Abstract
Objective:
The present study was undertaken to evaluate the protective effects of the fruit extract of Tribulus terrestris (TT) on the metronidazole (MTZ)-induced alterations in spermatogenesis, sperm count, testicular functions, and oxidative stress.
Materials and Methods:
Thirty adult Swiss strain mice were divided into six groups. Animals of Groups I and II served as untreated and vehicle-treated controls, while that of Groups III and IV were administered with MTZ (500 mg/kg BW/day) and TT (200 mg/kg BW/day) alone for 28 days, respectively. Low (100 mg/kg BW/day) and high (200 mg/kg BW/day) doses of TT along with MTZ (500 mg/kg BW/day) were administered for 28 days in the mice of Groups V and VI, respectively. Twenty four hours after the last treatment, all the animals were euthanized to study the histological changes in the testis and sperm count in the epididymis. Testicular functional markers, lipid peroxidation (LPO) and the activities of antioxidant enzymes, e.g., superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase, were also assessed in the mice of all the groups.
Results:
Metronidazole caused marked alterations in the testicular weight, spermatogenesis, activities of antioxidant enzymes, lactate dehydrogenase, alkaline phosphatase, and the level of LPO. The epididymal sperm count also declined significantly in MTZ-treated group. These changes were partially restored following co-administration of 500 mg/kg BW/day of MTZ and 100 mg/kg BW/day of TT. However, in the mice co-administered with 500 mg/kg BW/day of MTZ and 200 mg/kg BW/day of TT, the changes reverted back completely, similar to that of the controls.
Conclusion:
The fruit extract of TT ameliorates the MTZ-induced alterations in the testis.
KEY WORDS: Antioxidant enzymes, free radicals, metronidazole, testis, Tribulus terrestris
Introduction
The use of herbal medicines is extensively increasing in the world due to their natural property and minimum or no side effects. Plants and their derivatives play a key role in the world health and have long been known to possess biological activity. Tribulus terrestris (TT), a pubescent annual herb of family Zygophyllaceae, is regarded as a common weed found in many tropical and moderate areas of the world, including U.S., Mexico, Mediterranean region, and throughout Asia. The whole plant is of medicinal value as the leaves are considered to possess stomachic properties and paste prepared from them is used in the treatment of bladder stone. Root possesses aperient and tonic properties while the fruit is credited with diuretic, tonic, cooling, demulcent, and aphrodisiac properties. This indigenous medicinal plant is also used in the Indian and Chinese system of medicine for the treatment of various male reproductive disorders. Investigations carried out in the rat, rabbit, and primate suggest that TT improves the male reproductive functions.[1] The findings of Singh et al.[2] have validated the traditional use of TT as a sexual enhancer in the management of reproductive dysfunctions in the males.
Metronidazole (MTZ; 1-[2-hydroxyethyl]-2-methyl- 5-nitroimidazole), a widely used antiamoebic and antibacterial drug, is reported to induce marked impairments in the testis, epididymis, and fertility of the mice following its oral administration at the dose of 500 mg/kg BW/day, for 28 days.[3] MTZ-induced reproductive impairments in the males are reported to be restored following certain antioxidant supplementations such as curcumin,[4] vitamin E,[5] and α-tocopherol.[6]
Herbal products are being used extensively for infertility therapy. Among them, the involvement of TT in the improvement of male sexual dysfunctions is well-established. TT is reported to have a protective effect against cadmium[7] and cypermethrin[8]-induced testicular injury in the rat. Hence, based on the aphrodisiac and antioxidant properties of TT, the present study has been carried out with the objective to access the potentiality of the fruit extract of this plant against MTZ-induced changes in the testicular oxidative stress, functional markers, spermatogenesis, and the sperm count. The present study is an extension of our earlier published work.[3]
Materials and Methods
Animal Selection
Thirty Swiss strain adult (12 weeks old) male mice weighing about 23-30 g were used in the present investigation. The mice were selected from closed, randomly bred colony maintained in standard laboratory condition in the animal house of our department. They were fed with commercially available pelleted food and water ad libitum. The experimental plan was approved from the Animal Ethical Committee, Banaras Hindu University, Varanasi, India (No. Dean/11-12/CAEC/263).
Drug, Dosage, and Treatments
All the animals were divided into six groups of five each. After recording the initial body weights, the animals were treated as follows:
Group I: Untreated controls
Group II: Vehicle-treated controls (distilled water)
Group III: Administration of MTZ (500 mg/kg BW/day)
Group IV: Administration of the fruit extract of TT (200 mg/kg BW/day)
Group V: Co-administration of MTZ (500 mg/kg BW/day) and the fruit extract of TT (100 mg/kg BW/day)
Group VI: Co-administration of MTZ (500 mg/kg BW/day) and the fruit extract of TT (200 mg/kg BW/day).
Treatments in each group were continued for 28 days through oral route. MTZ was purchased from CDH, India while the fruit of TT was purchased from the local market of Varanasi, got identified from the Department of Botany, Banaras Hindu University (Voucher No. Zygo-2013-1) and its extract was prepared by adopting the method of Hussain et al.[9] The human therapeutic dose of MTZ was selected and translated to mice.[10] The doses of TT were standardized according to its efficacy on MTZ-induced impairments in spermatogenesis and sperm count.
Animal Sacrifice and Collection of Reproductive Organs
Twenty-eight days after the last treatment, the body weights of the animals were recorded and then sacrificed. Immediately, the heart was punctured to collect the blood for estimating the level of serum testosterone. Further, the testis and epididymis were dissected out and processed for the following studies:
Weight of testis
The testis was blotted free of blood and weighed to calculate the gonadosomatic index (GSI) using the following formula:
GSI = (Gonad weight/total body weight) ×100
Assessment of sperm count
Cauda epididymides of five mice from each group were minced thoroughly in physiological normal saline, maintained at 37°C. The suspension prepared was then used for assessment of the sperm count according to the WHO Laboratory Manual.[11]
Histological study
Bouin's fixed testis was dehydrated and embedded in paraffin. Sections of 5 μm thickness were taken from the mid portion of each testis and stained with Periodic Acid Schiff reagent followed by counterstaining with Ehrlich's Hematoxylin.
Quantitative study of the testis
Frequency of the stages was determined from one cross section of the testis of the five animals in each group. All the seminiferous tubules within a cross section of the testis were examined at ×40 and classified according to the stages of the cycle. The stages of the seminiferous tubules were classified according to the method of Hess and de Franca.[12] The accurate identification of each stage in some of the seminiferous tubules was not possible due to severe degenerative changes; therefore, the tubules were grouped as stages I-IV, V-VI, VII-VIII, IX-X, and XI-XII. The percentage frequency of the grouped stages was analyzed in one cross section of the testis in each of the five animals and calculated statistically. The relative number of each type of germ cells at stage VII of the spermatogenic cycle, that is, type-A spermatogonia, preleptotene spermatocytes, pachytene spermatocytes, and stage 7 spermatids was counted according to the method of Russell et al.[13]
Morphometric study of the seminiferous tubules
The diameter of the seminiferous tubules and height of the seminiferous epithelium were measured using ocular micrometer at ×40.
Serum Testosterone Assay
The level of serum testosterone was measured by ELISA, as described in the instructions provided in the kit (LDN, Nordhorn).
Estimations of the Antioxidant Enzyme Activities
The testis from each mouse was dissected out carefully and washed with the ice-cold physiological saline solution. The tissue was weighed, and 10% homogenate was prepared in ice-cold phosphate buffer (0.05M, pH 7.0). The homogenate was centrifuged at 10,000 ×g for 20 min at 4°C.[14] The supernatant was used for the enzyme assays after estimating the protein content by the method of Lowry et al.[15] using bovine serum albumin as a standard. Superoxide dismutase (SOD) (EC 1.15.1.1) was measured by the method of Marklund and Marklund[16] whereas the method of Claiborne[17] was used to determine the catalase (CAT) activity. The activities of glutathione peroxidase (GPx) and glutathione reductase (GR) were measured using the methods of Flohe and Gunzler[18] and Carlberg and Mannervick,[19] respectively.
Estimation of Lipid Peroxidation
The level of lipid peroxidation (LPO) was measured in the tissue supernatant using MDA concentration as a surrogate measure.[20]
Testicular Functional Markers
Lactate dehydrogenase
The activity of lactate dehydrogenase (LDH) was estimated in the tissue supernatant using LDH (P-L) kit (Mod. IFCC method).
Alkaline phosphatase
The activity of alkaline phosphatase (ALP) was estimated in the tissue supernatant using COGENT diagnostic kit.
Statistical Analysis
Results were expressed as mean and standard error of the mean in different groups. The differences between the mean values were evaluated by one-way ANOVA followed by Neuman–Keul's multiple range test. Body weight was analyzed using Student's t-test. The values for P < 0.05 were considered significant.
Results
Body Weight
No significant differences were observed between the initial and final body weights of all the treated groups when compared with their respective controls [Table 1].
Table 1.
Effect of the oral administration of MTZ (500 mg/kg BW/day) and co-administration of MTZ (500 mg/kg BW/day) and TT (100 mg/kg BW/day and 200 mg/kg BW/day) on the BW, weight of the testis, epididymal sperm count and serum testosterone level
Weight of the Testis
A significant decline was noticed in the weight of the testis in the MTZ-treated group as compared with the controls [Table 1]. Co-administration of MTZ and 100 mg/kg BW/day as well as of 200 mg/kg BW/day of the fruit extract of TT restored the weight of the testis significantly as compared with the MTZ-treated group, thus attained the values of the controls [Table 1].
Epididymal Sperm Count
A significant reduction was observed in the epididymal sperm count in the MTZ-treated groups as compared with the controls [Table 1]. Co-administration of MTZ and 100 mg/kg BW/day of the fruit extract of TT failed to increase the sperm count to the control values, whereas 200 mg/kg BW/day of the fruit extract caused a significant increase in the same as compared with the MTZ-treated group, thus attained the values of the controls [Table 1].
Histological Study
The testes of untreated and vehicle-treated controls [Figure 1a] showed normal histological features. Administration of MTZ induced marked regressive changes in the seminiferous tubules in the testis of all the animals, however, the extent of regression varied from individual to individual [Figure 1b]. The changes were indicated by shrinkage of the seminiferous tubules, depletion, disorganization, intraepithelial vacuolization, and exfoliation of the germ cells. Few giant cells containing round spermatids were also noticed in MTZ-treated mice. Co-administration of MTZ and 100 mg/kg BW/day of the fruit extract of TT caused partial recovery in spermatogenic activity [Figure 1c] however, majority of the seminiferous tubules in the testis of the mice co-administered with MTZ and 200 mg/kg BW/day of the extract, exhibited almost full recovery in spermatogenic activity, similar to that of the controls [Figure 1d].
Figure 1.
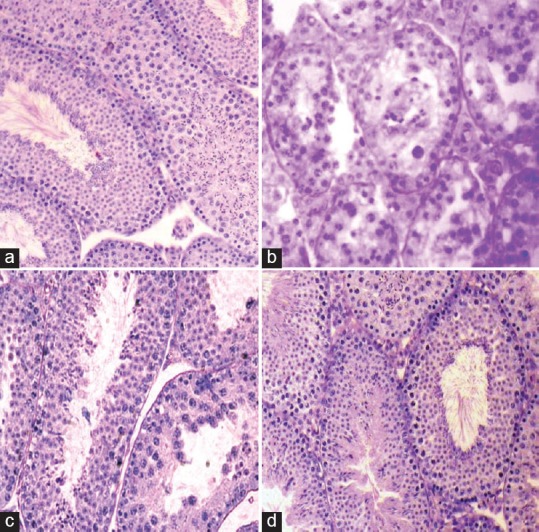
T.S. of the testis of (a) a control mouse showing full spermatogenic activity in the seminiferous tubules. (b) Metronidazole (MTZ) (500 mg/kg BW/day)-treated mouse for 28 days showing shrinkage of the seminiferous tubules, depletion, disorganization, vacuolization and sloughing of the germ cells and appearance of multinucleated giant cells. (c) A mouse co-administered with MTZ (500 mg/kg BW/day) and Tribulus terrestris (TT) (100 mg/kg BW/day) for 28 days presenting partial recovery in spermatogenic activity (d) a mouse co-administered with MTZ (500 mg/kg BW/day) and TT (200 mg/kg BW/day) for 28 days showing full recovery in spermatogenic activity
Quantitative Study of the Testis
Quantitative studies of the spermatogenic cycle revealed a significant decrease in the percentage frequency of stages I-VIII after MTZ-treatment, as compared with the controls [Table 2]. Treatment with MTZ also induced a significant decrease in the various types of germ cells in stage VII of the seminiferous tubules [Table 3]. These changes were partially recovered in the group co-administered with MTZ and the fruit extract of TT at the dose of 100 mg/kg BW/day, however, co-administration of MTZ and 200 mg/kg BW/day of the fruit extract resulted in almost full recovery in the spermatogenic cycle similar to that of the controls [Tables 2 and 3].
Table 2.
Effect of the oral administration of MTZ (500 mg/kg BW/day) and co-administration of MTZ (500 mg/kg BW/day) and TT (100 mg/kg BW/day and 200 mg/kg BW/day) on the percentage frequencies of the stages of spermatogenic cycle
Table 3.
Effect of the oral administration of MTZ (500 mg/kg BW/day) and co-administration of MTZ (500 mg/kg BW/day) and TT (100 mg/kg BW/day and 200 mg/kg BW/day) on the number of various types of germ cells of stage VII seminiferous tubules, diameter of seminiferous tubules and height of seminiferous epithelium
Morphometric Study of the Seminiferous Tubules
Administration of MTZ induced a significant decrease in the diameter of the seminiferous tubules as well as in the height of seminiferous epithelium as compared with the controls [Table 3]. These changes were restored to the control values when the mice were co-administered with MTZ and both the doses of the fruit extract of TT, respectively [Table 3].
Serum Testosterone Assay
No significant alteration was noticed in the level of serum testosterone in MTZ-treated mice as compared with the controls [Table 1]. Likewise, co-administration of MTZ and 100 mg/kg BW/day and 200 mg/kg BW/day of the fruit extract of TT, respectively, were ineffective in causing significant alteration in the testosterone level [Table 1].
Antioxidant Enzyme Activities in the Testis
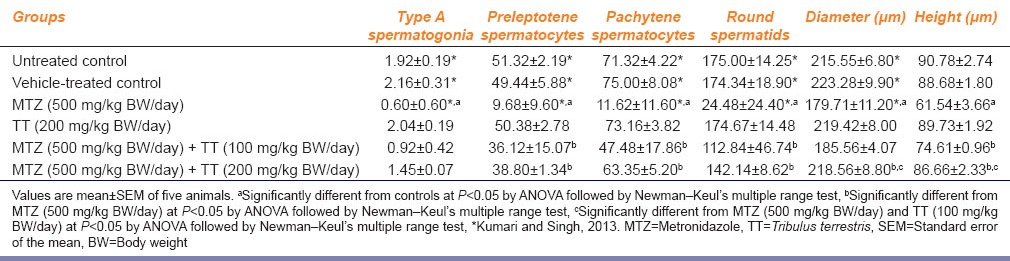
Superoxide dismutase (SOD)
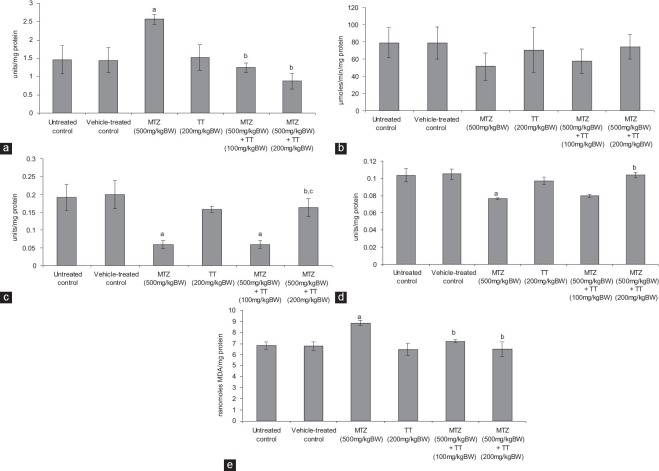
Metronidazole administration resulted in a significant increase in the activity of SOD as compared with the controls [Figure 2a]. By contrast, co-administration of MTZ and 100 mg/kg BW/day as well as 200 mg/kg BW/day of the fruit extract of TT, respectively, caused significant reduction in the activity of SOD as compared with MTZ-treated group, thus attained the values of the controls [Figure 2a].
Figure 2.
Effect of the oral administration of metronidazole (MTZ) (500 mg/kg BW/day) and co-administration of MTZ (500 mg/kg BW/day) and Tribulus terrestris (TT) (100 mg/kg BW/day and 200 mg/kg BW/day) on the (a) activity of superoxide dismutase (b) activity of catalase (c) activity of glutathione peroxidase (d) activity of glutathione reductase and, (e) the level of lipid peroxidation in the testis (values are mean ± standard error of the mean of five animals). aSignificantly different from the controls at P < 0.05 by ANOVA followed by Newman–Keul's multiple range test, bsignificantly different from MTZ (500mg/kg BW/day) at P < 0.05 by ANOVA followed by Newman–Keul's multiple range test, csignificantly different from MTZ (500mg/kg BW/day) co-administered with TT (100 mg/kg BW/day) at P < 0.05 by ANOVA followed by Newman–Keul's multiple range test
Catalase (CAT)
The activity of CAT was decreased following MTZ administration [Figure 2b] though the value was not significantly different as compared with the controls. Co-administration of MTZ and both the doses of the fruit extract of TT, respectively, increased the activity of CAT similar to that of the controls [Figure 2b].
Glutathione peroxidase (GPx)
The activity of GPx was decreased significantly following MTZ administration as compared with the controls [Figure 2c]. Co-administration of MTZ and 100 mg/kg BW/day of the fruit extract of TT failed to recover the activity of GPx as compared with the controls. However, a significant elevation was noticed in its activity in those MTZ-treated mice which were supplemented with 200 mg/kg BW/day of the fruit extract of TT as compared with the MTZ-treated group, thus attained the values of the control [Figure 2c].
Glutathione reductase (GR)
Administration of MTZ caused a significant reduction in the activity of GR comparable to that of the controls [Figure 2d]. Co-administration of MTZ and 100 mg/kg BW/day of the fruit extract of TT did not induce significant alteration, however, 200 mg/kg BW/day of the fruit extract caused a significant increase in the activity of GR as compared with the MTZ-treated group, thus attained the values of the controls [Figure 2d].
Lipid peroxidation (LPO)
Metronidazole administration induced significant increase in the lipid peroxidation (LPO) level, as indicated by the increased activity of MDA, comparable to that of the controls [Figure 2e]. Co-administration of MTZ and both the doses of the fruit extract of TT, respectively, reduced the LPO level similar to that of the controls [Figure 2e].
Testicular Functional Markers
Lactate dehydrogenase (LDH)
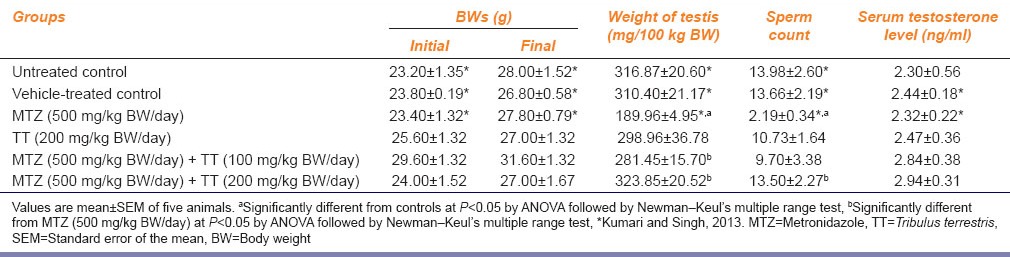
A significantly increased activity of LDH was noted in the testis of the MTZ-treated group as compared with the controls [Figure 3a]. Co-administration of MTZ and both the doses of the fruit extract of TT, respectively, reduced the activity of LDH similar to that of the controls [Figure 3a].
Figure 3.
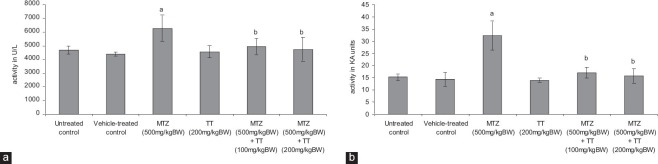
Effect of the oral administration of metronidazole (MTZ) (500 mg/kg BW/day) and co-administration of MTZ (500 mg/kg BW/day) and Tribulus terrestris (100 mg/kg BW/day and 200 mg/kg BW/day) on the (a) activity of lactate dehydrogenase (b) activity of alkaline phosphatase in the testis (values are mean ± standard error of the mean of five animals). aSignificantly different from the controls at P < 0.05 by ANOVA followed by Newman–Keul's multiple range test, bSignificantly different from MTZ (500mg/kg BW/day) at P < 0.05 by ANOVA followed by Newman–Keul's multiple range test
Alkaline phosphatase (ALP)
The activity of testicular ALP was increased significantly in the MTZ-treated group as compared with the controls [Figure 3b]. Co-administration of MTZ and both the doses of the fruit extract of TT, respectively, decreased the activity of ALP similar to that of the controls [Figure 3b].
Discussion
The present study reports the potentiality of TT against MTZ–induced alterations in the testicular weight, spermatogenesis, antioxidant enzyme activity, functional markers, and the epididymal sperm count.
In the present study, no significant alterations were noticed between the initial and final body weights in any of the treated group. However, administration of MTZ resulted in a significant decline in the testicular weight as well as in the epididymal sperm count depicted by regressive histological changes in the seminiferous tubules resulting in the suppression of spermatogenesis. Our earlier findings have already reported spermatogenic inhibition, significant reductions in stages I-VIII and in the number of various types of germ cells of stage VII seminiferous tubules with significant decline in the tubular diameter and reduction in the epididymal sperm count without altering the level of serum testosterone following administration of 500 mg/kg BW/day of MTZ for 28 days.[3]
The findings of the present study indicate the restoration of testicular weight following co-administration of MTZ and 100 mg/kg BW/day and 200 mg/kg BW/day of the fruit extract of TT, respectively. Further, the regressive histological alterations in the seminiferous tubules induced by MTZ administration were almost recovered in the group supplemented with 200 mg/kg BW/day of the fruit extract of TT, thereby enhancing the spermatogenic activity. However, co-administration of MTZ and 100 mg/kg BW/day of the fruit extract of TT ameliorated the regressive changes in the seminiferous tubules only to some extent. The property of TT in enhancing spermatogenic activity may be due to its anabolic action which is reported to increase the testicular profile in the albino rat.[21] The present study revealed that MTZ, co-administered with 100 mg/kg BW/day of the fruit extract of TT failed to recover the sperm count to the control extent, however, a significant increase in the count was evidenced following co-administration of MTZ and 200 mg/kg BW/day of the fruit extract. This marked increase in the sperm count may be due to the beneficial effect of TT which is reported earlier to increase the quality and quantity of spermatozoa due to the presence of an active phytochemical constituent, protodioscin, in the fruit extract.[21] According to Antonio et al.,[22] TT enhances sperm indices by increasing the testosterone level preceded by the increased gonadotropin-releasing hormone which in turn stimulates the production of luteinizing hormone and follicle-stimulating hormone. These gonadotrophins regulate the testicular function by acting on the seminiferous tubules thereby initiating and maintaining the spermatogenesis which in turn affects the quality of spermatozoa and increases the testosterone level. The increased diameter as noticed in the seminiferous tubules following co-administration of MTZ and the fruit extract of TT is reported to be indicative of its androgenic effect on the experimental animals.[23] However, the finding of the present study indicates no significant alteration in the level of serum testosterone in the treated groups.
Edwards et al.[24] have reported that the drugs belonging to nitroimidazole group act through reduction of the nitro group which oxidizes DNA thereby causing DNA strand breaks and subsequently the cell death. MTZ-induced oxidative stress in the testis has been reported by some authors.[8,25] The results of the present study also suggest significant alterations in the activities of testicular antioxidant enzymes indicating the MTZ-induced stress in the testis. Oxidative stress occurs when redox status within the cell is altered towards oxidative conditions. This imbalance may be due to an overproduction of reactive oxygen species or a deficiency in an antioxidant system. The enhanced LPO indicated by increased activity of MDA in the testis of MTZ-treated mice is suggestive of the membrane damage of the germ cells. The antioxidant enzyme activity in a MTZ-treated group indicates an increased activity of SOD and decreased activities of CAT, GPx, and GR. When SOD activity is high, the conversion of superoxide anion (O2−) to hydrogen peroxide (H2O2) is facilitated. High SOD activity in conjunction with low CAT and GPx activity leads to an increased levels of H2O2 and H2O2-derived reactive species like hydroxyl radical (•OH). The LPO destroys the structure of lipid matrix in the membranes of spermatozoa and impairs spermatogenesis.[26] Marked alterations noticed in the activities of SOD, CAT, GR, and GPx in the testis of MTZ-treated mice might have been initiating and propagating the oxidative damage. Subsequently, this oxidative damage might have destroyed most of the testicular germ cells either to the membrane damage or macromolecular degradation, caused by the imbalance of oxidative enzymes. In the present study, an increase in the activities of testicular LDH and ALP has been noticed following MTZ administration. The elevated LDH activity might be due to its leakage in the seminiferous tubular fluid from the germ cells which are damaged by the increased oxidative stress.[27,28] The increase in the ALP activity may also be attributed to the cellular leakage caused by chemical-induced injury of the tissue.[29]
The results of the present study suggest that administration of the fruit extract of TT (200 mg/kg BW/day) in mice prevents the free radical generation caused by MTZ and hence oxidative stress, as evidenced by restoring the levels of antioxidative enzymes and LPO to the control values. Protective effects of TT by inhibiting the free radicals generation against cadmium[7] and cypermethrin[8]-induced testicular damage have been reported in the rat. The whole plant extract of TT is reported to exhibit antioxidant property.[30] Further, the flavonoids present in fruit extract of TT contain the free radicals scavenging properties.[30,31] The present findings suggest that the flavonoids found in the fruit extract of TT might have acted as reductones by donating the electrons and reacted with the free radicals to convert them to more stable product and terminate free radical chain reaction,[32] thus ameliorating the spermatogenic activity and testicular functions, impaired by MTZ-induced oxidative stress.
Conclusion
The present findings, therefore, indicate that only high dose of the fruit extract of TT (200 mg/kg BW/day) restores the MTZ-induced spermatogenic inhibition and reduced epididymal sperm count. The restoring potentiality of the TT against MTZ-induced alterations in the spermatogenesis appears to be due to the presence of antioxidative flavonoids, rather than the steroidal saponins. The findings of the present study, therefore, may assist in the choice of the drugs for longer durations which can be prescribed safely without affecting the fertility potential in the males.
Acknowledgment
The authors are thankful to the Banaras Hindu University, Varanasi, for providing financial assistance through CRET, University Grants Commission, New Delhi to carry out the present study. The first author is also thankful to the University Grant Commission, New Delhi, for providing financial assistance to carry out the present work (No.R/Dev./Sch.(UGC-JRF-211)/S-01/14058).
Footnotes
Source of Support: Nill.
Conflict of Interest: No.
References
- 1.Gauthaman K, Ganesan AP. The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction – An evaluation using primates, rabbit and rat. Phytomedicine. 2008;15:44–54. doi: 10.1016/j.phymed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Singh S, Nair V, Gupta YK. Evaluation of the aphrodisiac activity of Tribulus terrestris Linn. in sexually sluggish male albino rats. J Pharmacol Pharmacother. 2012;3:43–7. doi: 10.4103/0976-500X.92512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumari M, Singh P. Study on the reproductive organs and fertility of the male mice following administration of metronidazole. Int J Fertil Steril. 2013;7:225–38. [PMC free article] [PubMed] [Google Scholar]
- 4.Karbalay-Doust S, Noorafshan A. Ameliorative effects of curcumin on the spermatozoon tail length, count, motility and testosterone serum level in metronidazole-treated mice. Prague Med Rep. 2011;112:288–97. [PubMed] [Google Scholar]
- 5.Ligha AE, Bokolo B, Didia BC. Antifertility potentials of metronidazole in male Wistar rats. Pak J Biol Sci. 2012;15:224–30. doi: 10.3923/pjbs.2012.224.230. [DOI] [PubMed] [Google Scholar]
- 6.Raji Y, Kunle-Alabi OT, Olaleye SB, Gbadegesin MA, Awobajo FO, Osonuga OA, et al. Impact of α-tocopherol on metronidazole and tetracycline-induced alterations in reproductive activities of male albino rats. J Biol Sci. 2007;7:41–6. [Google Scholar]
- 7.Rajendar B, Bharavi K, Rao GS, Kishore PV, Kumar PR, Kumar CS, et al. Protective effect of an aphrodisiac herb Tribulus terrestris Linn on cadmium-induced testicular damage. Indian J Pharmacol. 2011;43:568–73. doi: 10.4103/0253-7613.84974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, Huq AU, Singh R. Cypermethrin induced reproductive toxicity in male Wistar rats: Protective role of Tribulus terrestris. J Environ Biol. 2013;34:857–62. [PubMed] [Google Scholar]
- 9.Hussain AA, Muhammad AA, Ibrahim HH, Abbas AH. Study of the biological activities of Tribulus terrestris extracts. World Acad Sci Eng Technol. 2009;57:433–5. [Google Scholar]
- 10.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 11.New York, Cambridge: Cambridge University Press; 2010. World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction. [Google Scholar]
- 12.Hess RA, de Franca LR. Spermatogenesis and cycle of seminiferous epithelium. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Texas: Landes Bioscience and Springer Science Business Media; 2008. pp. 1–15. [Google Scholar]
- 13.Russell LD, Ettlin RA, Sinha HA, Clegg ED. Vienna, IL: Cache River Press; 1990. Histological and Histopathological Evaluation of the Testis. [Google Scholar]
- 14.Vaithinathan S, Saradha B, Mathur PP. Methoxychlor-induced alteration in the levels of HSP70 and clusterin is accompanied with oxidative stress in adult rat testis. J Biochem Mol Toxicol. 2009;23:29–35. doi: 10.1002/jbt.20262. [DOI] [PubMed] [Google Scholar]
- 15.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 16.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 17.Claiborne A. Catalase activity. In: Greenwald R, editor. CRC Handbook of Methods for Oxygen Radical Research. Boca Raton, FL: CRC Press; 1985. pp. 283–4. [Google Scholar]
- 18.Flohe L, Gunzler WA. Assay of glutathione peroxidase. In: Packer L, editor. Methods Enzymol. Vol. 105. New York: Academic Press; 1984. pp. 114–21. [DOI] [PubMed] [Google Scholar]
- 19.Carlberg I, Mannervick B. Glutathione reductase. Methods Enzymol. 1955;2:722–5. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 20.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 21.Singh PK, Singh AP, Gupta AK, Chaudhary S. Beneficial effects of aqueous fruit extract of Tribulus terrestris on testicular and serum biochemistry of albino rats. J Ecophysiol Occup Health. 2009;9:217–23. [Google Scholar]
- 22.Antonio J, Uelmen J, Rodriguez R, Earnest C. The effects of Tribulus terrestris on body composition and exercise performance in resistance-trained males. Int J Sport Nutr Exerc Metab. 2000;10:208–15. doi: 10.1123/ijsnem.10.2.208. [DOI] [PubMed] [Google Scholar]
- 23.Bashir A, Tahir M, Waqas S, Munir B. Effects of Tribulus terrestris on testicular development of immature albino rats. Biomedica. 2009;25:63–8. [Google Scholar]
- 24.Edwards DI, Tocher JH, Dale LD, Widdick DA, Virk NS. Effects on DNA of bioreducible nitroimidazole and benzotriazine drugs. In: Adams GE, Breccia A, Feilden M, Wardman P, editors. Selective Activation of Drugs by Redox Processes. Vol. 198. NY, USA: Plenum Press, NATO Advanced Study Institutes Series; 1990. pp. 275–83. [Google Scholar]
- 25.Ligha AE, Paul CW. Oxidative effect of metronidazole on the testis of Wistar rats. Aust J Basic Appl Sci. 2011;5:1339–44. [Google Scholar]
- 26.Said TM, Aziz N, Sharma RK, Lewis-Jones I, Thomas AJ, Jr, Agarwal A. Novel association between sperm deformity index and oxidative stress-induced DNA damage in infertile male patients. Asian J Androl. 2005;7:121–6. doi: 10.1111/j.1745-7262.2005.00022.x. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee BD, Seth V, Bhattacharya A, Pasha ST, Chakraborty AK. Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol Lett. 1999;107:33–47. doi: 10.1016/s0378-4274(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 28.El-Khawaga OA. Role of selenium on antioxidant capacity in methomyl-treated mice. J Physiol Biochem. 2005;61:501–6. doi: 10.1007/BF03168375. [DOI] [PubMed] [Google Scholar]
- 29.Manawadi SL, Kaliwal BB. Methomyl induced gonadal dusfunction, biochemical contents and enzyme activities in male albino mice. Int J Biotechnol Appl. 2010;10:20–32. [Google Scholar]
- 30.Mitra N, Mohammad-Mehdi D, Mohammad Reza Z. Tribulus terrestris L. (Zygophyllaceaa) flavonoid compounds. Int J Mod Bot. 2012;2:35–9. [Google Scholar]
- 31.Zheleva-Dimitrova D, Obreshkova D, Nedialkov P. Antioxidant activity of Tribulus terrestris – A natural product in infertility therapy. Int J Pharm Pharm Sci. 2012;4:508–11. [Google Scholar]
- 32.Patel S, Patel T, Parmar K, Patel B, Patel P. Evaluation of antioxidant activity, phenol and flavonoid contents of Momordica charantia Linn fruit. Adv Res Pharmacol Biol. 2011;1:120–9. [Google Scholar]