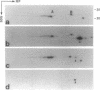
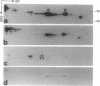
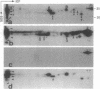
Abstract
For identification of Rab, Rac, Rho, Ral, Rap, and Arf proteins on two-dimensional polyacrylamide gels, we have expressed full-length cDNAs of members of these protein families with the T7 RNA polymerase-recombinant vaccinia virus expression system. Membrane preparations from cells expressing the cDNAs were subjected to high-resolution two-dimensional polyacrylamide gel electrophoresis followed by [alpha-32P]GTP ligand blotting. We have mapped 28 small GTP-binding proteins relative to their isoelectric points and according to their molecular weights and by immunoblotting with specific antibodies. Rab and Rho proteins could be specifically identified by extraction of streptolysin O-permeabilized Madin-Darby canine kidney (MDCK) cells with Rab- and Rho-GDP dissociation inhibitor. We applied the reference mapping to analyze the GTP-binding patterns of synaptosome fractions from rat brain. The purified synaptosomes exhibited specific enrichment of Rab3a, Rab5a, Ral, and several other GTPases. This approach and the map we have produced should provide a useful aid for the analysis of the expression and localization of members of all families of small GTP-binding proteins in various cell types and subcellular fractions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokoch G. M., Der C. J. Emerging concepts in the Ras superfamily of GTP-binding proteins. FASEB J. 1993 Jun;7(9):750–759. doi: 10.1096/fasebj.7.9.8330683. [DOI] [PubMed] [Google Scholar]
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Rasmussen H. H., Olsen E., Madsen P., Leffers H., Honoré B., Dejgaard K., Gromov P., Hoffmann H. J., Nielsen M. The human keratinocyte two-dimensional gel protein database: update 1993. Electrophoresis. 1993 Nov;14(11):1091–1198. doi: 10.1002/elps.11501401178. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Simons K., Zerial M. The complexity of the Rab and Rho GTP-binding protein subfamilies revealed by a PCR cloning approach. Gene. 1992 Mar 15;112(2):261–264. doi: 10.1016/0378-1119(92)90387-5. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Vingron M., Sander C., Simons K., Zerial M. Molecular cloning of YPT1/SEC4-related cDNAs from an epithelial cell line. Mol Cell Biol. 1990 Dec;10(12):6578–6585. doi: 10.1128/mcb.10.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. T., Holcomb C., Moore H. P. Expression and localization of two low molecular weight GTP-binding proteins, Rab8 and Rab10, by epitope tag. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6508–6512. doi: 10.1073/pnas.90.14.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti C. G., Sullivan C. A., Banker G. A. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988 Apr;8(4):1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink L. A., Anzai K., Scheller R. H. rab15, a novel low molecular weight GTP-binding protein specifically expressed in rat brain. J Biol Chem. 1992 Mar 25;267(9):5768–5775. [PubMed] [Google Scholar]
- Fischer von Mollard G., Mignery G. A., Baumert M., Perin M. S., Hanson T. J., Burger P. M., Jahn R., Südhof T. C. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K., Schreyer D. J., Skene J. H., Banker G. Development of neuronal polarity: GAP-43 distinguishes axonal from dendritic growth cones. Nature. 1988 Dec 15;336(6200):672–674. doi: 10.1038/336672a0. [DOI] [PubMed] [Google Scholar]
- Goud B., Zahraoui A., Tavitian A., Saraste J. Small GTP-binding protein associated with Golgi cisternae. Nature. 1990 Jun 7;345(6275):553–556. doi: 10.1038/345553a0. [DOI] [PubMed] [Google Scholar]
- Hall A. Ras-related proteins. Curr Opin Cell Biol. 1993 Apr;5(2):265–268. doi: 10.1016/0955-0674(93)90114-6. [DOI] [PubMed] [Google Scholar]
- Huber L. A., Beug H., Simons K., Reichmann E. Two-dimensional gel mapping of small GTPases reveals transformation-specific changes during oncogenesis. Electrophoresis. 1994 Mar-Apr;15(3-4):469–473. doi: 10.1002/elps.1150150164. [DOI] [PubMed] [Google Scholar]
- Huber L. A., Peter M. E. Mapping small GTP-binding proteins on high-resolution two-dimensional gels by a combination of GTP binding and labeling with in situ periodate-oxidized GTP. Electrophoresis. 1994 Feb;15(2):283–288. doi: 10.1002/elps.1150150148. [DOI] [PubMed] [Google Scholar]
- Huber L. A., Pimplikar S., Parton R. G., Virta H., Zerial M., Simons K. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol. 1993 Oct;123(1):35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G. J., Frutiger S., Paquet N., Pasquali C., Sanchez J. C., Tissot J. D., Bairoch A., Appel R. D., Hochstrasser D. F. Human liver protein map: update 1993. Electrophoresis. 1993 Nov;14(11):1216–1222. doi: 10.1002/elps.11501401181. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A., Kuroda S., Sasaki T., Kotani K., Hirata K., Katayama M., Takai Y. Functional interactions of stimulatory and inhibitory GDP/GTP exchange proteins and their common substrate small GTP-binding protein. J Biol Chem. 1992 Jul 25;267(21):14611–14615. [PubMed] [Google Scholar]
- Lapetina E. G., Reep B. R. Specific binding of [alpha-32P]GTP to cytosolic and membrane-bound proteins of human platelets correlates with the activation of phospholipase C. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2261–2265. doi: 10.1073/pnas.84.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi D., Soldati T., Riederer M. A., Goda Y., Zerial M., Pfeffer S. R. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J. 1993 Feb;12(2):677–682. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke A., Jansson S., Parton R. G., Chavrier P., Valencia A., Huber L. A., Lehtonen E., Zerial M. Rab17, a novel small GTPase, is specific for epithelial cells and is induced during cell polarization. J Cell Biol. 1993 May;121(3):553–564. doi: 10.1083/jcb.121.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maridonneau-Parini I., de Gunzburg J. Association of rap1 and rap2 proteins with the specific granules of human neutrophils. Translocation to the plasma membrane during cell activation. J Biol Chem. 1992 Mar 25;267(9):6396–6402. [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981 Dec;91(3 Pt 1):601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y., Kikuchi A., Araki S., Hata Y., Kondo J., Teranishi Y., Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for smg p25A, a ras p21-like GTP-binding protein. Mol Cell Biol. 1990 Aug;10(8):4116–4122. doi: 10.1128/mcb.10.8.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli M., Takei K., Cameron R., Hurlbut P., Johnston P. A., Südhof T. C., Jahn R., De Camilli P. Association of Rab3A with synaptic vesicles at late stages of the secretory pathway. J Cell Biol. 1991 Nov;115(3):625–633. doi: 10.1083/jcb.115.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Brennwald P. Friends and family: the role of the Rab GTPases in vesicular traffic. Cell. 1993 Nov 19;75(4):597–601. doi: 10.1016/0092-8674(93)90478-9. [DOI] [PubMed] [Google Scholar]
- Olkkonen V. M., Dupree P., Killisch I., Lütcke A., Zerial M., Simons K. Molecular cloning and subcellular localization of three GTP-binding proteins of the rab subfamily. J Cell Sci. 1993 Dec;106(Pt 4):1249–1261. doi: 10.1242/jcs.106.4.1249. [DOI] [PubMed] [Google Scholar]
- Olkkonen V. M., Peterson J. R., Dupree P., Lütcke A., Zerial M., Simons K. Isolation of a mouse cDNA encoding Rab23, a small novel GTPase expressed predominantly in the brain. Gene. 1994 Jan 28;138(1-2):207–211. doi: 10.1016/0378-1119(94)90809-5. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R. GTP-binding proteins in intracellular transport. Trends Cell Biol. 1992 Feb;2(2):41–46. doi: 10.1016/0962-8924(92)90161-f. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Simons K., Zerial M. Rab proteins and the road maps for intracellular transport. Neuron. 1993 Nov;11(5):789–799. doi: 10.1016/0896-6273(93)90109-5. [DOI] [PubMed] [Google Scholar]
- Stearns T., Willingham M. C., Botstein D., Kahn R. A. ADP-ribosylation factor is functionally and physically associated with the Golgi complex. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1238–1242. doi: 10.1073/pnas.87.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-Mortimer O., Clague M. J., Huber L. A., Chavrier P., Gruenberg J., Gorvel J. P. The N-terminal domain of a rab protein is involved in membrane-membrane recognition and/or fusion. EMBO J. 1994 Jan 1;13(1):34–41. doi: 10.1002/j.1460-2075.1994.tb06232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchot N., Chardin P., Tavitian A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: molecular cloning of YPT-related cDNAs from a rat brain library. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8210–8214. doi: 10.1073/pnas.84.23.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. C., Adamik R., Tsuchiya M., Chang P. P., Moss J., Vaughan M. Differential expression during development of ADP-ribosylation factors, 20-kDa guanine nucleotide-binding protein activators of cholera toxin. J Biol Chem. 1991 May 5;266(13):8213–8219. [PubMed] [Google Scholar]
- Ullrich O., Stenmark H., Alexandrov K., Huber L. A., Kaibuchi K., Sasaki T., Takai Y., Zerial M. Rab GDP dissociation inhibitor as a general regulator for the membrane association of rab proteins. J Biol Chem. 1993 Aug 25;268(24):18143–18150. [PubMed] [Google Scholar]
- Urbé S., Huber L. A., Zerial M., Tooze S. A., Parton R. G. Rab11, a small GTPase associated with both constitutive and regulated secretory pathways in PC12 cells. FEBS Lett. 1993 Nov 15;334(2):175–182. doi: 10.1016/0014-5793(93)81707-7. [DOI] [PubMed] [Google Scholar]
- Valencia A., Chardin P., Wittinghofer A., Sander C. The ras protein family: evolutionary tree and role of conserved amino acids. Biochemistry. 1991 May 14;30(19):4637–4648. doi: 10.1021/bi00233a001. [DOI] [PubMed] [Google Scholar]
- Volknandt W., Pevsner J., Elferink L. A., Scheller R. H. Association of three small GTP-binding proteins with cholinergic synaptic vesicles. FEBS Lett. 1993 Feb 8;317(1-2):53–56. doi: 10.1016/0014-5793(93)81490-q. [DOI] [PubMed] [Google Scholar]
- Zahraoui A., Touchot N., Chardin P., Tavitian A. The human Rab genes encode a family of GTP-binding proteins related to yeast YPT1 and SEC4 products involved in secretion. J Biol Chem. 1989 Jul 25;264(21):12394–12401. [PubMed] [Google Scholar]
- Zerial M., Stenmark H. Rab GTPases in vesicular transport. Curr Opin Cell Biol. 1993 Aug;5(4):613–620. doi: 10.1016/0955-0674(93)90130-i. [DOI] [PubMed] [Google Scholar]
- van der Sluijs P., Hull M., Huber L. A., Mâle P., Goud B., Mellman I. Reversible phosphorylation--dephosphorylation determines the localization of rab4 during the cell cycle. EMBO J. 1992 Dec;11(12):4379–4389. doi: 10.1002/j.1460-2075.1992.tb05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs P., Hull M., Webster P., Mâle P., Goud B., Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992 Sep 4;70(5):729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]