Abstract
Background:
Intracranial vascular complications following radiosurgery are extremely rare.
Case Description:
We report a case of stenosis in the internal carotid artery 5 years after gamma knife radiosurgery for a recurrent pituitary adenoma. Percutaneous transluminal angioplasty was performed successfully with anatomical and functional improvement.
Conclusion:
These results suggested the importance of monitoring for arterial stenosis in the long-term follow-up. Moreover, this is the first case of endovascular treatment as an effective therapy for intracranial arterial stenosis due to radiotherapy.
Keywords: Angioplasty, endovascular treatment, gamma knife radiosurgery, internal carotid artery stenosis, pituitary adenoma
INTRODUCTION
Gamma knife radiosurgery (GKS) has been considered as an effective and safe management strategy for recurrent pituitary adenoma.[25] A few side effects associated with this therapy have been described, such as pituitary insufficiency, visual dysfunction, dysfunction of the cranial nerves in the cavernous sinus, and vascular complication occasionally.[6] We report a case of symptomatic severe stenosis of the intracavernous segment of the internal carotid artery (ICA) 5 years after GKS. Percutaneous transluminal angioplasty (PTA) was performed successfully with anatomical and clinical improvement.
CASE REPORT
A 54-year-old male patient was admitted following a 3-month history of visual disturbance. He had no history of heart disease or metabolic disorder that could predispose to cerebrovascular complications. Neurological examination revealed a right homonymous hemianopia and decreased visual acuity. Magnetic resonance imaging (MRI) demonstrated a 5.0 × 5.3 cm pituitary tumor projecting toward the suprasellar region with encasement of both ICAs [Figure 1a]. The endocrinological findings were within the normal range. A transsphenoidal surgery with subtotal removal was achieved. The histological diagnosis was pituitary adenoma. Though the visual disturbance was temporarily improved after the operation, the residual tumor enlarged again in 1 year. The second transsphenoidal surgery was performed 3 years after the first operation. Preoperative magnetic resonance angiography (MRA) indicated that the cavernous segments of both ICAs were normal [Figure 1b]. Six months after the second surgery, the patient was treated with GKS for the residual tumor. The treatment was performed with a Leksell Gamma Knife model B (Elekta Instruments AB, Stockholm, Sweden). The tumor volume was 10.4 cm3. Dose planning was performed using MRI and computed tomography image fusion. The marginal dose to the tumor margin was 15 Gy at the 50% isodose curve [Figure 2a]. The irradiated dose of the cavernous segment of the right ICA was 20-22 Gy, retrospectively [Figure 2b].
Figure 1.
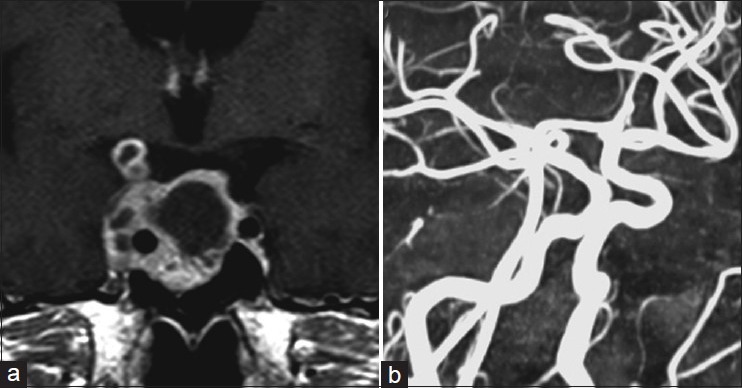
(a) Initial gadolinium-enhanced MRI showing irregular-shaped pituitary macroadenoma with multiple cyst formations invading the cavernous sinus, completely encasing the right ICA. (b) Preoperative MRA indicating no abnormal findings (right oblique projection)
Figure 2.
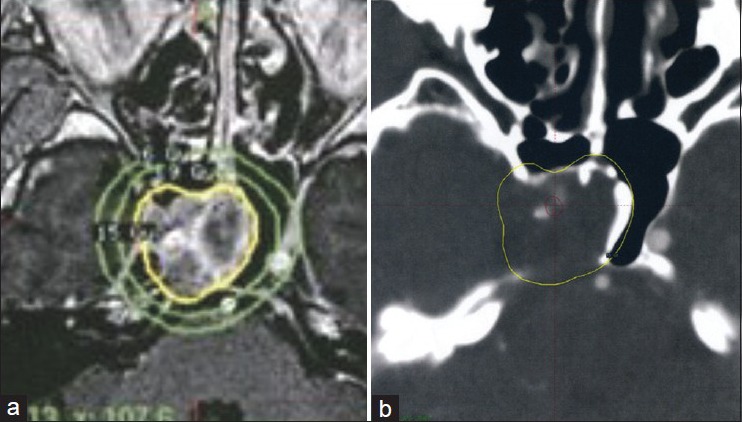
(a) Dosimetry of the GKS procedure. The marginal dose to the tumor margin was 15 Gy at the 50% isodose curve. (b) Isodose lines on the dosimetry planning showing the dose received by the intracavernous segment of the ICA, retrospectively (red line = 20-22 Gy isodose line, yellow line = 15 Gy isodose line)
Five years after the GKS, the MRA demonstrated severe stenosis of the cavernous segment of the right ICA with a remarkable reduction of the residual pituitary adenoma [Figure 3]. The following day, however, the patient had amaurosis. Diagnostic angiography revealed 95% stenosis of the cavernous ICA. With the progression of stenosis and being symptomatic, despite antiplatelet therapy, it was decided to proceed with ICA PTA. Initially 5000 U of heparin was administrated intravenously, activated clotting time was maintained at more than two times the control. A 7-Fr balloon guiding catheter (Optimo; Tokai Medical Products, Aichi, Japan) was emplaced selectively into the midcervical ICA. Next, a 0.014-inch regular microguidewire was navigated through the stenotic lesion into the supraclinoid segment of the ICA. Because the diameter of the normal part of cavernous segment of ICA was smaller (4.2-mm), a 2.5 × 9.0-mm Gateway Balloon Catheter (Boston Scientific Corporation, Natick, MA, USA) was advanced over the wire into the stenotic cavernous segment of the ICA. Careful low-pressure dilatations to a 2.5-mm diameter were performed under proximal flow control with inflation of the balloon guide catheter. Posttreatment angiography showed improvement in vessel diameter [Figure 4]. After the procedure, patient was managed through antiplatelet therapy for 3 months. Angiography 1 year after the PTA indicated slight restenosis of target lesion [Figure 5]; the patient remained asymptomatic.
Figure 3.
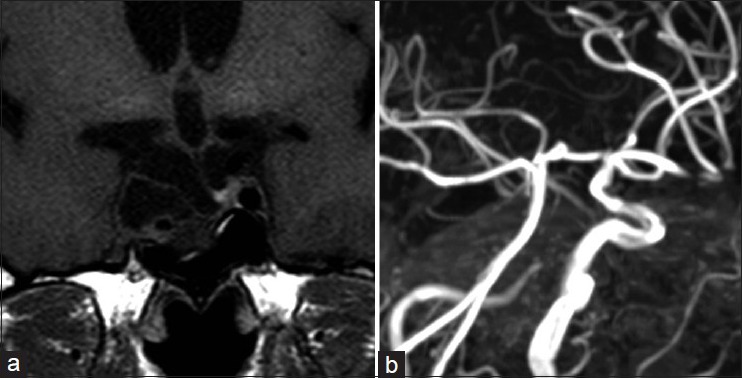
Five years postradiosurgical MRI and MRA. (a) Nonenhanced MRI demonstrating a remarkable reduction of the tumor volume. (b) MRA indicating severe stenosis and disappearance at the distal portion of the intracavernous segment of the right ICA (right oblique projection)
Figure 4.
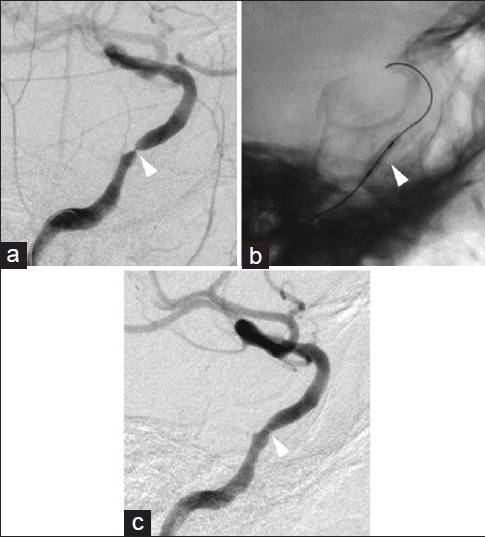
Pretreatment and posttreatment right ICA angiograms. The pretreatment angiogram indicating severe stenosis in the cavernous segment of the ICA (a, arrowhead). A microguidewire was advanced through the stenotic lesion and into the supraclinoid segment of the ICA. Inflation of a contrast-filled gateway PTA balloon (b, arrowhead) within the stenotic cavernous segment of the ICA dilated the vessel lumen, improving flow (c, arrowhead)
Figure 5.
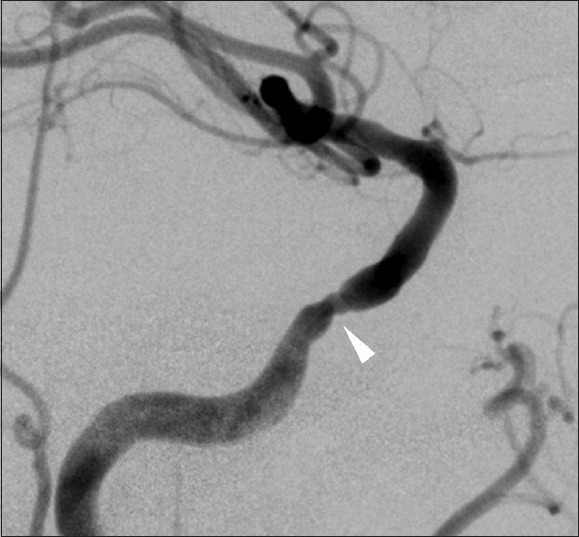
Angiogram of the right common carotid artery showing slight restenosis of cavernous segment of the ICA (arrowhead) one year after the treatment
DISCUSSION
Several therapeutic options are recommended for the management of pituitary adenomas, for example, medical treatment, surgery, or radiotherapy.[3,20] Especially, in most cases of residual tumor or recurrence, radiotherapy has been used effectively.[11,30] However, postoperative conventional radiotherapy has a high complication rate, including panhypopituitarism, visual disturbances, and cerebral stroke.[12,14] Recently, GKS, among other types of radiosurgery, has gained wide acceptance as an effective and safe adjuvant postoperative treatment modality.[18,25] Although few complications relative to conventional radiation therapy in the treatment of pituitary adenoma have been reported, it may induce modifications in the vessel walls after irradiation.[17,22]
Accelerated atherosclerosis in the extracranial ICA is a well-recognized complication of irradiation for head and neck diseases.[9] The rate of severe extracranial ICA has ranged from 6.3% to 16.0% in different series.[7,10] And cerebrovascular accidents with intracranial artery occlusion after conventional radiation therapy have been reported in about 4.7-7.2%.[12,14] In reviewing a series of patients receiving radiation therapy for pituitary adenoma, Hashimoto et al.[14] reported 10 ischemic strokes in the long-term follow-up of 139 patients who received doses that varied between 50 and 60 Gy. Flickinger et al.[12] reported 7 patients who suffered strokes due to intracranial arterial occlusion in 156 patients, and the occurrence of these strokes was delayed 3.2-14.6 years after irradiation. These authors reported an inability to find a clear correlation between ischemic strokes and radiation doses, fractions, and durations.
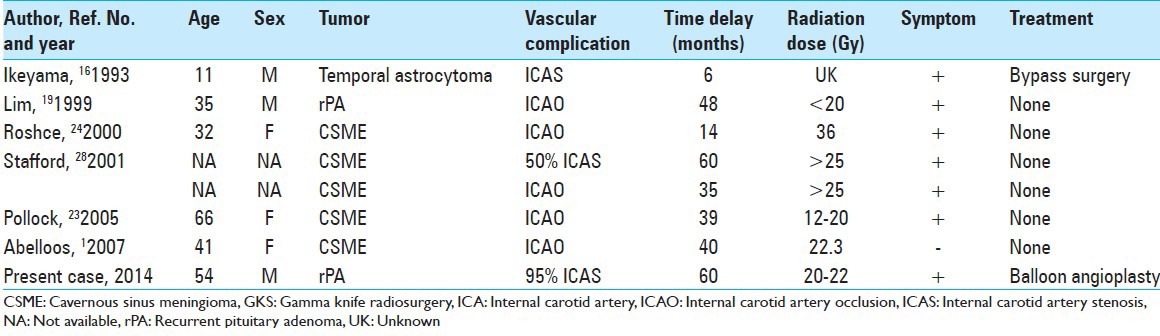
Intracranial vascular complications following radiosurgery seem to be extremely rare. The first case report of ICA stenosis after GKS was published by Ikeyama et al.[16] They described multifocal stenosis in bilateral ICA, MCA, and ACA 6 months after GKS for an astrocytoma located at the left temporal lobe. Barami et al.[4] summarized the vascular complications that occur after radiosurgery for meningiomas. Four cases with occlusive complications after GKS for cavernous sinus meningiomas were reported. Those reports indicated that the incidence of ICA occlusion or stenosis was 1.0-2.0% in a delayed fashion, usually 14-60 months after radiosurgery, and that the margin dose was 13-18 Gy, and the calculated radiation dose to the affected arteries was 25-36 Gy. Abeloos et al.[1] also reported a case of ICA occlusion at 40 months after GKS for cavernous sinus meningioma. The prescribed margin dose was 13 Gy at the 50% isodose, and the estimated dose delivered to the cavernous ICA was 22.3 Gy. Only Lim et al.[19] reported one patient, treated with GKS for a residual pituitary adenoma, who developed a cerebral infarction with cavernous ICA occlusion 4 years after GKS. The maximum dose was 40 Gy, and the dose to the right ICA was below 20 Gy. Cases with steno-occlusive complications to the ICA after GKS are summarized in the Table 1. Almost all patients had ischemic symptoms or suffered a stroke; therefore, there might also have been asymptomatic cases with a stenotic arterial lesion.
Table 1.
Literature review of steno-occlusive complications to the ICA after GKS
Extracranial carotid artery angioplasty and stenting have clear advantages in treating patients with radiation-induced extracranial carotid artery stenosis.[2,29] Even though intracranial PTA or stenting have been effective treatment options and more commonly performed in the setting of intracranial atherosclerotic disease, the efficacy and safety for radiation-induced arterial stenosis remains unknown.[5,8]
The resteonsis rate of a stenting for symptomatic intracranial stenosis has been reported to be from 7.5% to 30%,[13,21,27] and that of a PTA was from 20.0% to 50.0%.[15,21,26] Though stenting may lower the rate of postoperative restenosis compared with PTA, Siddiq et al.[26] reported that no benefits of stenting over PTA in reducing stroke or death could be identified from their multicenter study. In the present case, only PTA was performed because the Japanese national insurance did not approve the use of intracranial stent devices for the first treatment. To avoid ischemic stroke, as part of follow-up, imaging should be closely performed to find restenosis. To our knowledge, this is the first reported case of PTA as an effective therapy for intracranial ICA stenosis due to radiotherapy. The present case suggests that arterial occlusion or stenosis could occur following GKS as delayed complications; therefore, the irradiation dose to the artery should be reduced as much as possible. Patients who undergo GKS for lesions involving major vessels should be monitored for arterial stenosis in the long-term follow-up even if the original disease is under control, and endovascular intervention including PTA and intracranial stenting may be of significant benefit to avoid ischemic stroke for these asymptomatic patients.
CONCLUSION
We reported a rare case of ICA stenosis 5 years after GKS for a recurrent pituitary adenoma. The irradiated vessels could be affected with stenosis in a delayed fashion; therefore, surveillance imaging should be performed even if the patient is asymptomaic up until that time. Endovascular angioplasty may most likely be the treatment of choice for steno-occlusive arteries following GKS.
ACKNOWLEDGMENTS
The authors thank Robert E. Brandt, Founder, CEO, and CME, of MedEd Japan, for editing the manuscript.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2015/6/8/279/157795
Contributor Information
Hidemichi Ito, Email: hdmcito@yahoo.co.jp.
Hidetaka Onodera, Email: dera@marianna-u.ac.jp.
Taigen Sase, Email: sasetaigen@marianna-u.ac.jp.
Masashi Uchida, Email: m2uchida@marianna-u.ac.jp.
Hiroyuki Morishima, Email: h2mori@marianna-u.ac.jp.
Kotaro Oshio, Email: koshio@marianna-u.ac.jp.
Takashi Shuto, Email: shuto@yokohamah.rofuku.go.jp.
Yuichiro Tanaka, Email: tanaka@marianna-u.ac.jp.
REFERENCES
- 1.Abeloos L, Levivier M, Devriendt D, Massager N. Internal carotid occlusion following gamma knife radiosurgery for cavernous sinus meningioma. Stereotact Funct Neurosurg. 2007;85:303–6. doi: 10.1159/000107365. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ali F, Cree T, Hall S, Louis S, Major K, Smoker S, et al. Predictors of unfavorable outcome in intracranial angioplasty and stenting in a single-center comparison: Results from the Borgess Medical Center-Intracranial Revascularization Registry. AJNR Am J Neuroradiol. 2011;32:1221–6. doi: 10.3174/ajnr.A2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews DW. Pituitary adenomas. Curr Opin Oncol. 1994;6:53–9. doi: 10.1097/00001622-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Barami K, Grow A, Brem S, Dagnew E, Sloan AE. Vascular complications after radiosurgery for menigiomas. Neurosurg Focus. 2007;22:E9. doi: 10.3171/foc.2007.22.3.10. [DOI] [PubMed] [Google Scholar]
- 5.Bose A, Hartmann M, Henkes H, Liu HM, Teng MM, Szikora I, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenosis: The Wingspan study. Stroke. 2007;38:1531–7. doi: 10.1161/STROKEAHA.106.477711. [DOI] [PubMed] [Google Scholar]
- 6.Bowen J, Paulsen CA. Stroke after pituitary irradiation. Stroke. 1992;23:908–11. doi: 10.1161/01.str.23.6.908. [DOI] [PubMed] [Google Scholar]
- 7.Cheng SW, Ting AC, Lam LK, Wei WI. Carotid stenosis after radiotherapy for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126:517–21. doi: 10.1001/archotol.126.4.517. [DOI] [PubMed] [Google Scholar]
- 8.Connors JJ, 3rd, Wojak JC. Percutaneous transluminal angioplasty for intracranial atherosclerotic lesions: Evolution of technique and short-term results. J Neurosurg. 1999;91:415–23. doi: 10.3171/jns.1999.91.3.0415. [DOI] [PubMed] [Google Scholar]
- 9.Conomy JP, Kellermeyer RW. Delayed cerebrovascular consequences of therapeutic radiation. A clinicopathologic study of a stroke associated with radiation-related carotid arteriopathy. Cancer. 1975;36:1702–8. doi: 10.1002/1097-0142(197511)36:5<1702::aid-cncr2820360525>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Elerding SC, Fernandez RN, Grotta JC, Lindberg RD, Causay LC, McMurtrey MJ. Carotid artery disease following external cervical irradiation. Ann Surg. 1981;194:609–15. doi: 10.1097/00000658-198111000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flickinger JC, Nelson PB, Martinez AJ, Deutsch M, Taylor F. Radiotherapy of nonfunctional adenomas of the pituitary gland. Results with long-term follow-up. Cancer. 1989;63:2409–14. doi: 10.1002/1097-0142(19890615)63:12<2409::aid-cncr2820631206>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Flickinger JC, Nelson PB, Taylor FH, Robinson A. Incidence of cerebral infarction after radiotherapy for pituitary adenoma. Cancer. 1989;63:2404–8. doi: 10.1002/1097-0142(19890615)63:12<2404::aid-cncr2820631205>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Groschel K, Schnaudigel S, Pilgram SM, Wasser K, Kastrup A. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke. 2009 doi: 10.1161/STROKEAHA.108.532713. 40-e340-7. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto N, Handa H, Yamashita J, Yamagami T. Long-term follow-up of large or invasive pituitary adenomas. Surg Neurol. 1986;25:49–54. doi: 10.1016/0090-3019(86)90114-x. [DOI] [PubMed] [Google Scholar]
- 15.Hyodo A, Matsumaru Y, Anno I, Sato H, Kato N, Nakai Y, et al. Percutaneous transluminal angioplasty for atherosclerotic stenosis of the intracranial cerebral arteries. Special reference to the device for reducing the complications drawn from the analysis of our complicated cases. Interv Neuroradiol. 1998;4(Suppl 1):S57–62. doi: 10.1177/15910199980040S110. [DOI] [PubMed] [Google Scholar]
- 16.Ikeyama Y, Abiko S, Kurokawa Y, Okamura T, Watanabe K, Inoue S, et al. Radiation-induced cerebrovasculopathy: A case report and review of the literature (in Japanese) No Shinkei Geka. 1993;21:751–7. [PubMed] [Google Scholar]
- 17.Jahan R, Solberg TD, Lee D, Medin P, Tateshima S, Sayre J, et al. Stereotactic radiosurgery of the rete mirabile in swine: A longitudinal study of histopathological changes. Neurosurgery. 2006;58:551–8. doi: 10.1227/01.NEU.0000197335.93538.BD. [DOI] [PubMed] [Google Scholar]
- 18.Kong DS, Lee JI, Lim do H, Kim KW, Shin HJ, Nam DH, et al. The efficacy of fractionated radiotherapy and stereotactic radiosurgery for pituitary adenomas: Long-term results of 125 consecutive patients treated in a single institution. Cancer. 2007;110:854–60. doi: 10.1002/cncr.22860. [DOI] [PubMed] [Google Scholar]
- 19.Lim YJ, Leem W, Park JT, Kim TS, Rhee BA, Kim GK. Cerebral infarction with ICA occlusion after Gamma Knife radiosurgery for pituitary adenoma: A case report. Stereotact Funct Neurosurg. 1999;72(Suppl 1):S132–9. doi: 10.1159/000056449. [DOI] [PubMed] [Google Scholar]
- 20.Marks LB. Conventional fractionated radiation therapy vs. radiosurgery for selected benign intracranial lesions (arteriovenous malformations, pituitary adenomas, and acoustic neuromas) J Neurooncol. 1993;17:223–30. doi: 10.1007/BF01049978. [DOI] [PubMed] [Google Scholar]
- 21.Mazighi M, Yadav JS, Abou-Chebl A. Durability of endovascular therapy for symptomatic intracranial atherosclerosis. Stroke. 2008;39:1766–9. doi: 10.1161/STROKEAHA.107.500587. [DOI] [PubMed] [Google Scholar]
- 22.Murros KE, Toole JF. The effect of radiation on carotid arteries. A review article. Arch Neurol. 1989;46:449–55. doi: 10.1001/archneur.1989.00520400109029. [DOI] [PubMed] [Google Scholar]
- 23.Pollock BE, Stafford SL. Results of stereotactic radiosurgery for patients with imaging defined cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2005;62:1427–31. doi: 10.1016/j.ijrobp.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 24.Roche PH, Regis J, Dufour H, Fournier HD, Delsanti C, Pellet W, et al. Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurusurg. 2000;93(Suppl 3):S68–73. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 25.Sheehan JP, Starke RM, Mathieu D, Young B, Sneed PK, Chiang VL, et al. Gamma Knife radiosurgery for the management of nonfunctioning pituitary adenomas: A multicenter study. J Neurosurg. 2013;119:446–56. doi: 10.3171/2013.3.JNS12766. [DOI] [PubMed] [Google Scholar]
- 26.Siddiq F, Vazquez G, Memon MZ, Suri MF, Taylor RA, Wojak JC, et al. Comparison of primary angioplasty with stent placement for treating symptomatic intracranial atherosclerotic diseases: A multicenter study. Stroke. 2008;39:2505–10. doi: 10.1161/STROKEAHA.108.515361. [DOI] [PubMed] [Google Scholar]
- 27.SSYLVIA Study Investigators. Stenting of symptomatic atherosclerotic lesions in the vertebral or intracranial arteries (SSYLVIA): Study results. Stroke. 2004;35:1388–92. doi: 10.1161/01.STR.0000128708.86762.d6. [DOI] [PubMed] [Google Scholar]
- 28.Stafford SL, Pollock BE, Foote RL, Link MJ, Gorman DA, Schomberg PJ, et al. Meningioma radiosurgery: Tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49:1029–37. doi: 10.1097/00006123-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Tallarita T, Oderich GS, Lanzino G, Cloft H, Kallmes D, Bower TC, et al. Outcomes of carotid artery stenting versus historical surgical controls for radiation-induced carotid stenosis. J Vasc Surg. 2011;53:629–36. doi: 10.1016/j.jvs.2010.09.056. [DOI] [PubMed] [Google Scholar]
- 30.Tran LM, Blount L, Horton D, Sadeghi A, Parker RG. Radiation therapy of pituitary tumors: Results in 95 cases. Am J Clin Oncol. 1991;14:25–9. doi: 10.1097/00000421-199102000-00005. [DOI] [PubMed] [Google Scholar]


