomeric proteins TbTRF or TbTRF-interacting factor 2 (TbTIF2) increased VSG switching rates in T. brucei BF (38, 39). The loss of these proteins appears to have enhanced repair and recombination. Overall, these results indicate that perturbation of the IP pathway may precipitate antigenic switching by both switching transcription among telomeric ESs or by recombination, perhaps by affecting associations and functions of proteins that associate with telomeric ESs.
A general model that embraces knowledge from other systems (19, 20, 22) is that certain IP metabolites may specifically bind to proteins that have roles in telomeric silencing and affect their interactions with other proteins and/or their functions, e.g., histone modification and chromatin remodeling. The ultimate consequence of the derepression is that the chromatin at multiple ESs is permissive for the elongation of transcription by Pol I through the VSG genes. Our results suggest that the control of VSG gene allelic exclusion is the result of the properties of the cells molecular components, which are functionally integrated within the cell. The locations and levels of IP enzymes and metabolites are determined by these properties and may maintain a dynamic equilibrium that results in silencing of all but one ES. Disruption of the equilibrium alters the associations and/or functions of proteins, perhaps involving specific interactions with the IP metabolites, resulting in the loss of silencing of all ESs. Reestablishment of the equilibrium results in silencing of all but one ES but a likely switch to a different ES. This hypothesis implies that the equilibrium maintains the BF cells in a state that is poised for antigenic switching.
By way of perspective, the IP pathway functions to regulate silencing of telomeric ESs and antigenic switching in T. brucei and thus controls allelic exclusion of VSG genes. The stage-specific control of VSG gene expression implies that the IP pathway may also participate in the control and coordination of stage-specific processes during the life cycle in African trypanosomes. In addition, the IP pathway is conserved from yeast to humans (18); hence, the IP pathway may function in telomeric silencing and allelic exclusion in other organisms such as the expression of var genes in Plasmodium spp., or perhaps in the allelic exclusion of odorant receptors in mammals (40, 41). T. cruzi and Leishmania, which are related to T. brucei, conserve the IP pathway but lack telomeric ESs and may have adapted the pathway for alternate functions, e.g., control the expression of numerous surface proteins.
Materials and Methods
Cell Lines.
Cell lines that conditionally express IP pathway genes, i.e., CN cells, were generated as previously described (42); see Dataset S1 for primers. Cells that conditionally express C-terminal V5-tagged TbPLC, TbPIP5K, or TbPIP5Pase were generated using the pLEW100-3V5 vector, and cells that constitutively express HA-tagged TbRAP1, TbTRF, or TbNUP1 were generated by replacing an endogenous allele with one that has a 3′ terminal tag using PMOTag2H vector as previously published (43, 44). Growth curves in the presence and absence of tet were determined by seeding 25-cm2 cell culture flasks with 5.0 × 104 parasites/mL in 10 mL HMI-9 medium supplemented with 10% (vol/vol) FBS, 2.0 µg/mL G418, and 2.5 µg/mL of phleomycin to select for the tet regulatable allele. Parasites were counted using a Neubauer chamber.
RNA Quantification.
CN cell lines were grown with or without 0.5 µg/mL tet for 18 or 24 h and harvested at room temperature (10 min, 1,300 × g), RNA was extracted using TRIzol (Life Technologies), and cDNA preparation and qRT-PCR were done as previously described (42). The absolute RNA levels were determined by qRT-PCR relative to a standard curve using the β-tubulin gene fragment in the pHD1344-tub vector, and the amounts of VSG, ESAG, and Pol II mRNAs and 18S rRNA were calculated as previously described (45).
IF and FISH.
T. brucei midlog phase cells were fixed with 2% (wt/vol) paraformaldehyde in PBS and adhered to poly-l-lysine–treated 2-mm cover glass (Fisher) and permeabilized with 0.2% Nonidet P-40 in PBS for 5 min. Cells were blocked for 1 h with 3% (wt/vol) BSA in PBS and incubated for 2 h at room temperature or overnight at 4 °C with specific antibodies (SI Materials and Methods). An FITC-conjugated PNA (CCCTTA)3 telomere C FISH probe (Biosynthesis) was used for FISH as previously described (11). DNA was stained with 1 µg/mL DAPI (Sigma), and slides were mounted with ProLong Gold Antifade Reagent (Life Technologies). Images were obtained using a Deltavision 3D deconvolution microscope (Olympus IX70) and analyzed with Softworks software (Applied Precision).
VSG Switching.
Clonal TbPIP5K and TbIPMK CN cells that express VSG2 were grown for 24 h without tet for gene knockdown, followed by reexpression by adding 0.5 µg/mL tet to medium. Cells (1.0 × 105) were immediately cloned by limited dilution into 96-well plates after five times 10-fold serial dilution. The clones were expanded for an additional 3–5 d, and the RNAs were isolated as above. The VSG expressed was assayed by qRT-PCR (SI Materials and Methods). VSG switching was calculated as previously published (26).
Western and Glycerol Gradient.
Western and glycerol gradient analyses were performed as described previously (43). Briefly, lysates from 5.0 × 108 cells were obtained with 1% Triton-X-100 in Tris 50 mM/NaCl 150 mM with EDTA-free protease inhibitor mixture (Roche), loaded on 10–30% (vol/vol) continuous glycerol gradients, and centrifuged for 10 h at 38,000 × g in a SW40 Beckman ultracentrifuge. Fractions of 500 µL each were collected from the top and analyzed by Western analysis.
Immunoprecipitation.
T. brucei BF cells (5.0 × 107) that express tet-regulatable TbPIP5Pase-V5, and endogenous TbRAP1-HA were lysed in 50 mM Tris, 150 mM NaCl, 1% Triton-X-100, 0.5% sodium deoxycolate, and 0.2% Nonidet P-40 with EDTA-free protease inhibitor mixture (Roche). The cleared lysates were obtained after a 5-min centrifugation at 16,000 × g and incubated with 2 µg/mL Mabs anti-V5 (Life Technologies) cross-linked to Protein A Mag Sepharose according to the manufacturer’s instructions (GE Healthcare Life Sciences). An anti-mouse IgG (Bio-Rad) cross-linked to Protein A Mag Sepharose was used as a negative control. After a 2-h incubation at 4 °C, the mix was washed in washing buffer (50 mM Tris, 150 mM NaCl, and 0.2% Nonidet P-40) and eluted in 2× Tricine Sample Buffer (Bio-Rad) without reducing agent. The eluted fractions were supplemented with 1.4 M β-mercaptoethanol and analyzed by Western analysis.
Enzymology.
BF cells that conditionally express TAP-tagged TbPIP5Pase were produced, and TbPIP5Pase-CBP (250 ng) was expressed, purified, and assayed with soluble 50 µM inositol phosphates or diC8 phosphatidylinositol phosphates (Echelon) as previously described (27, 43). Enzyme kinetic reactions were prepared using 1–300 µM of I(1,4,5)P3, diC8-PI(4,5)P2 or diC8-PI(3,4,5)P3 and incubating for 30 min at 37 °C, and kinetics were calculated by nonlinear regressions using GraphPad Prism 5.03 (GraphPad Software).
Statistical Analysis.
Data are shown as means ± SEM, and a two-tailed Student t test was calculated using GraphPad Prism 5.03 (GraphPad Software). Statistical analyses of gene expression were performed by a pairwise fixed reallocation randomization test (46). P values of 0.05 with confidence intervals of 95% were considered statistically significant. Pearson coefficient of correlation for colocalization analysis was performed with Softworks software (Applied Precision) according to the manufacturer’s instructions.
Supplementary Material
Acknowledgments
We thank members of the K.S. laboratory, John Aitchison, and Peter Myler for helpful comments on this work; Anna Sokolov for administrative support; and Keith Gull for anti-Pol I antibody. The work received support from National Institutes of Health Grant R01AI078962 and Supplement R01AI014102-37S1 (to K.S. and I.C.), American Heart Association Fellowship 14POST18970046 (to I.C.), and the Seattle Biomedical Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1501206112/-/DCSupplemental.
References
- 1.Borst P. Antigenic variation and allelic exclusion. Cell. 2002;109(1):5–8. doi: 10.1016/s0092-8674(02)00711-0. [DOI] [PubMed] [Google Scholar]
- 2.Zomerdijk JC, et al. The promoter for a variant surface glycoprotein gene expression site in Trypanosoma brucei. EMBO J. 1990;9(9):2791–2801. doi: 10.1002/j.1460-2075.1990.tb07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hertz-Fowler C, et al. Telomeric expression sites are highly conserved in Trypanosoma brucei. PLoS ONE. 2008;3(10):e3527. doi: 10.1371/journal.pone.0003527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pays E, Lips S, Nolan D, Vanhamme L, Pérez-Morga D. The VSG expression sites of Trypanosoma brucei: Multipurpose tools for the adaptation of the parasite to mammalian hosts. Mol Biochem Parasitol. 2001;114(1):1–16. doi: 10.1016/s0166-6851(01)00242-0. [DOI] [PubMed] [Google Scholar]
- 5.Cross GA, Kim HS, Wickstead B. Capturing the variant surface glycoprotein repertoire (the VSGnome) of Trypanosoma brucei Lister 427. Mol Biochem Parasitol. 2014;195(1):59–73. doi: 10.1016/j.molbiopara.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Myler PJ, Allison J, Agabian N, Stuart K. Antigenic variation in African trypanosomes by gene replacement or activation of alternate telomeres. Cell. 1984;39(1):203–211. doi: 10.1016/0092-8674(84)90206-x. [DOI] [PubMed] [Google Scholar]
- 7.Kassem A, Pays E, Vanhamme L. Transcription is initiated on silent variant surface glycoprotein expression sites despite monoallelic expression in Trypanosoma brucei. Proc Natl Acad Sci USA. 2014;111(24):8943–8948. doi: 10.1073/pnas.1404873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navarro M, Gull K. A pol I transcriptional body associated with VSG mono-allelic expression in Trypanosoma brucei. Nature. 2001;414(6865):759–763. doi: 10.1038/414759a. [DOI] [PubMed] [Google Scholar]
- 9.Yang X, Figueiredo LM, Espinal A, Okubo E, Li B. RAP1 is essential for silencing telomeric variant surface glycoprotein genes in Trypanosoma brucei. Cell. 2009;137(1):99–109. doi: 10.1016/j.cell.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueiredo LM, Janzen CJ, Cross GA. A histone methyltransferase modulates antigenic variation in African trypanosomes. PLoS Biol. 2008;6(7):e161. doi: 10.1371/journal.pbio.0060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DuBois KN, et al. NUP-1 Is a large coiled-coil nucleoskeletal protein in trypanosomes with lamin-like functions. PLoS Biol. 2012;10(3):e1001287. doi: 10.1371/journal.pbio.1001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denninger V, Fullbrook A, Bessat M, Ersfeld K, Rudenko G. The FACT subunit TbSpt16 is involved in cell cycle specific control of VSG expression sites in Trypanosoma brucei. Mol Microbiol. 2010;78(2):459–474. doi: 10.1111/j.1365-2958.2010.07350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes K, et al. A novel ISWI is involved in VSG expression site downregulation in African trypanosomes. EMBO J. 2007;26(9):2400–2410. doi: 10.1038/sj.emboj.7601678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsford S, Kawahara T, Isamah C, Horn D. A sirtuin in the African trypanosome is involved in both DNA repair and telomeric gene silencing but is not required for antigenic variation. Mol Microbiol. 2007;63(3):724–736. doi: 10.1111/j.1365-2958.2006.05553.x. [DOI] [PubMed] [Google Scholar]
- 15.Horn D, Cross GA. A developmentally regulated position effect at a telomeric locus in Trypanosoma brucei. Cell. 1995;83(4):555–561. doi: 10.1016/0092-8674(95)90095-0. [DOI] [PubMed] [Google Scholar]
- 16.Gasser SM, Hediger F, Taddei A, Neumann FR, Gartenberg MR. The function of telomere clustering in yeast: The circe effect. Cold Spring Harb Symp Quant Biol. 2004;69:327–337. doi: 10.1101/sqb.2004.69.327. [DOI] [PubMed] [Google Scholar]
- 17.Kueng S, Oppikofer M, Gasser SM. SIR proteins and the assembly of silent chromatin in budding yeast. Annu Rev Genet. 2013;47:275–306. doi: 10.1146/annurev-genet-021313-173730. [DOI] [PubMed] [Google Scholar]
- 18.Michell RH. Inositol derivatives: Evolution and functions. Nat Rev Mol Cell Biol. 2008;9(2):151–161. doi: 10.1038/nrm2334. [DOI] [PubMed] [Google Scholar]
- 19.Mellman DL, et al. A PtdIns4,5P2-regulated nuclear poly(A) polymerase controls expression of select mRNAs. Nature. 2008;451(7181):1013–1017. doi: 10.1038/nature06666. [DOI] [PubMed] [Google Scholar]
- 20.Yildirim S, et al. Involvement of phosphatidylinositol 4,5-bisphosphate in RNA polymerase I transcription. J Cell Sci. 2013;126(Pt 12):2730–2739. doi: 10.1242/jcs.123661. [DOI] [PubMed] [Google Scholar]
- 21.Watson PJ, Fairall L, Santos GM, Schwabe JW. Structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nature. 2012;481(7381):335–340. doi: 10.1038/nature10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millard CJ, et al. Class I HDACs share a common mechanism of regulation by inositol phosphates. Mol Cell. 2013;51(1):57–67. doi: 10.1016/j.molcel.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irvine RF. Nuclear lipid signalling. Nat Rev Mol Cell Biol. 2003;4(5):349–360. doi: 10.1038/nrm1100. [DOI] [PubMed] [Google Scholar]
- 24.Berriman M, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309(5733):416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- 25.Essen LO, Perisic O, Cheung R, Katan M, Williams RL. Crystal structure of a mammalian phosphoinositide-specific phospholipase C delta. Nature. 1996;380(6575):595–602. doi: 10.1038/380595a0. [DOI] [PubMed] [Google Scholar]
- 26.Boothroyd CE, et al. A yeast-endonuclease-generated DNA break induces antigenic switching in Trypanosoma brucei. Nature. 2009;459(7244):278–281. doi: 10.1038/nature07982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trésaugues L, et al. Structural basis for phosphoinositide substrate recognition, catalysis, and membrane interactions in human inositol polyphosphate 5-phosphatases. Structure. 2014;22(5):744–755. doi: 10.1016/j.str.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Rudenko G, Blundell PA, Dirks-Mulder A, Kieft R, Borst P. A ribosomal DNA promoter replacing the promoter of a telomeric VSG gene expression site can be efficiently switched on and off in T. brucei. Cell. 1995;83(4):547–553. doi: 10.1016/0092-8674(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 29.Graham SV, Barry JD. Transcriptional regulation of metacyclic variant surface glycoprotein gene expression during the life cycle of Trypanosoma brucei. Mol Cell Biol. 1995;15(11):5945–5956. doi: 10.1128/mcb.15.11.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horn D, Cross GA. Position-dependent and promoter-specific regulation of gene expression in Trypanosoma brucei. EMBO J. 1997;16(24):7422–7431. doi: 10.1093/emboj/16.24.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B, Espinal A, Cross GA. Trypanosome telomeres are protected by a homologue of mammalian TRF2. Mol Cell Biol. 2005;25(12):5011–5021. doi: 10.1128/MCB.25.12.5011-5021.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freitas-Junior LH, et al. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell. 2005;121(1):25–36. doi: 10.1016/j.cell.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 33.Galy V, et al. Nuclear pore complexes in the organization of silent telomeric chromatin. Nature. 2000;403(6765):108–112. doi: 10.1038/47528. [DOI] [PubMed] [Google Scholar]
- 34.Boss WF, Im YJ. Phosphoinositide signaling. Annu Rev Plant Biol. 2012;63:409–429. doi: 10.1146/annurev-arplant-042110-103840. [DOI] [PubMed] [Google Scholar]
- 35.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 36.Aline RF, Jr, Scholler JK, Nelson RG, Agabian N, Stuart K. Preferential activation of telomeric variant surface glycoprotein genes in Trypanosoma brucei. Mol Biochem Parasitol. 1985;17(3):311–320. doi: 10.1016/0166-6851(85)90005-2. [DOI] [PubMed] [Google Scholar]
- 37.Aymard F, et al. Transcriptionally active chromatin recruits homologous recombination at DNA double-strand breaks. Nat Struct Mol Biol. 2014;21(4):366–374. doi: 10.1038/nsmb.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jehi SE, Wu F, Li B. Trypanosoma brucei TIF2 suppresses VSG switching by maintaining subtelomere integrity. Cell Res. 2014;24(7):870–885. doi: 10.1038/cr.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jehi SE, et al. Suppression of subtelomeric VSG switching by Trypanosoma brucei TRF requires its TTAGGG repeat-binding activity. Nucleic Acids Res. 2014;42(20):12899–12911. doi: 10.1093/nar/gku942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirkman LA, Deitsch KW. Antigenic variation and the generation of diversity in malaria parasites. Curr Opin Microbiol. 2012;15(4):456–462. doi: 10.1016/j.mib.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serizawa S, Miyamichi K, Sakano H. One neuron-one receptor rule in the mouse olfactory system. Trends Genet. 2004;20(12):648–653. doi: 10.1016/j.tig.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Merritt C, Stuart K. Identification of essential and non-essential protein kinases by a fusion PCR method for efficient production of transgenic Trypanosoma brucei. Mol Biochem Parasitol. 2013;190(1):44–49. doi: 10.1016/j.molbiopara.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cestari I, et al. A multiple aminoacyl-tRNA synthetase complex that enhances tRNA-aminoacylation in African trypanosomes. Mol Cell Biol. 2013;33(24):4872–4888. doi: 10.1128/MCB.00711-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oberholzer M, Morand S, Kunz S, Seebeck T. A vector series for rapid PCR-mediated C-terminal in situ tagging of Trypanosoma brucei genes. Mol Biochem Parasitol. 2006;145(1):117–120. doi: 10.1016/j.molbiopara.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Haanstra JR, et al. Control and regulation of gene expression: Quantitative analysis of the expression of phosphoglycerate kinase in bloodstream form Trypanosoma brucei. J Biol Chem. 2008;283(5):2495–2507. doi: 10.1074/jbc.M705782200. [DOI] [PubMed] [Google Scholar]
- 46.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becker M, et al. Isolation of the repertoire of VSG expression site containing telomeres of Trypanosoma brucei 427 using transformation-associated recombination in yeast. Genome Res. 2004;14(11):2319–2329. doi: 10.1101/gr.2955304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolev NG, Ramey-Butler K, Cross GA, Ullu E, Tschudi C. Developmental progression to infectivity in Trypanosoma brucei triggered by an RNA-binding protein. Science. 2012;338(6112):1352–1353. doi: 10.1126/science.1229641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.