Abstract
Background
Human papillomavirus (HPV) positive oropharyngeal squamous cell carcinomas are increasing in incidence and are becoming significant public health concerns. Periodontitis is a chronic condition in which the affected tissue may facilitate oral HPV infection and persistence. The purpose of this study was to determine if an association of the presence of HPV in oral rinse specimens and periodontal disease exists.
Methods
The National Health and Nutrition Examination Survey data for years 2009–10 and 2011–12 were combined. Participants ages 30–69 years with clinically assessed periodontal and HPV data were included in the study (n=6,004). The data were analyzed with Rao Scott Chi Square and logistic regression.
Results
There were 498 participants who had the presence of HPV in oral rinse specimens. The adjusted odds ratio for the presence of HPV in oral rinse specimens with relation to periodontal disease was 1.04 (95% confidence interval: 0.63, 1.73), adjusting for sex, race/ethnicity, education, age, income to poverty ratio, smoking, alcohol use and number of lifetime sex partners.
Conclusions
The researchers failed to reject the hypothesis of no association of the presence of HPV in oral rinse specimens and periodontitis.
Practical Implications
Although oral HPV infection is a serious concern, periodontitis was not shown to be related to the presence of HPV in oral rinse specimens in adjusted analyses in this study.
Keywords: Periodontal diseases, risk assessment, public health/community dentistry, National Health and Nutrition Examination Survey (NHANES)
Worldwide, head and neck carcinomas are the 6th most common cancer with an incidence of 400,000–500,000 annual cases,1–5 and nearly $3.2 billion in treatment costs. Even with aggressive treatment, there is significant morbidity and mortality.6 In 1983, human papilloma virus (HPV) was identified as a risk factor for oropharyngeal squamous cell carcinoma.7 Between 5–20% of head and neck squamous cell carcinomas and 40–90% of oropharyngeal carcinomas are related to HPV.1,8 There are over 150 different HPV types9 (or strains), most of which are not considered high risk types.
The high risk types are: HPV-16, 18, 31, 33, 35, 45, 51, 52, 56, 68, 59, 68, 73, and 82.1 High risk HPV types have been found in nearly one-fourth of oral and laryngeal squamous cell carcinomas;10 HPV-16 was associated with 45–90% of oropharyngeal carcinomas.1,11 In contrast to the incidence of HPV negative (HPV−) oropharyngeal squamous cell carcinomas, HPV positive (HPV+) oropharyngeal squamous cell carcinomas have been increasing in incidence in the U.S.,12–15 from 0.8/100,000 in 1998 to 2.6/100,000 in 2004.8 HPV is an 8,000 base-pair DNA genome in an icosahedral 55nm capsid.16 The virus is small, non-enveloped, and epitheliotrophic.17
Although there is a growing awareness of the association of HPV and oropharyngeal squamous cell carcinomas, there is a lack of information about oral HPV infection and infectivity. D’Souza et al. reported that the prevalence of HPV-16 infection in exfoliated oral epithelial cells increased the odds of oropharyngeal carcinoma 13 times.18
Periodontitis is a complex chronic oral condition involving teeth, bone, epithelial cells, a biofilm community of bacteria, fungi, and viruses,16,19,20 and the immune system’s response to the biofilm community. Periodontitis has been associated with other chronic inflammatory diseases such as diabetes, and cardiovascular disease.15 In periodontitis, there is a continuous release of inflammatory cytokines and biomarkers which adversely affects systemic health,5 and is related to a modulation in epithelial barrier function protection.
It is unknown if the biofilm and chronic inflammation of periodontitis with a decrease in epithelial barrier protection may be associated with oral HPV infectivity. The purpose of this study is to determine if there is an association of the presence of HPV in oral rinse specimens and periodontitis. The null hypothesis is that the odds ratio for the presence of HPV in oral rinse specimens is equal in individuals who have periodontitis as it is in individuals who do not have periodontitis. Previous studies have been equivocal. Some were limited by the use of self-reports for identifying periodontitis, others used self-reported oral health as the periodontal variable. Other studies have also had limitations as small sample sizes, or hospital-based cases. This study adds to the literature results from a large, nationally represented sample in which clinical assessment of all teeth was performed and the definition used for periodontitis was based on the definition for population surveillance of the Centers for Disease Control and Prevention in partnership with the American Academy of Periodontology.21
METHODS
Study design and data source
This study was acknowledged by the West Virginia University Institutional Review Board (protocol 1408392538). The researchers used a cross-sectional study design to determine the association of the presence of HPV in oral rinse specimens (HPV+ or HPV−) and periodontitis. The data source was the National Health and Nutrition Examination Survey (NHANES), years 2009–2012. The NHANES is a national population study of the non-institutionalized and civilian public which has a stratified, multistage probability sampling design. The National Center for Health Statistics Institutional Review Board approved the NHANES study. Participants received written informed consent. Interviewers surveyed a household member and requested a physical evaluation of the participant at an NHANES mobile examination center. Eligible participants in the NHANES were ages 14 to 69 years. Details of the NHANES are available at http://www.cdc.gov/nchs/nhanes.htm.
Study sample
The eligible sample for this study was a subset of the NHANES, years 2009–2012. It included NHANES participants with complete, clinically assessed periodontal and HPV data. Edentulous individuals, individuals with heart transplants, artificial heart valves, congenital heart disease, rheumatic fever, hemophilia, kidney disease with dialysis, a pacemaker or younger than age 30 years were not included. The data were available for participants ages 30–69 years (n= 6,004).
Outcome/Dependent Variable
The study outcome was the presence of HPV in oral rinse specimens (HPV+ or HPV−). The HPV samples were obtained during oral evaluations in which the participants swished and gargled 10 mL of a mouth rinse or saline for 30 seconds. The samples were stored at 4°C and evaluated with polymerase chain reaction for HPV at Ohio State University. Details are available at the NHANES website.
Key variable of interest/exposure
The key variable of interest was periodontal status as mild, moderate, or severe periodontitis (a combined category) or no periodontitis. The definition of periodontitis used in this study was based on the definition for population surveillance of the Centers for Disease Control and Prevention in partnership with the American Academy of Periodontology.21 Their researchers defined mild periodontitis as either one periodontal site having a probing depth of 5 mm or above; or having at least 2 interproximal periodontal sites with 3 mm or more in attachment loss and at least 2 interproximal periodontal sites with at least 4mm probing depth which does not occur on the same tooth.21 The definition for moderate periodontitis was at least 2 interproximal sites with probing depths of at least 5 mm which do not occur on the same tooth or at least 2 interproximal sites which are not on the same tooth and which have an attachment loss of at least 4 mm.21 Severe periodontitis was defined as at least one interproximal site having a probing depth of at least 5 mm with at least 2 interproximal sites which are on different teeth having attachment loss of at least 4 mm. The data in this study were dichotomized (mild, moderate, or severe periodontitis v. no periodontitis) due to sample size availability.
The periodontal evaluations were conducted by licensed dentists with halogen illumination and the use of a mirror and HuFriedy™ periodontal probes. Details are available at the NHANES website.
Other variables/potential confounders of Epidemiological importance
Other variables have been indicated to having had an epidemiologic role in HPV+ status. The other variables used for this study included sociodemographic and behavioral variables. The variables were self-reported to the interviewer and included: sex (male v. female); race/ethnicity (Non-Hispanic black, Mexican American, Other v. Non-Hispanic white); education (high school or less than high school v. more than high school); age (30–44 v. 45–69 years); family income to poverty ratio (less than 2 v. 2 and above); smoking (current, or former smoker v. never smoker); alcohol consumption (moderate, heavy v. no consumption); and lifetime sex partners (2–10, 11 and above v. 0–1). These variables were selected as having had previously been shown to have an association with HPV infectivity;6,22–25 and sex, race/ethnicity, education, age, income, and smoking were also previously shown to be related to periodontal disease.26–31
Statistical analyses
The NHANES has a complex study design. The design and sample weights were used in the statistical analyses of this study. Statistical analyses included descriptive statistics (frequency determinations), univariate Rao Scott Chi Square, and unadjusted and adjusted logistic regression on HPV. Three models were built. The full adjusted logistic regression included sex, race/ethnicity, education, age, family income to poverty ratio, alcohol consumption, and lifetime sex partners in addition to the key variable of interest, periodontitis, as these variables were significant in the univariate analysis and are considered important in epidemiological studies. A reduced model included only the variables which were significant in the full model in addition to the periodontitis variable. These variables were sex, smoking, lifetime sex partners, and race/ethnicity. A third parsimonious model was made in which the variable, race/ethnicity, was removed as it failed to reach significance in the reduced model. Interactions of the variables with periodontitis and presence of HPV in oral rinse specimens (HPV+ or HPV−) were also analyzed, but did not significantly change the relationship and were not included in the three models presented. SAS 9.3® (Cary, NC) software was used to analyze the data.
RESULTS
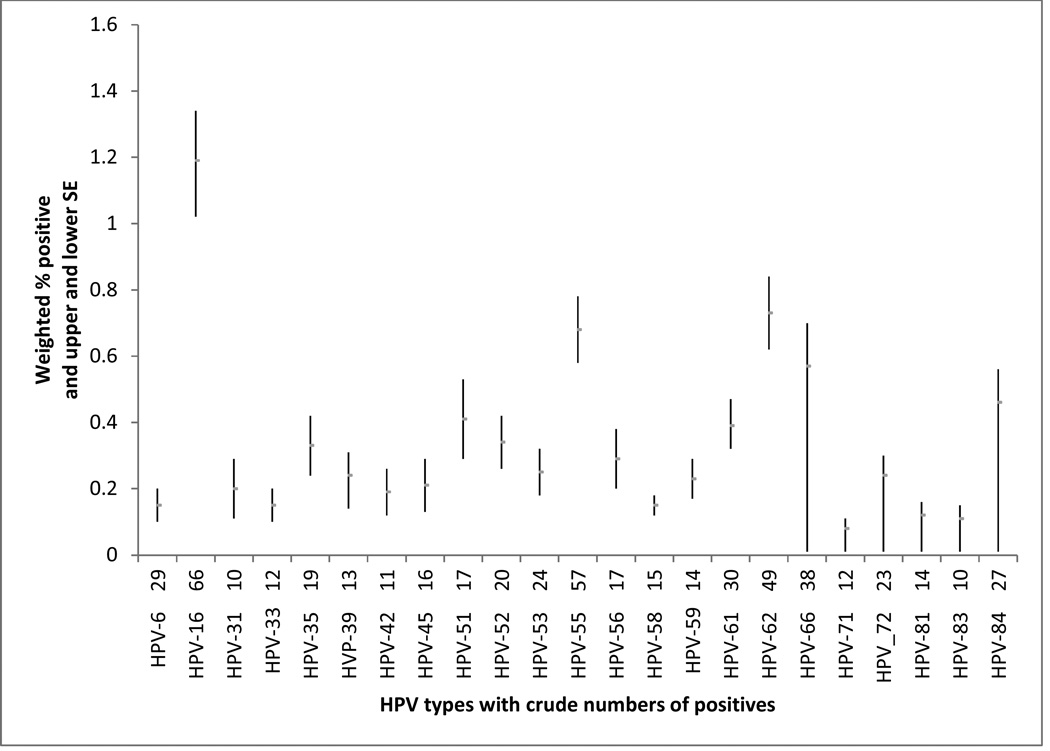
Of the 6,004 participants, 50.1% were male, and 66.8% were non-Hispanic white. The participants were evenly distributed in age. Most (63.7%) had some college or above; had an income to poverty ratio above 2.0 (69.2%); and were never-smokers (56.3%). There were 28.2% who reported moderate alcohol use and 43.7% who reported having 2–10 lifetime sex partners. There were 59.7% who did not have periodontitis and 92.5% who were did not have the presence of HPV in their oral rinse specimens (HPV−). Of the 498 participants who had the presence of HPV in their oral rinse specimens (HPV+), 66 were HPV-16 positive, 57 were HPV-55 positive, and 49 were HPV-62 positive. Details are presented in the figure.
Figure.
Prevalence of oral human papillomavirus (HPV) by HPV type. HPV types with fewer than 10 were suppressed.
The results of Chi Square analyses are present in Table 1. There were significant differences in the presence of HPV in oral rinse specimens (HPV+ or HPV−) with relation to periodontitis. Of the HPV+ participants, there were 41.3% who did not have periodontitis, and 58.7% who had periodontitis; whereas, 61.3% of HPV− participants did not have periodontitis, and 38.7% had periodontitis. These differences were statistically significant (p<0.0001).
Table 1.
Presence of HPV in oral rinse specimens (HPV+ or HPV−) versus periodontal disease and other variables of interest, NHANES 2009–12: Rao Scott Chi Square Analysis
| Variable | HPV+ N |
HPV+ wt N |
wt column % |
HPV− N |
HPV− wt N |
wt column % |
p-value |
|---|---|---|---|---|---|---|---|
| Periodontal Disease | <.0001* | ||||||
| No disease | 189 | 7,680,364 | 41.3 | 2,870 | 141,244,692 | 61.3 | |
| Yes disease | 309 | 10,919,274 | 58.7 | 2,636 | 89,292,955 | 38.7 | |
| Sex | <.0001* | ||||||
| Male | 384 | 14,682,623 | 78.9 | 2,612 | 109,515,598 | 47.5 | |
| Female | 114 | 3,917,016 | 21.1 | 2,894 | 121,022,048 | 52.5 | |
| Race/ethnicity | .0568 | ||||||
| Non-Hispanic white | 176 | 11,765,083 | 63.3 | 2,147 | 154,894,716 | 67.2 | |
| Non-Hispanic black | 150 | 2,911,913 | 15.7 | 1,201 | 25,268,865 | 11.0 | |
| Mexican American | 84 | 1,719,076 | 9.2 | 882 | 19,381,458 | 8.4 | |
| Other | 88 | 2,203,567 | 11.8 | 1,276 | 30,992,608 | 13.4 | |
| Age | .0677 | ||||||
| 30–34 years | 66 | 2,261,033 | 15.5 | 624 | 25.844,936 | 14.1 | |
| 35–39 years | 41 | 1,316,833 | 9.0 | 607 | 25,164,592 | 13.8 | |
| 40–44 years | 41 | 1,859,750 | 12.8 | 640 | 26,580,912 | 14.5 | |
| 45–49 years | 41 | 1,670,795 | 11.5 | 589 | 27,158,291 | 14.9 | |
| 50–54 years | 64 | 2,156,027 | 14.8 | 580 | 25,196,983 | 13.8 | |
| 55–60 years | 53 | 2,142,149 | 14.7 | 456 | 21,368,017 | 11.7 | |
| 60–64 years | 74 | 2,214,353 | 15.2 | 575 | 17,631,893 | 9.6 | |
| 65–69 years | 33 | 953,617 | 6.5 | 397 | 13,903,242 | 7.6 | |
| Education | .0008* | ||||||
| Less than HS | 131 | 3,186,241 | 17.1 | 1,339 | 36,039,431 | 15.6 | |
| HS graduate | 130 | 4,823,129 | 25.9 | 1,137 | 45,888,992 | 19.9 | |
| Some coll/tech | 157 | 6,482,892 | 34.9 | 1,512 | 66,699,681 | 29.0 | |
| Coll/tech graduate | 80 | 4,107,376 | 22.1 | 1,512 | 81,744,421 | 35.5 | |
| Family income to poverty ratio | .0016* | ||||||
| 0 to less than 1.25 | 167 | 4,280,819 | 24.1 | 1,438 | 36,141,939 | 16.8 | |
| 1.25 to less than 2.00 | 86 | 2,738,831 | 15.4 | 835 | 28,295,693 | 13.2 | |
| 2.00 to less than 4.00 | 103 | 4,717,321 | 26.6 | 1,231 | 58,958,012 | 27.4 | |
| 4.00 and above | 104 | 5,995,035 | 33.8 | 1.519 | 91,557,015 | 42.6 | |
| Alcohol consumption | <.0001* | ||||||
| Non-use | 70 | 1,772,617 | 12.0 | 1,277 | 41,425,319 | 21.4 | |
| Moderate use | 149 | 6,135,307 | 41.6 | 1,954 | 100,024,901 | 51.7 | |
| Heavy use | 170 | 6,826,175 | 46.3 | 1,245 | 51,862,437 | 26.8 | |
| Smoking status | <.0001* | ||||||
| Current | 186 | 6,652,863 | 35.8 | 1,079 | 40,640,365 | 17.6 | |
| Former | 120 | 4,950,246 | 26.7 | 1,234 | 56,584,439 | 24.6 | |
| Never | 192 | 6,996,529 | 37.6 | 3,190 | 133,250,544 | 57.8 | |
| Lifetime sex partners | <.0001* | ||||||
| 0 | ** | ** | 157 | 4,110,015 | 2.0 | ||
| 1 | 11 | 350,645 | 2.1 | 701 | 29,720,647 | 14.3 | |
| 2–5 | 80 | 2,347,233 | 13.9 | 1,582 | 69,712,801 | 33.5 | |
| 6–10 | 102 | 3,836,682 | 22.7 | 1017 | 46,783,958 | 22.5 | |
| 11–20 | 82 | 3,383,768 | 20.0 | 662 | 30,383,430 | 14.6 | |
| Above 20 | 156 | 6,859,791 | 40.6 | 690 | 34,451,329 | 13.2 | |
indicates significant differences in p-values between the HPV seropositive and HPV seronegative groups in relation to the specific variable.
Abbreviations: HPV+ HPV seropositive; HPV−: HPV seronegative; N-number; wt-weighted; HS, high school; and coll/tech, college technical school. Tech-technical school.
cell has too few to report
Wt column % indicates weighted percentage to account for complex sample design. Consequentially, dividing cell size, N, by total sample will not result in weighed percentage; however dividing wt N, by weighted sample will provide wt column%.
Other variables with significant relationships with the presence of HPV in oral rinse specimens (HPV+ or HPV−) were male sex, less education, lower income to poverty ratio increased alcohol consumption, smoking, and higher number of lifetime sex partners.
The results of the logistic regression of periodontitis on the presence of HPV in oral rinse specimens are presented in Table 2. In unadjusted logistic regression, the odds ratio for presence of HPV in oral rinse specimens with periodontitis was 2.25 (95% confidence interval: 1.61, 3.13, p<0.001) as compared with participants who did not have periodontitis.
Table 2.
Logistic regression on presence of HPV in oral rinse specimens, NHANES 2009–12
| Variable | Model 1 AOR (95%CI) |
Model 2 AOR (95%CI) |
Model 3 AOR (95%CI) |
|---|---|---|---|
| Periodontal disease | |||
| Yes | 1.38 (0.92, 2.08) | 1.33 (0.88, 2.00) | 1.04 (0.63, 1.73) |
| No | reference | reference | reference |
| Sex | |||
| Male | 3.13 (2.27, 4.31) | 3.15 (2.28, 4.35) | 3.35 (2.35, 4.77) |
| Female | reference | reference | reference |
| Smoking | |||
| Current | 1.90 (1.40, 2.60) | 1.92 (1.41, 2.61) | 2.08 (1.47, 2.95) |
| Former smoker | 1.10 (0.76, 1.59) | 1.14 (0.79, 1.66) | 1.26 (0.80, 1.98) |
| Never smoker | reference | reference | reference |
| Lifetime sex partners | |||
| 2–10 | 3.80 (1.68, 8.60) | 3.86 (1.75, 8.55) | 4.04 (1.86, 8.75) |
| 11 or more | 8.57 (4.04, 18.16) | 8.69 (4.21, 17.94) | 8.44 (4.37, 16.30) |
| 0–1 | reference | reference | reference |
| Race/ethnicity | |||
| Non-Hispanic Black | 1.36 (0.95, 1.95) | 1.66 (1.14, 2.43) | |
| Mexican American | 1.24 (0.85, 1.80) | 1.29 (0.87, 1.91) | |
| Other | 1.36 (0.88, 2.09) | 1.46 (0.96, 2.28) | |
| Non-Hispanic White | reference | reference | |
| Education | |||
| High School education or less | 1.03 (0.72, 1.47) | ||
| More than high school education | reference | ||
| Age | |||
| 30–44 years | 0.72 (0.47, 1.12) | ||
| 45 –69 years | reference | ||
| Family income to poverty ratio | |||
| Less than 2 | 1.04 (0.76, 1.44) | ||
| 2 and above | reference | ||
| Alcohol consumption | |||
| Moderate | 1.01(0.61, 1.67) | ||
| Heavy | 1.08 (0.62, 1.90) | ||
| No consumption | reference | ||
AOR= Adjusted odds ratio; CI=confidence interval.
Model 1 is a parsimonious model including periodontal disease, the variable of interest, and significant epidemiological variables; Model 2 additionally includes race/ethnicity; and Model 3 is the full model considered with epidemiological rationale.
When sex, race/ethnicity, education, age, income to poverty ratio, smoking, alcohol use and number of lifetime sex partners were included in a full model of the variables of epidemiological importance, the adjusted odds ratio for the presence of HPV in oral rinse specimens with periodontitis was 1.04 (95% confidence interval: 0.63, 1.73, p=0.8684). (Interaction analyses of periodontitis with sex, race/ethnicity, education, income to poverty ratio, smoking, alcohol use and number of lifetime sex partners were not significant in their relationship with the presence of HPV in oral rinse specimens and were not included in the full model.)
A reduced model, retaining the periodontitis variable and the significant variables from the full model (sex, race/ethnicity, smoking, and lifetime sex partners), had an adjusted odds ratio of 1.33 (95% confidence interval: 0.88, 2.00, p=0.1715) for periodontitis. A parsimonious model retaining the periodontitis variable and removing race/ethnicity (which failed to reach significance in the reduced model) had an adjusted odds ratio of 1.38 (95% confidence interval: 0.92, 2.08, p=0.1182).
DISCUSSION
In summary, the primary result of this study is that the odds ratio for the presence of HPV in oral rinse specimens (HPV+ or HPV−) with relation to periodontitis adjusted for sex, race/ethnicity, education, age, income to poverty ratio, smoking, alcohol use and number of lifetime sex partners was 1.04 (95% confidence interval: 0.63, 1.73, p=0.8684), indicating there was no independent association between the presence of HPV in oral rinse specimens (HPV+ or HPV−) and periodontitis. Though not the focus of the study, the sex of the participant, smoking status, and number of lifetime sex partners were independently associated with the presence of HPV in oral rinse specimens. The data used were NHANES 2009–12 data which were nationally representative. There were 498 participants who had the presence of HPV in oral rinse specimens of 6,004 participants; and of these participants, there were 66 who were HPV-16 positive.
These results are important and add to the body of subject knowledge in that the role of periodontitis in the presence of HPV in oral rinse specimens was not shown to be significant. Although periodontitis has been shown to be associated with many other chronic diseases, it was not shown to be associated with oral HPV in this cross-sectional study.
In addressing the context and related studies with the results of this study, there were similar results found by researchers in a small study of 56 participants with chronic periodontitis, 26 participants with gingivitis, and 22 controls who were healthy.16 The researchers did not find HPV-16 in the gingival samples.16
Similarly, Escalona et al. conducted a study using the gingival crevicular fluid of 20 HIV+ participants who had periodontitis, 7 HIV− participants who had periodontitis, and 7 controls.32 They found no significant difference in periodontal status in HPV-HIV infection with the patients with HIV; and HPV was found only in the crevicular fluid of HIV+ patients on antiretroviral therapy.32
In addition, in a study of 102 healthy participants with periodontal disease, no HPV DNA was found in the gingival biopsy specimens.33
However, positive associations of periodontitis and HPV were found in several other studies. Hormia, et al. studied 31 individuals with periodontitis.20 They used polymerase chain reaction on gingival biopsy specimens and found 26% were positive for high risk type HPV, indicating that the periodontal pocket was a possible reservoir of HPV.20 Other researchers, using the Southern blot method with 32P-radiolabeled DNA found HPV in 30% of gingival tissue with gingivitis or periodontitis, which also suggested gingival epithelium was a potential reservoir for HPV;34 and 92.3% of 13 biopsies of gingival overgrowth lesions in renal allograft recipients were HPV +.17
Tezal, et al. examined tongue cancer biopsy specimens for HPV+ or HPV− status from 30 participants and determined periodontal status by panoramic radiographic alveolar bone loss and found an association with HPV+ status and alveolar bone loss.35 Tezal, et al. also examined biopsy specimens of primary squamous cell carcinoma of the oral cavity, oropharynx, and larynx for HPV+ or HPV− status from 124 participants and determined periodontal status with the same panoramic radiographic alveolar bone loss technique.37 They also found an association of HPV+ status and alveolar bone loss.36 Although the positive associations were determined in the aforementioned small studies, Hang, et al. evaluated 5410 healthy adults in China and found a self-reported association of a history of oral disease and HPV infectivity;31 and, Bui, et al. found a self-reported association of: poor or fair oral health; possibility of gum disease; use of mouthwash to treat dental problems in the previous week; and higher number of teeth lost to be significantly associated with HPV+ status in the 2009–2010 National Health and Nutrition Examination Survey.29
The current research results and the studies which showed positive associations had differences in study variables and methodologies. Several of the studies in which positive associations were determined, except for the Hang, et al., and Bui, et al., studies, were very small studies in which there is a potential for type 2 (beta error) due to the small sample size. There were methodology differences in the definition of periodontitis. Researchers in two of the studies in which positive associations were found used radiographic bone loss to define periodontitis, whereas, in this study, full mouth periodontal probings were used as the basis to define periodontitis. Similarly, in the large studies of Hang, et al.31, and Bui, et al.29, periodontitis was either self-reported, or a self-reported proxy variable was used rather than full mouth probings to determine periodontitis. Gingival tissue samples were used in several of the studies, whereas oral rinse specimens were used in this study. These differences in study variables and methodologies explain some of the different results. This is the first study, to our knowledge, to use full mouth periodontal probings and the definition of periodontitis from the Centers for Disease Control and Prevention in partnership with the American Academy of Periodontology to identify periodontitis in HPV studies. Future research is needed using full mouth periodontal probings and the definition of periodontitis from the Centers for Disease Control and Prevention in partnership with the American Academy of Periodontology and large data sources to support the results of this study.
The study has several strengths. The researchers in this current study used clinical periodontal data from the National Health and Nutrition Examination Survey, 2009–2012, rather than self-report reported data which have the potential to be subject to social bias. The data for periodontitis included clinical evaluations of all of the teeth of the participants, rather than partial mouth evaluations, and were analyzed with an established definition. The HPV analyses were strictly conducted with polymerase chain reaction testing. The study is not hospital-based, or case-based. The sample size was large (N=6004), and nationally representative. It had a high quality data source, NHANES, and combined years to adequately develop a large sample.
Nevertheless, this study has limitations in that causal or temporal relationships cannot be determined by the study design. The variables considered were based on previous epidemiological studies and there may be other social determinants or biological factors which could confound or be effect modifiers in the relationship. Although the sample was large and used combined years of the NHANES, there were 498 participants who had the presence of HPV in oral rinse specimens and there is the potential of significant results with a larger sample.
CONCLUSION
There is an urgent need to learn more about HPV, its infectivity, persistence, remission, recurrence, and its role in malignancy.37 The presence of HPV in oral rinse specimens is a concern and requires future studies.
The researchers failed to reject the hypothesis of no association of the presence of HPV in oral rinse specimens and periodontitis. Although oral HPV infection is a serious concern, periodontitis was not shown to be related to the presence of HPV in oral rinse specimens in adjusted analyses in this study.
Acknowledgments
Drs. Wiener, Sambamoorthi, and Jurevic received research support under award U54GM104942 from the National Institute of General Medical Sciences, National Institutes of Health.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure. None of the authors reported any disclosures.
Contributor Information
R. Constance Wiener, Dental Practice and Rural Health, School of Dentistry, West Virginia University and Department of Epidemiology, School of Public Health, West Virginia University.
Usha Sambamoorthi, Department of Pharmaceutical Systems and Policy, School of Pharmacy, West Virginia University, Robert C. Byrd Health Sciences Center, Morgantown, WV.
Richard J. Jurevic, Department of Oral Medicine, School of Dentistry, West Virginia University, Morgantown, WV.
References
- 1.Zaravinos A. An updated overview of HPV-associated head and neck carcinomas. Oncotarget. 2014;5:3956–3969. doi: 10.18632/oncotarget.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krüger M, Pabst AM, Walter C, et al. The prevalence of human papilloma virus (HPV) infections in oral squamous cell carcinomas: A retrospective analysis of 88 patients and literature overview. J Craniomaxillofac Surg. 2014 May 10; doi: 10.1016/j.jcms.2014.04.022. pii: S1010-5182(14)00145-0. doi: 10.1016/j.jcms.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123–141. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sathish N, Wang X, Yuan Y. Human Papillomavirus (HPV)-associated Oral Cancers and Treatment Strategies. J Dent Res. 2014;93:29S–36S. doi: 10.1177/0022034514527969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen W, Sakamoto N, Yang L. Cancer-Specific Mortality and Competing Mortality in Patients with Head and Neck Squamous Cell Carcinoma: A Competing Risk Analysis. Annals of Surgical Oncology. 2014 doi: 10.1245/s10434-014-3951-8. [DOI] [PubMed] [Google Scholar]
- 6.Tezal M, Sullivan MA, Hyland A, et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:2406–2412. doi: 10.1158/1055-9965.EPI-09-0334. [DOI] [PubMed] [Google Scholar]
- 7.Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418–424. doi: 10.1016/s0300-9785(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 8.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of Oral HPV Infection in the United States, 2009–2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalianis T. Human papillomavirus and oropharyngeal cancer, the epidemics, and significance of additional clinical biomarkers for prediction of response to therapy (Review) Int J Oncol. 2014;44:1799–1805. doi: 10.3892/ijo.2014.2355. Epub 2014 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 11.George M. Should patients with HPV-positive or negative tumors be treated differently? Curr Oncol Rep. 2014;16:384. doi: 10.1007/s11912-014-0384-2. [DOI] [PubMed] [Google Scholar]
- 12.Deschler DG, Richmon JD, Khariwala SS, Ferris RL, Wang MB. The "New" Head and Neck Cancer Patient-Young, Nonsmoker, Nondrinker, and HPV Positive: Evaluation. Otolaryngol Head Neck Surg. 2014 Jun 12; doi: 10.1177/0194599814538605. pii: 0194599814538605. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Psychogios G, Alexiou C, Agaimy A, et al. Epidemiology and survival of HPV-related tonsillar carcinoma. Cancer Med. 2014;3:652–659. doi: 10.1002/cam4.212. Epub 2014 Mar 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380–386. doi: 10.1016/j.oraloncology.2013.12.019. Epub 2014 Jan 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han YW, Houcken W, Loos BG, Schenkein HA, Tezal M. Periodontitis, atherosclerosis, adverse pregnancy outcomes, and head-and-neck cancer. Adv Dent Res. 2014;26:47–55. doi: 10.1177/0022034514528334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horewicz VV, Feres M, Rapp GE, Yasuda V, Cury PR. Human papillomavirus-16 prevalence in gingival tissue and its association with periodontal destruction: a case-control study. J Periodontol. 2010;81:562–568. doi: 10.1902/jop.2009.090571. [DOI] [PubMed] [Google Scholar]
- 17.Bustos DA, Grenón MS, Benitez M, de Boccardo G, Pavan JV, Gendelman H. Human papillomavirus infection in cyclosporin-induced gingival overgrowth in renal allograft recipients. J Periodontol. 2001 Jun;72(6):741–744. doi: 10.1902/jop.2001.72.6.741. [DOI] [PubMed] [Google Scholar]
- 18.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 19.Habra-Rizk MA. Pathogenesis of Polymicrobial Biofilms. The Open Mycology Journal. 2011;5:39–43. [Google Scholar]
- 20.Hormia M1, Willberg J, Ruokonen H, Syrjänen S. Marginal periodontium as a potential reservoir of human papillomavirus in oral mucosa. J Periodontol. 2005 Mar;76(3):358–363. doi: 10.1902/jop.2005.76.3.358. [DOI] [PubMed] [Google Scholar]
- 21.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the Case Definitions for Population-Based Surveillance of Periodontitis. J Periodontol. 2012;83:1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Görögh T, Quabius ES, Meyer P, Hoffmann M. Characterisation of seven newly established head and neck squamous cell carcinoma cell lines. Eur Arch Otorhinolaryngol. 2014 May 4; doi: 10.1007/s00405-014-3073-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Markowitz LE, Dunne EF, Saraiya M, et al. Human Papillomavirus Vaccination: Recommendations of the Advisory Committee on Immunization Practices. MMWR. 2014;63:1–30. [PubMed] [Google Scholar]
- 24.Gillison ML, Castellsague X, Chaturvedi A, et al. Eurogin Roadmap: Comparative epidemiology of HPV infection and associated cancers of the head and neck and cervix. Int J Cancedr. 2014;134:497–507. doi: 10.1002/ijc.28201. [DOI] [PubMed] [Google Scholar]
- 25.Troeltzsch M, Knosel T, Eichinger C, et al. Clinicopathologic Features of Oral Squamous Cell Carcinoma: Do They Vary in Different Age Groups? J Oral Maxillofac Surg. 2014:1–10. doi: 10.1016/j.joms.2014.01.009. http:/dx.doi.org/10.1016/j.joms.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Thornton-Evans G, Eke P, Wei L, Palmer A, Moeti R, Hutchins S, Borrell LN. Periodontitis among adults aged ≥30 years - United States, 2009–2010. MMWR Surveill. 2013;62S:129–135. [PubMed] [Google Scholar]
- 27.Holtfreter B, Demmer RT, Bernhardt O, et al. A comparison of periodontal status in the two regional, population-based studies of SHIP and INVEST. J Clin Periodontol. 2012;39:1115–1124. doi: 10.1111/jcpe.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutton JD, Ranney LM, Wilder RS, Sanders AE. Environmental tobacco smoke and periodontitis in U.S. non-smokers. J Dent Hyg. 2012;86:185–194. [PubMed] [Google Scholar]
- 29.Bui TC, Markham CM, Ross MW, Mullen PD. Examining the Association between Oral Health and Oral HPV infection. American Association for Cancer Research. 2013 doi: 10.1158/1940-6207.CAPR-13-0081. [DOI] [PubMed] [Google Scholar]
- 30.Broadbent JM, Thomson WM, Boyens JV, Poulton R. Dental plaque and oral health during the first 32 years of life. JADA. 2011;142:415–426. doi: 10.14219/jada.archive.2011.0197. [DOI] [PubMed] [Google Scholar]
- 31.Hang D, Liu F, Liu M, et al. Oral Human Papillomavirus Infection and Its Risk Factors among 5,410 Healthy Adults in China, 2009–2011. Cancer Epidemiol Biomarkers Pre. 2014;23:2101. doi: 10.1158/1055-9965.EPI-14-0084. [DOI] [PubMed] [Google Scholar]
- 32.Escalona L, Correnti M, Weitia D, Perrone M. Detection of human papillomavirus in gingival fluid of Venzeuelan HIV patients with periodontal disease. Invest Clin Abstract. 2011;52:207–215. [PubMed] [Google Scholar]
- 33.Jacob A, Janam P, Vijayamma JMB. Prevalence of human papilloma virus in marginal periodontium and its association with periodontitis: A cross sectional study. J Indian Soc Periodontol. 2014;18:447–450. doi: 10.4103/0972-124X.138682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madinier I, Doglio A, Cagnon L, Lefèbvre JC, Monteil RA. Southern blot detection of human papillomaviruses (HPVs) DNA sequences in gingival tissues. J Periodontol. 1992 Aug;63(8):667–673. doi: 10.1902/jop.1992.63.8.667. [DOI] [PubMed] [Google Scholar]
- 35.Tezal M, Nasca MS, Stoler DL, et al. Chronic Periodontitis-Human Papillomavirus Synergy in Base of Tongue Cancers. Arch Otolaryngol Head Neck Surg. 2009;135:391–396. doi: 10.1001/archoto.2009.6. [DOI] [PubMed] [Google Scholar]
- 36.Tezal M, Scannapieco FA, Wactawski-Wende J, et al. Local Inflammation and Human Papillomavirus Status of Head and Neck Cancers. Arch Otolaryngol Head Neck Surg. 2013;138:669–675. doi: 10.1001/archoto.2012.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fried JL. Confronting human papilloma virus/oropharyngeal cancer: a model for interprofessional collaboration. J Evid Based Dent Pract. 2014 Jun 14;(Suppl):136.e1–146.e1. doi: 10.1016/j.jebdp.2014.03.005. [DOI] [PubMed] [Google Scholar]