Abstract
Objectives:
Ellagic acid (EA) has shown antinociceptive and anti-inflammatory effects. Inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2) enzymes and also cytokines play a key role in many inflammatory conditions. This study was aimed to investigate the mechanisms involved in the anti-inflammatory effect of EA.
Materials and Methods:
Carrageenan-induced mouse paw edema model was used for induction of inflammation.
Results:
The results showed that intraplantar injection of carrageenan led to time-dependent development of peripheral inflammation, which resulted in a significant increase in the levels of tumor necrosis factor α (TNF-α) and interleukin 1 (IL-1) β, nitric oxide (NO) and prostaglandin E2 (PGE2) and also iNOS and COX-2 protein expression in inflamed paw. However, systemic administration of EA (1–30 mg/kg, intraperitoneal [i.p.]) could reduce edema in a dose-dependent fashion in inflamed rat paws with ED50 value 8.41 (5.26–14.76) mg/kg. It decreased the serum concentration of NO, PGE2, aspartate aminotransferase and alanine aminotransferase, and suppress the protein expression of iNOS, COX-2 enzymes, and attenuated the formation of PGE2, TNF-α and IL-1 β in inflamed paw tissue. We also demonstrated that EA significantly decreased the malondialdehyde (MDA) level in liver at 5 h after carrageenan injection. Moreover, histopathological studies indicated that EA significantly diminished migration of polymorphonuclear leukocytes into site of inflammation, as did indomethacin.
Conclusions:
Collectively, the anti-inflammatory mechanisms of EA might be related to the decrease in the level of MDA, iNOS, and COX-2 in the edema paw via the suppression of pro-inflammatory cytokines (TNFα, IL1 β), NO and PGE2 overproduction.
KEY WORDS: Carrageenan, ellagic acid, inducible nitric oxide synthase, prostaglandin E2, Rat
Introduction
Inflammation is the body's response against invading pathogens, which is typically characterized by redness, swelling, pain, and heat. Several reports have provided evidence that inflammation is involved in the pathogenesis of many diseases including aging,[1] cancer,[2] cardiovascular dysfunction,[3] and other life-threatening and debilitating disorders.
Acute inflammation is a process that involved the overproduction of free radicals, activation of a complex enzymes, and release of several inflammatory and pro-inflammatory mediators. The carrageenan-induced paw edema is a well-known acute model of inflammation that is widely used for screening novel anti-inflammatory compounds. Carrageenan injection into the subplantar surface of rat paw induced a biphasic edema. The early phase observed around 1 h is related to the release of histamine, serotonin, bradykinin, and to a less extent prostaglandins produced by cyclooxygenase enzymes (COX), whereas the delayed phase (after 1 h) is attributed to neutrophil infiltration, and the continuing of the prostaglandin generation.[4] Release of the neutrophil-derived free radicals, nitric oxide (NO) and pro-inflammatory cytokines such as tumor necrosis factor (TNF-α), and interleukin-1 β (IL-1 β) also involved in the delayed phase of carrageenan-induced acute inflammation.[5] Different observations suggest that drugs targeting COX enzyme, free radical formation and pro-inflammatory protein expression (e.g. inducible nitric oxide synthase; iNOS) might provide a better control over inflammatory states than the therapeutic agents currently available.[6]
Plant polyphenols play an important role in human nutrition. There is increasing evidence that dietary phenolic compounds have biological properties including antioxidant, anti-inflammatory, anticancer and antiatherosclerotic effects.[7] Among these phytochemicals, ellagic acid (EA; 2,3,7,8 tetrahydroxy[1] benzopyranol 5,4,3-cde][1] benzopyran-5,10-dione) is a naturally-synthesized polyphenolic compound that present in fruits and nuts such as raspberries, strawberries, pomegranates, cranberries, walnuts, pecans, and other plant foods as EA-glycosides, or bound as ellagitannins.[8] Extracts from red raspberry leaves or seeds, pomegranates, or various other sources containing high levels of EA are commercially available as dietary supplements. This compound exhibits anti-carcinogenic,[9] anti-oxidative,[10] antinociceptive and anti-inflammatory[11,12] activities. Furthermore, it has been shown that EA suppress the release of NO and prostaglandine E2 (PGE2) by down-regulating inducible NOS and COX, respectively in the endothelial cells and also rat model of colon cancer.[13]
Based on the mentioned above, this study was aimed to assess the mechanisms involved in the anti-inflammatory effect of EA in carrageenan-induced paw edema in rats.
Materials and Methods
Animals
Adult male Wistar rats (160–200 g) were housed at controlled temperature (22 ± 2°C) and allowed free access to food and drinking water. Testing took place in the middle of the light period of a 12 h/12 h light/dark cycle. All animal experiments were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. The Institutional Animal Ethical Committee of Jundishapur University, formed under Committee for Purpose of Control and Supervision of Experiments on Animals (CPCSEA, Reg. No. PRC150) approved the pharmacological protocols. The animals were used only once and then euthanized.
Drugs and Chemicals
Ellagic acid, 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), phenylmethylsulfonyl fluoride (PMSF), λ-carrageenan, thiobarbituric acid (TBA), aprotinin, pepstatin, and leupeptin were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Indomethacin was donated by Iran Daru Pharmaceutical Co. (Tehran, Iran). TNF-α and IL1 β kits were purchased from eBioscience, USA. Anti-iNOS, anti-COX-2, and anti-β-actin antibody were obtained from Abcam Biochemical Co. (Cambridge, UK).
Carrageenan-induced Rat Paw Edema
Paw edema was induced in a right hind paw of each rat by intraplantar injection of 100 μl of 1% (suspension in saline) lambda carrageenan. EA (1, 3, 10 and 30 mg/kg) and indomethacin (5 mg/kg) were intraperitoneally administered 30 min before the carrageenan injection. The paw volume of rats was measured by plethysmometer (Ugo Basile, Varese, Italy), before and after injection of carrageenan at different time intervals (1, 2, 3, 4, and 5 h). The degree of edema induced was assessed by a − b, where a and b were the volume of the right hind paw after and before carrageenan treatment, respectively. After 5 h, the animals were sacrificed and the carrageenan-induced edema feet were dissected and stored at − 80°C. The whole liver tissue was rinsed in ice-cold normal saline, and immediately were homogenized in cold KCl solution (1.5%) to give a 10% homogenate suspension used for measuring malondialdehyde (MDA). Furthermore, blood was withdrawn and serum expressed from the clotted blood was kept at −80°C for measuring PGE2 and NO levels.
Measurement of the Tumor Necrosis Factor α and Interleukin 1 β Level in Paw Tissue
Paw tissue were homogenized in 500 μl of buffer containing protease inhibitors, and IL-1 β and TNF-α levels were determined using a commercially available ELISA kit (eBioscience, USA) according to the instructions of the manufacturer.
Western Blot Analysis of Inducible Nitric Oxide Synthase and Cyclooxygenase 2
Soft tissues were removed from rats paw and homogenized in a lysis solution containing 10 mM CHAPS, 1 mM PMSF, 5 μg/ml aprotinin, 1 μM pepstatin, and 10 μM leupeptin. The homogenates were centrifuged at 12,000 g for 20 min, and 30 μg of protein from the supernatants was then separated on 10% SDS-PAGE using a standard method and transferred to polyvinylidene difluoride membranes. The membranes were then incubated with anti-iNOS, anti-COX-2 or anti-β-actin antibodies in 5% skim milk in TBST for 2 h at room temperature. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibody. The immunoreactive protein bands were detected by enhanced chemiluminescence (ECL) using hyperfilm and ECL reagent.
Measurement of Prostaglandine E2 and Nitric Oxide Level in Paw Tissue and Serum
Tissue levels of PGE2 were determined using a commercially available ELISA kit, following the manufacturer's guidelines (Abcam Biochemical, UK). Furthermore, nitrate reductase enzyme was used to measure NO products following the manufacture's guidelines (Cayman, USA). Values obtained by this procedure represented the sums of nitrite and nitrate. Similarly, PGE2 and NO levels in serum were measured.
Hepatic Malondialdehyde Assay
Malondialdehyde levels, an index of lipid peroxidation, produced by free radicals were measured to check the hepatoprotective and antioxidant activity of EA in carrageenan-induced liver injury. MDA reacts with TBA to produce a red colored complex that has peak absorbance at 532 nm. 1,1,3,3-tetramethoxypropane was used as a standard.[14]
Hepatic Assessment of the Aspartate Aminotransferase and Alanine Aminotransferase Levels
Plasma levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured as indicators of liver function parameters using commercial diagnostic assay kits from Bionik (Tehran, Iran) following the instructions of the manufacturer.
Histopathological Examination
After 5 h of carrageenan treatment, rats were sacrificed, and the tissue of the paws was removed and fixed in 10% neutral-buffered formaldehyde solution. Each sample was embedded in paraffin wax, sectioned at 5 μm and stained with H and E. A representative area was selected for qualitative light microscopic analysis of the inflammatory cellular response with a ×40 objective.
Statistical Analysis
All experimental results are given as means ± standard error of the mean for 6–8 animals per group. The statistical analyses were performed using one-way ANOVA, followed by Tukey's post-hoc test. The ED50 value for the anti-inflammatory effect of EA accompanied by its respective 95% confidence limits were determined by linear regression from individual experiments using GraphPad Prism 5.0 software (San Diego, CA, USA).
Results
Effects of Ellagic Acid on Carrageenan-induced Rat Paw Edema
Intraplantar injection of carrageenan in rats resulted in a time-dependent increase in paw volume [Figure 1]. However, treatment with EA reduced the paw edema in a dose-dependent manner at 1, 2, 3, 4 and 5 h after an injection of carrageenan. ED50 values with 95% confidence intervals for the anti-inflammatory effect of EA were 8.41 (5.26–14.76) mg/kg. As expected, the reference drug, indomethacin (5 mg/kg), caused a significant inhibition of postcarrageenan edema [Figure 1].
Figure 1.
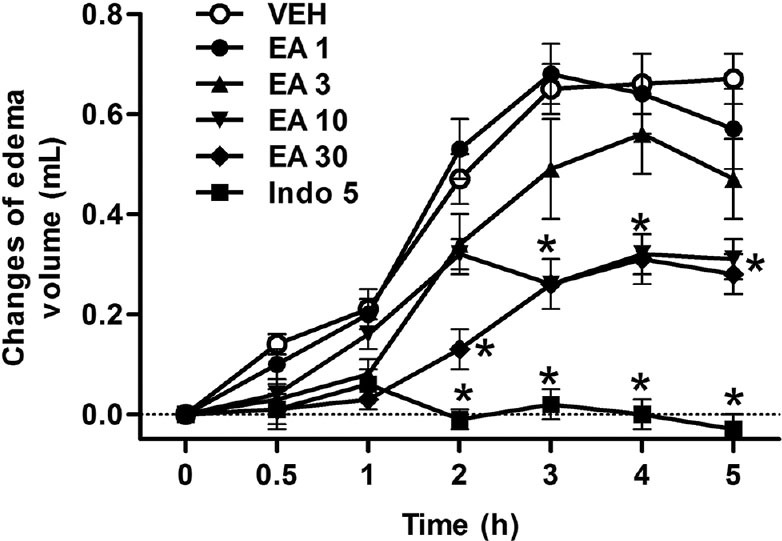
Effects of ellagic acid (EA) on hind paw edema induced by carrageenan (Carr) in rats. Each value represents the mean ± standard error of the mean. *P < 0.05 as compared to the Carr group (one-way ANOVA followed by Tukey's test). VEH: Vehicle, EA: Ellagic acid (1–30 mg/kg, intraperitoneal [i.p.]), Indo: Indomethacin (5 mg/kg, i.p.)
Effects of Ellagic Acid on Cytokine Secretion
As shown in Figure 2a and b, carrageenan increased the level of TNF-α and IL-1 β in paw edema, respectively. Meanwhile, in the range dose 1–30 mg/kg, EA could inhibit the level of TNF-α and IL-1 β to 40–17% and 68–36% of that observed in carrageenan group, respectively. Moreover, indomethacin (5 mg/kg) significantly decreased TNF-α level in the rat edema paw.
Figure 2.
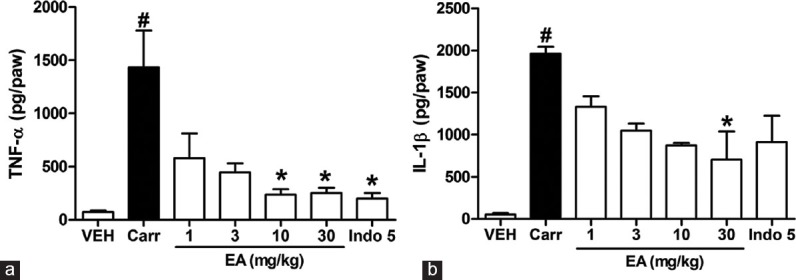
Effects of ellagic acid (EA) on carrageenan (Carr)-induced (A) tumor necrosis factor-α and (B) interleukin 1 β concentrations in paw tissue at 5 h in rats. Each value represents the mean ± standard error of the mean. #P < 0.05 and *P < 0.05 as compared to the VEH and Carr group, respectively (one-way ANOVA followed by Tukey's test). VEH: Vehicle, EA: Ellagic acid, Indo: Indomethacin (5 mg/kg, intraperitoneal)
Effects of Ellagic Acid on Inducible Nitric Oxide Synthase and Cyclooxygenase 2 Expression
To determine whether the inhibition of NO and PGE2 production was due to a decreased iNOS and COX-2 protein level, the effect of EA on iNOS and COX-2 protein expression was studied using Western blotting analysis. The results showed that treatment with EA at 30 mg/kg for 5 h after carrageenan injection reduced iNOS and COX-2 proteins expression in the rat paw edema [Figure 3]. In addition, the protein expression showed a reduction of iNOS and COX-2 protein expression after treatment with indomethacin at 5 mg/kg compared with the carrageenan-induced alone [Figure 3].
Figure 3.
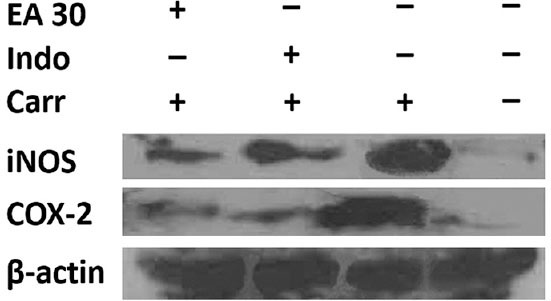
Inhibition of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) protein expression by ellagic acid (EA) induced by carrageenan (Carr) in rat paw edema for 5 h. Tissue hemogenated were then prepared and subjected to western blotting using an antibody specific for iNOS and COX-2. β-actin was used as an internal control. EA: Ellagic acid (30 mg/kg, intraperitoneal [i.p.]), Indo: Indomethacin (5 mg/kg, i.p.)
Effects of Ellagic Acid on the Nitric Oxide Level
As shown in Figure 4a and b, injection of carrageenan into the rat hind paw induced a marked increase in the hind paw and also serum NO level 5 h after injection. The i.p. treatment of rats with EA (10 and 30 mg/kg) and indomethacin (5 mg/kg, i.p.) caused a significant reduction of increased NO generation by carrageenan in serum (P < 0.05). However, EA had no effect on NO level in edema paw tissue (Figure 4a, P > 0.05).
Figure 4.
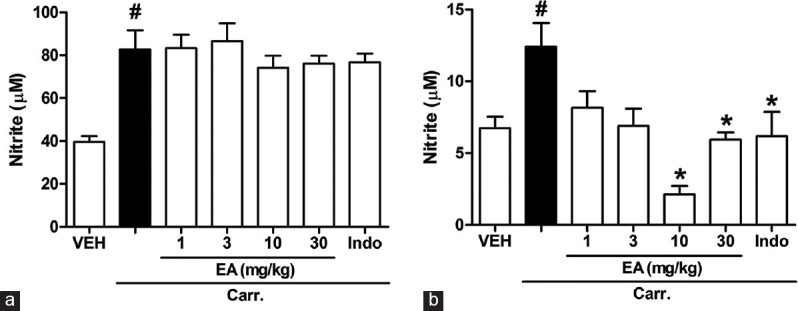
Effects of ellagic acid (EA) on carrageenan (Carr)-induced nitric oxide (NO) in (a) paw tissue and (b) serum at 5 h in rats. Each value represents the mean ± standard error of the mean. #P < 0.05 and *P < 0.05 as compared to the VEH and Carr group, respectively (one-way ANOVA followed by Tukey's test). VEH: Vehicle, EA: Ellagic acid, Indo: Indomethacin (5 mg/kg, intraperitoneal)
Effects of Ellagic Acid on the Prostaglandine E2 Level
Figure 5a and b show the PGE2 level increased significantly in the paw tissue and serum 5 h after carrageenan injection (P < 0.05). The i.p. treatment of rats with EA (10 and 30 mg/kg) and indomethacin (5 mg/kg, i.p.) caused a significant reduction of increased PGE2 level by carrageenan in serum (P < 0.05). However, the effect of EA in paw tissue was more potent than that of serum.
Figure 5.
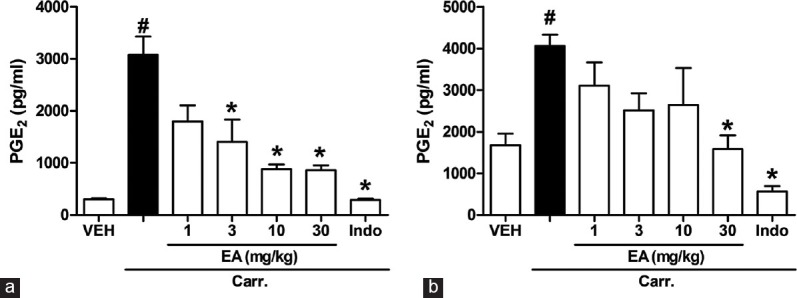
Effects of ellagic acid (EA) on carrageenan (Carr)-induced prostaglandine E2 in (a) paw tissue and (b) serum at 5 h in rats. Each value represents the mean ± standard error of the mean. #P < 0.05 and *P < 0.05 as compared to the VEH and Carr group, respectively (one-way ANOVA followed by Tukey's test). VEH: Vehicle, EA: Ellagic acid, Indo: Indomethacin (5 mg/kg, intraperitoneal)
Effects of Ellagic Acid on the Malondialdehyde, Alanine Aminotransferase and Aspartate Aminotransferase Level
To determine whether the anti-inflammatory effect of EA was partly due to antioxidant activity, the hepatoprotective properties of EA were assessed by measurement the MDA levels as an index of lipid peroxidation and plasma contents of AST and ALT enzymes. As shown in Figure 6a, The MDA level increased significantly in liver tissue of rats at 5 h after carrageenan injection (P < 0.05). However, the MDA level was decreased significantly by treatment with EA. Furthermore, injection of carrageenan into the rat hind paw induced an increase in the serum levels of AST and ALT enzymes 5 h after injection compared with control group (P < 0.05). EA (1-30 mg/kg, i.p.) resulted in a significant reduction in AST and ALT levels [Figure 6b and c].
Figure 6.
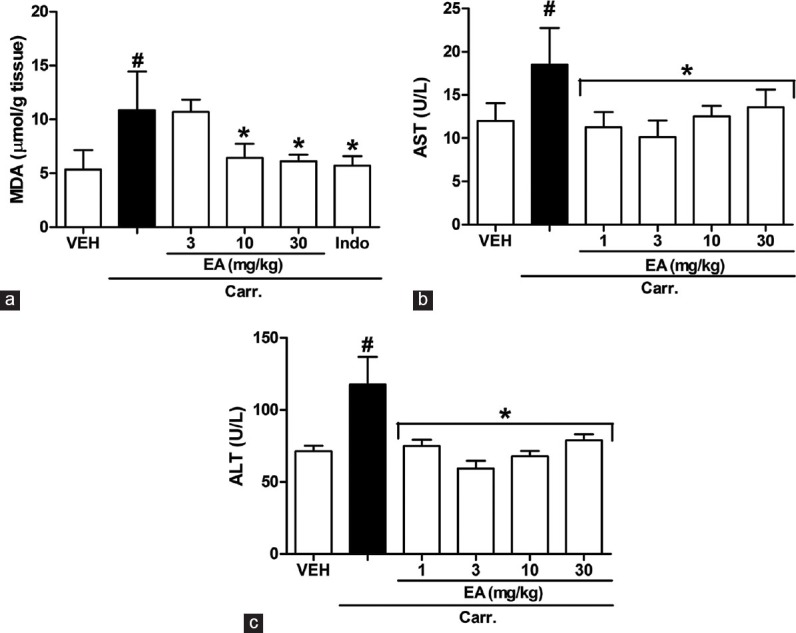
Effects of ellagic acid (EA) on carrageenan (Carr)-induced (a) malondialdehyde (MDA), (b) aspartate aminotransferase (AST) and (c) alanine aminotransferase (ALT) concentrations at 5 h in rats. MDA was measured in liver homogenate. AST and ALT were measured in serum. Each value represents the mean ± standard error of the mean. #P < 0.05 and *P < 0.05 as compared to the VEH and Carr group, respectively (one-way ANOVA followed by Tukey's test). VEH: Vehicle, EA: Ellagic acid, Indo: Indomethacin (5 mg/kg, intraperitoneal)
Histopathological Examination
As shown in Figure 7a, there was no sign of inflammation in the paw tissue of normal rats. Paw tissue of vehicle-treated rats showed an acute inflammation with extensive extravasations, mainly polymorphonuclear leukocytes [Figure 7b]. As observed in Figure 7c and d, treatment of rats with EA at 30 mg/kg and indomethacin (5 mg/kg, i.p.) exhibited a significant decrease in the number of cellular infiltrates.
Figure 7.
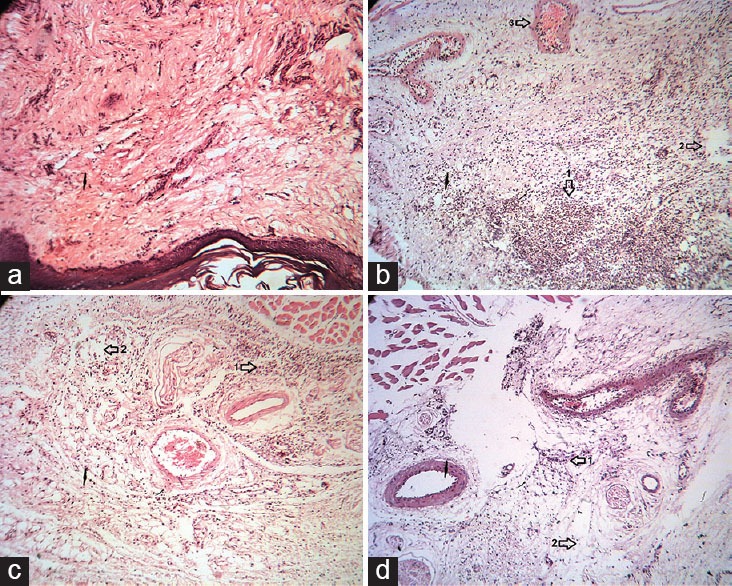
Histopathologic examination of paw tissue of rats treated with EA, 5 h after injection of carrageenan (Carr). (a) Normal rats show the normal appearance of epidermis and dermis without any lesion. (b) Carrageenan-injected paw tissue in control group. Vasodilatation with edema, and migration of neutrophil were observed. (c and d) Carrageenan-injected paw of rats treated with EA (30 mg/kg, intraperitoneal [i.p.]) and indomethacin (5 mg/kg, i.p.), respectively. The migration of neutrophil and edema reduced. Sections were stained with H and E, ×40. 1: Neutrophil, 2: Edema, 3: Vessel
Discussion
The results of this study showed that EA significantly suppressed the rat hind paw edema induced by intraplantar injection of carrageenan, indicating marked anti-acute inflammatory efficacy. In the current study, the anti-inflammatory effect of EA was further evaluated to elucidate the underlying mechanisms involved in this animal model. We demonstrated that the effect may be due to inhibition of the pro-inflammatory cytokines release, inflammatory enzymes (iNOS and COX-2) expression and also their products (NO and PGE2). Furthermore, we showed that EA could prevent liver damage induced by carrageenan through the anti-oxidative mechanisms.
The carrageenan-induced paw edema is a well-defined model of acute inflammation that a variety of inflammatory mediators involves in its development and has wildly been used to evaluate the anti-edematous effect of natural products. In the present study, we showed that EA produced anti-inflammatory effects in carrageenan-induced rat paw edema dose-dependently. Our results confirmed previous findings that EA exhibit a noticeable anti-inflammatory effect in experimental models.[15] On the other hand, it is well characterized that neutrophil infiltration plays a key role in the inflammation induced by carrageenan in hind paw.[4] Our results indicated that EA elicited a marked reduction in the infiltration of neutrophils into the carrageenan-treated paws, according to a histopathological examination.
A growing lines of evidence have demonstrated that flavonoids, phenolic acids, and triterpenoid possessed antinociceptive and anti-inflammatory effects in animal models.[16] Studies have also reported that flavonoids such as rutin, quercetin, luteolin produced significant antinociceptive and anti-inflammatory activities.[17] Hence, it was suggested that the antioxidant and anti-inflammatory activities of EA may be related to its phenolic content.
The expression of the inducible isoform of NO synthase (iNOS) has been proposed as an important mediator of inflammation. Moreover, a large amount of NO is produced from L-arginine by iNOS, thereby causing detrimental effects. Although physiological NO production has beneficial anti-microbial and anti-tumor effects, excessive NO produced by iNOS is a mediator of the inflammatory diseases and causes cell injury by generating reactive radicals, such as peroxynitrite.[18] Our finding revealed that EA suppressed carrageenan-induced production of iNOS and its product, NO. So, we suggest the anti-inflammatory mechanism of EA may be through the L-arginine-NO pathway because EA significantly inhibits the NO production by iNOS. In accordance with this data, we previously showed the involvement of L-arginine-NO pathway in the anti-nociceptive effect of EA in acetic acid-induced visceral pain.[11] Furthermore, Dhingra and Chhillar suggested the role of iNOS in antidepressant-like activity of EA.[19] Moreover, it has been indicated that EA inhibit the release of NO by down-regulating iNOS.[13]
In vitro and in vivo studies have been indicated an existing cross talk between the release of NO and PGs in the modulation of inflammation.[20] Data indicate that NO has a profound effect on COX-2 catalytic activity.[21] However, the mechanisms of this effect have not been elucidated. One possibility is that NO increases COX-2 half-life by generating free radicals and inhibiting COX-2 autoinactivation.[20] Another possibility is that lipid peroxidation initiated by peroxynitrite, the product of the reaction of NO with superoxide, liberates arachidonic acid from the cell membrane, which in turn activates COX-2.[22] PGE2, a product of COX-2, is considered as one of the strongest inflammatory mediators in the inflammatory response. Our study showed that EA could inhibit PGE2 production and reduce the COX-2 expression significantly in carrageenan-induced rat paw edema suggested that the anti-inflammatory effect of EA might be associated to its inhibitive effect on PGE2 production through blocking COX-2 protein expression. On the other hand, carrageenan injection also provokes the release of some important pro-inflammatory cytokines such as TNF-α and IL-1 β.[23] TNF-α triggers the release of IL-1 β and cytokine-induced neutrophil chemo-attractant 1. These mediators are responsible for stimulation of the PGs synthesis by COX-2. Moreover, TNF-α induces NO synthesis by activating iNOS and augments the responses of neutrophils to inflammatory stimuli.[5,24] Our findings demonstrated that EA considerably reduced the TNF-α and IL-1 β levels in the carrageenan-injected paw tissues. Inhibition of cytokine production by EA might account for the inhibition of COX-2 expression because both cytokines induce it.[25] Also, EA has been found to inhibit the activation of transcription factor NF-κB and, therefore, reduced expression of COX-2 protein.[26] Karin and Ben-Neriah showed that transcription of pro-inflammatory mediators such as iNOS, COX-2, TNF-α, and IL-1 β is regulated by activation of NF-κB.[27]
Free radical has been proposed to play an important role in the carrageenan-induced acute inflammatory response.[28] In a number of pathophysiological conditions associated with inflammation or oxidative stress, free radical have been proposed to mediate cell damage in the liver.[29] As a marker of oxidative damage, lipid peroxidation indicates changes in membrane fluidity and permeability and thus increases in rates of protein degradation, which will eventually lead to cell lysis. One of the consequences of lipid peroxidation can also result in enzyme activity changes.[30] MDA is a metabolic product of lipid peroxidation, the level of which is raised in oxidative stress. The present results showed that EA reduce the severity of liver damage by lowering MDA level. Furthermore, there were significant decreases in AST and ALT level with EA treatment. We assume that the suppression of AST and ALT level is probably due to the inhibition of lipid peroxidation. A growing body of evidence suggests the anti-oxidative effect of EA, which is consistent with our results.[8,10]
In conclusion, the results of the current study suggested that EA possessed anti-inflammatory effects in carrageenan-induced rat paw edema, which is comparable to indomethacin. The anti-inflammatory mechanism of EA may be related to the reduction of TNF-α and IL-1 β that could result in inhibition of iNOS and COX-2 expression and its product (NO and PGE2). Furthermore, the protective effect of EA on liver damage may result from anti-oxidative effects. Our findings provide new perspectives on the therapeutic use EA in the management of inflammatory diseases.
Acknowledgments
This paper is issued from Ph.D. thesis of (Behnam Ghorbanzadeh) and financial support was provided by the Vice Chancellor of Research, Ahvaz Jundishapur University of Medical Sciences (grant no. PRC150).
Footnotes
Source of Support: Nill.
Conflict of Interest: No.
References
- 1.Finch CE. Developmental origins of aging in brain and blood vessels: An overview. Neurobiol Aging. 2005;26:281–91. doi: 10.1016/j.neurobiolaging.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- 3.Rus H, Niculescu FI. Inflammation, aspirin, and the risk of cardiovascular disease. N Engl J Med. 1997;337:423. [PubMed] [Google Scholar]
- 4.Gilligan JP, Lovato SJ, Erion MD, Jeng AY. Modulation of carrageenan-induced hind paw edema by substance P. Inflammation. 1994;18:285–92. doi: 10.1007/BF01534269. [DOI] [PubMed] [Google Scholar]
- 5.Halici Z, Dengiz GO, Odabasoglu F, Suleyman H, Cadirci E, Halici M. Amiodarone has anti-inflammatory and anti-oxidative properties: An experimental study in rats with carrageenan-induced paw edema. Eur J Pharmacol. 2007;566:215–21. doi: 10.1016/j.ejphar.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 6.Ronchetti D, Borghi V, Gaitan G, Herrero JF, Impagnatiello F. NC×2057, a novel NO-releasing derivative of ferulic acid, suppresses inflammatory and nociceptive responses in in vitro and in vivo models. Br J Pharmacol. 2009;158:569–79. doi: 10.1111/j.1476-5381.2009.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handique JG, Baruah JB. Polyphenolic compounds: An overview. React Funct Polym. 2002;52:163–88. [Google Scholar]
- 8.Priyadarsini KI, Khopde SM, Kumar SS, Mohan H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J Agric Food Chem. 2002;50:2200–6. doi: 10.1021/jf011275g. [DOI] [PubMed] [Google Scholar]
- 9.Glauert HP, Calfee-Mason K, Stemm DN, Tharappel JC, Spear BT. Dietary antioxidants in the prevention of hepatocarcinogenesis: A review. Mol Nutr Food Res. 2010;54:875–96. doi: 10.1002/mnfr.200900482. [DOI] [PubMed] [Google Scholar]
- 10.Park SH, Kim JL, Lee ES, Han SY, Gong JH, Kang MK, et al. Dietary ellagic acid attenuates oxidized LDL uptake and stimulates cholesterol efflux in murine macrophages. J Nutr. 2011;141:1931–7. doi: 10.3945/jn.111.144816. [DOI] [PubMed] [Google Scholar]
- 11.Taghi Mansouri M, Naghizadeh B, Ghorbanzadeh B, Farbood Y. Central and peripheral antinociceptive effects of ellagic acid in different animal models of pain. Eur J Pharmacol. 2013;707:46–53. doi: 10.1016/j.ejphar.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Mansouri MT, Naghizadeh B, Ghorbanzadeh B. Sildenafil enhances the peripheral antinociceptive effect of ellagic acid in the rat formalin test. Indian J Pharmacol. 2014;46:404–8. doi: 10.4103/0253-7613.135952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umesalma S, Sudhandiran G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-kappaB, iNOS, COX-2, TNF-alpha, and IL-6 in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin Pharmacol Toxicol. 2010;107:650–5. doi: 10.1111/j.1742-7843.2010.00565.x. [DOI] [PubMed] [Google Scholar]
- 14.Naghizadeh B, Mansouri MT, Ghorbanzadeh B, Farbood Y, Sarkaki A. Protective effects of oral crocin against intracerebroventricular streptozotocin-induced spatial memory deficit and oxidative stress in rats. Phytomedicine. 2013;20:537–42. doi: 10.1016/j.phymed.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Mansouri MT, Naghizadeh B, Ghorbanzadeh B. Involvement of opioid receptors in the systemic and peripheral antinociceptive actions of ellagic acid in the rat formalin test. Pharmacol Biochem Behav. 2014;120:43–9. doi: 10.1016/j.pbb.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Arslan R, Bektas N, Ozturk Y. Antinociceptive activity of methanol extract of fruits of Capparis ovata in mice. J Ethnopharmacol. 2010;131:28–32. doi: 10.1016/j.jep.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 17.Deliorman Orhan D, Hartevioglu A, Küpeli E, Yesilada E. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J Ethnopharmacol. 2007;112:394–400. doi: 10.1016/j.jep.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Huang GJ, Bhaskar Reddy MV, Kuo PC, Huang CH, Shih HC, Lee EJ, et al. A concise synthesis of viscolin, and its anti-inflammatory effects through the suppression of iNOS, COX-2, ERK phosphorylation and proinflammatory cytokines expressions. Eur J Med Chem. 2012;48:371–8. doi: 10.1016/j.ejmech.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Dhingra D, Chhillar R. Antidepressant-like activity of ellagic acid in unstressed and acute immobilization-induced stressed mice. Pharmacol Rep. 2012;64:796–807. doi: 10.1016/s1734-1140(12)70875-7. [DOI] [PubMed] [Google Scholar]
- 20.Salvemini D, Settle SL, Masferrer JL, Seibert K, Currie MG, Needleman P. Regulation of prostaglandin production by nitric oxide; an in vivo analysis. Br J Pharmacol. 1995;114:1171–8. doi: 10.1111/j.1476-5381.1995.tb13330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu KK. Inducible cyclooxygenase and nitric oxide synthase. Adv Pharmacol. 1995;33:179–207. doi: 10.1016/s1054-3589(08)60669-9. [DOI] [PubMed] [Google Scholar]
- 22.Davidge ST, Baker PN, Laughlin MK, Roberts JM. Nitric oxide produced by endothelial cells increases production of eicosanoids through activation of prostaglandin H synthase. Circ Res. 1995;77:274–83. doi: 10.1161/01.res.77.2.274. [DOI] [PubMed] [Google Scholar]
- 23.Nacife VP, Soeiro Mde N, Gomes RN, D’Avila H, Castro-Faria Neto HC, Meirelles Mde N. Morphological and biochemical characterization of macrophages activated by carrageenan and lipopolysaccharide in vivo . Cell Struct Funct. 2004;29:27–34. doi: 10.1247/csf.29.27. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian N, Bray MA. Interleukin 1 releases histamine from human basophils and mast cells in vitro . J Immunol. 1987;138:271–5. [PubMed] [Google Scholar]
- 25.Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 2005;102:1755–60. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marín M, María Giner R, Ríos JL, Recio MC. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J Ethnopharmacol. 2013;150:925–34. doi: 10.1016/j.jep.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-[kappa] B activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 28.Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur J Pharmacol. 1996;303:217–20. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- 29.Huang GJ, Huang SS, Lin SS, Shao YY, Chen CC, Hou WC, et al. Analgesic effects and the mechanisms of anti-inflammation of ergostatrien-3beta-ol from Antrodia camphorata submerged whole broth in mice. J Agric Food Chem. 2010;58:7445–52. doi: 10.1021/jf1013764. [DOI] [PubMed] [Google Scholar]
- 30.Rauchová H, Drahota Z, Koudelová J. The role of membrane fluidity changes and thiobarbituric acid-reactive substances production in the inhibition of cerebral cortex Na+/K+-ATPase activity. Physiol Res. 1999;48:73–8. [PubMed] [Google Scholar]


