Abstract
Aims:
This study examined the setting time, compressive strength, and pH of white mineral trioxide aggregate (MTA) mixed with various additives: Calcium chloride (CaCl2), calcium formate (CaF), disodium hydrogen orthophosphate (Na2HPO4).
Materials and Methods:
Group 1 (Control) was obtained by mixing MTA with distilled water. In Groups 2 and 3, MTA containing 10% CaCl2 and 20% CaF, respectively, was mixed with distilled water. In Group 4, MTA was mixed with 15% Na2HPO4. Setting time, compressive strength, and pH of each group were examined.
Statistical Analysis Used:
Analysis was done using Statistical Package for Social Sciences (SPSS) version 14. A P-value < 0.05 was considered statistically significant. Comparison of mean values was done using analysis of variance (ANOVA) with post-hoc Games-Howell test.
Results:
The setting time of test groups were significantly shorter than that of control group (P < 0.001). The compressive strengths of test groups were lower than that of control group (P < 0.001). The pH value obtained for Groups 3 and 4 were higher than that of the control group (P < 0.001).
Conclusions:
Study result showed that additives significantly reduced the setting time of MTA and also maintained the pH at a high value. However, there was not much improvement in the compressive strength of the material.
Keywords: Additives, calcium chloride, calcium formate, compressive strength, disodium hydrogen orthphosphate, MTA, pH, physical properties, setting time
INTRODUCTION
The quest for newer materials is never ending in the field of restorative dentistry and endodontics. Efforts to develop a new material with “ideal” characteristics lead to the introduction of mineral trioxide aggregate (MTA) by Torabinejad et al., in 1993.[1] MTA was recommended initially as a root-end filling material; however, its use has been subsequently expanded to pulp capping, pulpotomy, apexogenesis, apical barrier formation in teeth with open apexes, repair of root perforations, and as root canal filling material.[2,3]
MTA is composed of fine hydrophilic particles; its main components are tricalcium silicate, tricalcium aluminate, tricalcium oxide, silicate oxide, and other mineral oxides.[4] The original formulation, grey MTA, was introduced first; however, white MTA was developed later as this version improved esthetics.[5,6] MTA powder sets in presence of water and results in formation of a colloidal gel, which solidifies to a hard structure in approximately 3-4h.[5,6,7]
MTA is a biocompatible material, promotes cementum formation and apical root closure, it is an effective material in preventing leakage, as perforation repair and root-end filling material.[8,9] Hydrophilic nature of MTA enables it to set in the presence of moisture.[10] The pH of MTA is approximately 12.5 and is similar to that of calcium hydroxide, a material with proven antibacterial activity.[11]
Despite its favorable properties, MTA presents some shortcomings like long setting time.[12] A shorter setting time would be beneficial because it would allow less time for contaminants in the oral environment to adversely affect the material, allow safer placement of restorative material over it (pulp capping), and also shorten the period when washout of cement can occur.[13] It is thus of interest to attempt to use chemicals to accelerate the setting time of MTA. Also MTA has low compressive strength compared with most other materials, a reason for its limited application in restorative dentistry as this parameter is of major significance for indication of MTA as coronal restorative material.[14,15]
The extended endodontic use and its potential of use in restorative dentistry necessitates the development of new formulation of the material, which optimizes both its strength and its setting time without compromising its pH and biocompatibility.[14]
MTA and construction grade Portland cement (PC) are very similar in chemical and physical characters.[11] Since setting time of PC can be accelerated by addition of certain accelerating admixtures; addition of these construction grade PC setting accelerators to MTA may induce a faster set. Some of the commonly used cement accelerators are calcium chloride (CaCl2), calcium formate (CaF), disodium hydrogen orthophosphate (Na2HPO4), and potassium chloride.[8] However, it is important that these accelerants should not adversely affect the other properties of MTA.[11]
Therefore, this study was designed to evaluate the effect of 10% CaCl2, 20% CaF, and 15% Na2HPO4 on the setting time, pH, and compressive strength of white MTA.
MATERIALS AND METHODS
Samples were prepared by mixing 0.8g of white MTA (Pro Root MTA; Dentsply Tulsa Dental, OK) powder with various additives. Each mix was prepared using a stainless steel cement spatula on a glass slab and was then immediately transferred into the molds. Before mixing, the test materials, molds, pluggers, spatulas, and glass slabs were kept at room temperature for a period of 24 h.
The samples were divided into four groups:
Group 1 (Control): 0.8 g of MTA mixed with 0.3ml distilled water (n = 10).
Group 2: 0.8 g MTA with 0.08 g of CaCl2 (Lab chem., Kanpur, India) mixed with 0.3 ml distilled water (n = 10).
Group 3: 0.8 g MTA with 0.16 g of CaF (Otto, Mumbai, India) mixed with 0.3 ml of distilled water (n = 10).
Group 4: 0.8 g MTA mixed with 0.3 ml of 15% Na2HPO4 (Fisher Scientific, Mumbai, India; n = 10).
Sample preparation
The setting time was determined according to the ISO 9917-1 method.[16] Samples were prepared by mixing cement and placing them in circular acrylic mold (10.0 mm inner diameter and 5.0 mm height). The assembly was placed in a cabinet at 37°C and relative humidity of 95%.
For pH, 10specimens were prepared for each group by filling hydrated samples into a cylindrical Teflon mold (6.0 mm in diameter and 12.0 mm high). Specimens were individually immersed in holders containing 60 ml of deionized distilled water, sealed, and stored for 24 hours at 37°C.[17]
The compressive strengths were determined by using the ISO 9917-1 method.[16] Each material was mixed and placed in a split stainless steel mold (4.0 mm inner diameter and 6.0 mm height). Cement was then compacted into each mold using a spatula and further compacted using a dental plugger to ensure dense uniform sample with minimal porosity. Once filled, the excess was scraped off with the edge of a glass microscopic slide to leave a flat uniform surface. No later than 120 s after mixing, the complete assembly was transferred to a cabinet maintained at 37°C for 6 h, after which they were removed from the molds and checked visually for any air voids or chipped edges. All defective specimens were discarded, and 10 accepted samples were prepared for each time interval. The specimens were immersed in distilled water for 24 h, 3 days, and 7 days and maintained at 37°C.
Setting time
Setting time was determined using a Vicat apparatus (Aimil, New Delhi, India). The Vicat indenter is 400 ± 5g in weight containing a needle with a flat end and 1.0 ± 0.1 mm in diameter. Ninety seconds after mixing, the indenter needle was lowered vertically onto the surface of the test material. The process was repeated every 10 s until the needle failed to make a complete circular indentation in the test material. Setting time was defined as the time elapsed between the end of mixing and the time when the needle failed to make a complete circular indentation on the test material.
pH
After 24 h, the immersed solution was mixed by vortexing for 30 s and pH was measured using a pHmeter (Si, Mainz, Germany), which was calibrated using standard solutions of pH 4.0, 7.0, and 12.0.
Compressive strength
Compressive strength was measured by using a universal testing machine (Instron 1195, Norwood, MA, USA) at a crosshead speed of 1.0 mm/min. The maximum load needed to fracture each specimen was measured, and the compressive strength (C) was calculated in megapascals (MPa) according to formula:
C=4P/πD2
Where P is the maximum load applied in Newton and D is the mean diameter of the specimen in millimeters.
Statistical analysis
Analyses were done using Statistical Package for Social Sciences (SPSS) version 14. A P-value < 0.05 was considered statistically significant. Comparison of mean values was done using analysis of variance (ANOVA) with post-hoc Games-Howell test.
RESULTS
The setting time of MTA mixed with the additives was significantly shorter than that of MTA mixed with water (P < 0.001) [Table 1]. Setting time observed was shortest for Group 3.
Table 1.
Setting time of the test materials in minutes (n = 10 for each group)
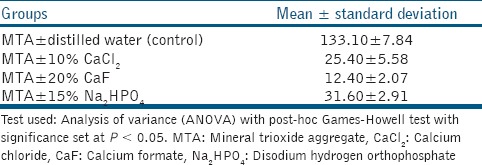
The pH of MTA mixed with CaCl2 was lower than that of the control group (P < 0.001); however, when CaF and Na2HPO4 was added to MTA, pH value obtained was higher than that of the control group (P < 0.001) [Table 2]. pH observed was highest for control group.
Table 2.
pH of test materials (n = 10 for each group)

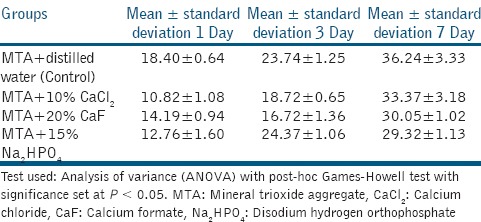
The compressive strength of all the groups increased during a 7-day period. The compressive strength of MTA mixed with the additives was lower than that of MTA mixed with water (P < 0.001) [Table 3]. For both day 1 and day 7, compressive strength of Group 1 was highest. However, on day 3 the compressive strength of Group 4 was found higher than other groups.
Table 3.
Compressive strengths of test materials in MPa (n = 10 for each group at each time interval)
DISCUSSION
It was the intent of this research to incorporate additives used by the cement industry to MTA to try to decrease its setting time and improve its compressive strength, without affecting its pH. MTA mixed with distilled water was used as control in this study. The various additives used included 10% CaCl2, 20% CaF, and 15% Na2HPO4.
In this study, MTA mixed with distilled water had a setting time of 133.1 ± 7.84 min. This result is in accordance with previous studies.[16,18,19] However, the setting time observed in this study was significantly shorter than the 2 h and 45 min as reported by Torabinejad et al. This difference can be attributed to changes that may have been incorporated into the MTA powder since it was first introduced.[20] Asgary et al., found that a significant change in composition has occurred since MTA was first introduced.[21]
CaCl2 accelerated the setting time of MTA to 25.40 ± 5.58 minutes. This result is comparable to values obtained in previous studies by Kogan et al., Bortoluzzi et al., and Wiltbank et al.[8,10,11] According to Bortoluzzi et al., the penetration of CaCl2 in the pores of cements could accelerate the reaction due to the hydration of silicates which reduces their crystallization time, thus hastening the final setting time of the material.[10] CaCl2 mixed with Portland cement has already been confirmed to be nontoxic to human cells in vitro.[22]
Addition of Na2HPO4 reduced the setting time of MTA to 31.60 ± 2.91 min, a result similar to that obtained by Ding et al., and Huang et al.[12,23] Na2HPO4 solution can be used as a cement liquid to accelerate the setting of the cement prepared from tricalcium phosphate as a cement powder. Phosphate increases the rate of hydroxyapatite formation. Strong ionic interactions between phosphate and calcium ions lead to the formation of calcium silicate hydrate phase.[23]
CaF resulted in markedly decreased setting time, that is, 12.40 ± 2.07 min. This result is in accordance with Wiltbank et al.[11] CaF is a highly soluble calcium compound that completely dissociates in solution to produce calcium ions. This might accelerate the dissociation step in hydration of cement, thus accelerating its setting time.[17]
pH of MTA mixed with distilled water was 12.54 ± 0.27. When MTA was mixed with CaCl2, pH decreased to 11.22 ± 0.15. This is similar to results obtained by Lee et al.[16] This decrease may be because of the addition of calcium-based electrolytes like CaCl2 which tends to suppress ionization of Ca(OH)2, thus the percentage dissociation of Ca(OH)2 decreases. The pH of solution will decrease because of common ion effect.[17] However, such pH difference at an alkaline condition, might not affect the antimicrobial property of modified MTA. MTA mixed with Na2HPO4 resulted in a pH value of 12.77 ± 0.09, which is similar to result obtained by Ding et al., and when mixed with CaF the pH is 12.56 ± 0.19, which is similar to result of Wiltbank et al.[11,12]
The compressive strength for MTA mixed with water after 7 days was 36.24 ± 3.33 MP a. This result is contrary to those obtained originally by Torabenajed et al., which maybe because of change in composition of MTA since the time it was first introduced.[20] With the addition of CaCl2 to MTA, compressive strength obtained was 33.37 ± 3.18, this result was similar to that obtained by Lee et al.[16] The compressive strength of MTA obtained with the addition of CaF was 30.05 ± 1.02 and that with addition of Na2HPO4 was 29.32 ± 1.13. Huang et al., observed that 15% Na2HPO4 used as liquid phase for MTA resulted in a good diametral tensile strength value.[23]
Although these additives reduced the setting time, they also reduced the compressive strength of MTA; which could be a disadvantage of these additives. Compressive strength is an important parameter to consider when placing a filling material in a cavity that bears occlusal pressure. On the contrary, when used as root-end filling where minimal forces are applied, reduced compressive strength will not be a major drawback.
One drawback of our study is that distilled water has been used as a storage medium for modified MTA samples. However, previous studies have shown that different storage methods and media can influence dentine characteristics as well as physical properties of dental materials.[24] Parirokh et al., and and Asgary et al., have shown that placing MTA in tissue fluids such as phosphate buffer saline(PBS) storage medium results in formation of hydroxyapatite crystals over MTA and causes a significant decrease in bacterial penetration.[24,25] Hence, future in vitro studies can use PBS as a storage medium to simulate environment in human body.
CONCLUSIONS
Within the limitation of this study, 10% CaCl2, 20% CaF, and 15% Na2HPO4 significantly reduced the setting time of white MTA and also maintained the pH at a high value. However, there was no improvement in the compressive strength of the material.
Based on the results, MTA mixed with these additives can be recommended for single-visit procedures where compressive strength is not a major concern. However, further studies regarding their biocompatibility, antimicrobial properties, sealing abilities, dimensional stability, etc., are indicated before any recommendation for clinical use.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rao A, Rao A, Shenoy R. Mineral trioxide aggregate-A review. J Clin Pediatr Dent. 2009;34:1–7. doi: 10.17796/jcpd.34.1.n1t0757815067g83. [DOI] [PubMed] [Google Scholar]
- 2.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review-Part I: Chemical, physical, and antibacterial properties. J Endod. 2010;36:16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Roberts HW, Toth JM, Berzins DW, Charlton DG. Mineral trioxide aggregate material use in endodontic treatment: A review of the literature. Dent Mater. 2008;24:149–64. doi: 10.1016/j.dental.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Kang JY, Lee BN, Son HJ, Koh JT, Kang SS, Son HH, et al. Biocompatibility of mineral trioxide aggregate mixed with hydration accelerators. J Endod. 2013;39:497–500. doi: 10.1016/j.joen.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 5.Walker MP, Diliberto A, Lee C. Effect of setting conditions on mineral trioxide aggregate flexural strength. J Endod. 2006;32:334–6. doi: 10.1016/j.joen.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Storm B, Eichmiller, Tordik PA, Goodell GG. Setting expansion of gray and white mineral trioxide aggregate and Portland cement. J Endod. 2008;34:80–2. doi: 10.1016/j.joen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Torabinejad M, Hong CU, Pitt TR, Kettering JD. Cytotoxicity of four root end filling materials. J Endod. 1995;21:489–92. doi: 10.1016/s0099-2399(06)80518-2. [DOI] [PubMed] [Google Scholar]
- 8.Kogan P, He J, Glickman GN, Watanabe I. The effect of various additives on setting properties of MTA. J Endod. 2006;32:569–72. doi: 10.1016/j.joen.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Gandolfi MG, Siboni F, Polimeni A, Bossu M, Riccitiello F, Rengo S, et al. In Vitro screening of the apatite-forming ability, biointeractivity and physical properties of a Tricalcium silicate material for Endodontics and Restorative Dentistry. Den J. 2013;1:41–60. [Google Scholar]
- 10.Bortoluzzi EA, Broon NJ, Bramante CM, Felippe WT, Tanomaru Filho M. The influence of calcium chloride on the setting time, solubility, disintegration and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. J Endod. 2009;35:550–4. doi: 10.1016/j.joen.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Wiltbank KB, Scwartz SA, Schindler WG. Effect of selected accelerants on the physical properties of Mineral trioxide aggregate and Portland cement. J Endod. 2007;33:1235–8. doi: 10.1016/j.joen.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Ding SJ, Kao CT, Shie MY, Hung CJ, Huang TH. The physical and cytological properties of white MTA mixed with Na 2 HPO 4 as an accelerant. J Endod. 2008;34:748–51. doi: 10.1016/j.joen.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 13.Ber BS, Hatton JF, Stewart GP. Chemical modification of Proroot MTA to improve handling characteristics and decrease setting time. J Endod. 2007;33:1231–4. doi: 10.1016/j.joen.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Camilleri J. Modification of mineral trioxide aggregate. Physical and mechanical properties. Int Endod J. 2008;41:843–9. doi: 10.1111/j.1365-2591.2008.01435.x. [DOI] [PubMed] [Google Scholar]
- 15.Machado DF, Bertassoni LE, de Souza EM, de Almeida JB, Rached RN. Effect of additives on the compressive strength and setting time of Portland cement. Braz Oral Res. 2010;24:158–64. doi: 10.1590/s1806-83242010000200006. [DOI] [PubMed] [Google Scholar]
- 16.Lee BN, Hwang YC, Jang JH, Chang HS, Hwang IN, Yang SY, et al. Improvement of the properties of mineral trioxide aggregate by mixing with hydration accelerants. J Endod. 2011;37:1433–6. doi: 10.1016/j.joen.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Ji DY, Wu HD, Hsieh SC, Teng NC, Chen CC, Ke ES, et al. Effects of a novel hydration accelerant on the biological and mechanical properties of white mineral trioxide aggregate. J Endod. 2011;37:851–5. doi: 10.1016/j.joen.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed HM, Saini R, Rahman IS, Saini D. Effect of bee products on the setting properties of mineral trioxide aggregate mixed with calcium chloride dihydrate. A preliminary study. J Api Product Api Med Sci. 2011;3:123–7. [Google Scholar]
- 19.Islam I, Chng HK, Yap AU. Comparison of the physical and mechanical properties of MTA and Portland cement. J Endod. 2006;32:193–6. doi: 10.1016/j.joen.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 20.Torabinejad M, Hong CU, McDonald F, Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349–53. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 21.Asgary S, Parirokh M, Eghbal MJ, Brink F. Chemical differences between white and gray mineral trioxide aggregate. J Endod. 2005;31:101–3. doi: 10.1097/01.don.0000133156.85164.b2. [DOI] [PubMed] [Google Scholar]
- 22.Abdullah D, Ford TR, Papaioannou S, Nicholson J, McDonald F. An evaluation of accelerated Portland cement as a restorative material. Biomaterials. 2002;23:4001–10. doi: 10.1016/s0142-9612(02)00147-3. [DOI] [PubMed] [Google Scholar]
- 23.Huang TH, Shie MY, Kao CT, Ding SJ. The effect of setting accelerator on properties of Mineral trioxide aggregate. J Endod. 2008;34:590–3. doi: 10.1016/j.joen.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J. Effect of two storage solutions on surface topography of two root-end fillings. Aust Endod J. 2008;35:147–52. doi: 10.1111/j.1747-4477.2008.00137.x. [DOI] [PubMed] [Google Scholar]
- 25.Parirokh M, Askarifard S, Mansouri S, Haghdoost AA, Raoof M, Torabinejad M. Effect of phosphate buffer saline on coronal leakage of mineral trioxide aggregate. J Oral Sci. 2009;51:187–91. doi: 10.2334/josnusd.51.187. [DOI] [PubMed] [Google Scholar]


