Abstract
A recent randomized trial (TOPPS) compared prophylactic platelet transfusions (for counts <10×109/L) with a strategy of no-prophylaxis in adults with hematologic malignancies. Seventy percent of enrolled patients received an autologous hematopoietic stem cell transplant. Statistical models were developed to explore which patient factors or clinical characteristics are important prognostic factors for bleeding. These models were presented for baseline characteristics and for recurrent analysis of bleeding to assess the risks of World Health Organization grade 2–4 bleeding on any given day. Additional analyses explored the importance of fever. Treatment plan (chemotherapy/allogeneic hematopoietic stem cell transplant), female sex, and treatment arm (no-prophylaxis) were significantly associated with an increased number of days of bleeding. The number of days with a platelet count <10×109/L was significantly associated with a grade 2–4 bleed (P<0.0001). Patients with a temperature of at least 38°C had the highest hazard of a grade 2–4 bleed (hazard ratio: 1.7, 95% confidence interval: 1.3 to 2.4, compared with the risk in patients with a temperature <37.5°C). There was no evidence that minor bleeding predicted a grade 2–4 bleed. The results highlighted the limited role of correction of thrombocytopenia by platelet transfusion in reducing the risk of bleeding. Clinically stable patients undergoing autologous hematopoietic stem cell transplantation had the lowest risk of bleeding and benefited least from prophylactic platelet transfusions. Prospective studies are required to address the usefulness of risk factors to support better targeted platelet transfusions. TOPPS Controlled-Trials.com number ISRCTN08758735.
Introduction
Thrombocytopenia is a common complication of hematologic malignancies or their treatment and many patients experience clinically significant bleeding during these periods of thrombocytopenia. Despite the use of prophylactic platelet transfusions, bleeding remains an important complication. In a large platelet dose trial, within a 30-day period, up to 70% of patients developed clinically significant bleeding [World Health Organization (WHO) grade 2–4] and up to 10% had severe/life-threatening hemorrhage (WHO grade 3–4).1 Comparable rates of bleeding were found in two recent trials that assessed the role of prophylactic platelet transfusions.2,3 Our current management of these patients to prevent bleeding is therefore sub-optimal and to improve it we need to understand better which patients are at higher or lower risk of bleeding.
Relationships between the severity of thrombocytopenia in predicting bleeding and the role of platelet transfusions in preventing bleeding remain unclear. In a retrospective study. Freidmann et al.4 identified clinical characteristics such as previous transplantation as more significant determinants of bleeding risk than platelet count. A retrospective analysis of a much earlier platelet threshold trial5,6 suggested that occurrence of lower grades of bleeding might predict higher grades of bleeding. However, comparisons of rates of bleeding between different clinical studies are limited by the different methods used to record and grade bleeding.7,8
The recent prospective TOPPS trial (Trial of Prophylactic vs. No-Prophylactic Platelet Transfusions in Patients with Hematological Malignancies) provides a resource to explore to what degree different patient factors or clinical characteristics are important prognostic factors for bleeding.2 However, analyzing bleeding data can be challenging due to factors such as multiple bleeding events per patient. In a post hoc analysis of data published by Rebulla et al.,6 Cook et al.9 demonstrated a 12% increase in the risk of bleeding in patients receiving platelet transfusions at a threshold of 10×109/L versus 20×109/L, although the 95% confidence intervals (95% CI) were wide (0.69 to 1.83), and suggested that the original study may have been underpowered to test for significant differences between treatment arms. Cook et al.9 discussed different analytical measures and proposed that a recurrent event analysis may be a more robust method to analyze bleeding data over time. This type of analysis allows the investigation of all bleeding data that are available, rather than just focusing on measures such as rates of bleeding in patients or time to first bleeding event.
This study is a further analysis of the TOPPS trial data and assesses patients’ baseline characteristics at enrolment, burden of thrombocytopenia, presence of fever and presence of minor hemorrhage as potential risk factors for WHO grade 2 to 4 bleeding. Recurrent event analyses have been used to allow assessment of bleeding over the 30-day trial period and to account for multiple bleeds per patient.
Methods
Details of the UK and Australian TOPPS randomized trial have been published previously.2,8,10 Six hundred patients were randomized to receive either prophylactic platelet transfusions if the platelet count was <10×109/L (n=299), or no prophylaxis (n=301). The trial was approved by independent ethics committees. Although the median number of days with complete data was 30, bleeding data were not complete for all 30 days for all patients.2 The vast majority of bleeds were inpatient bleeds8 and, as incomplete records often related to when the patient was at home, no bleeding was assumed on days for which no data were reported.
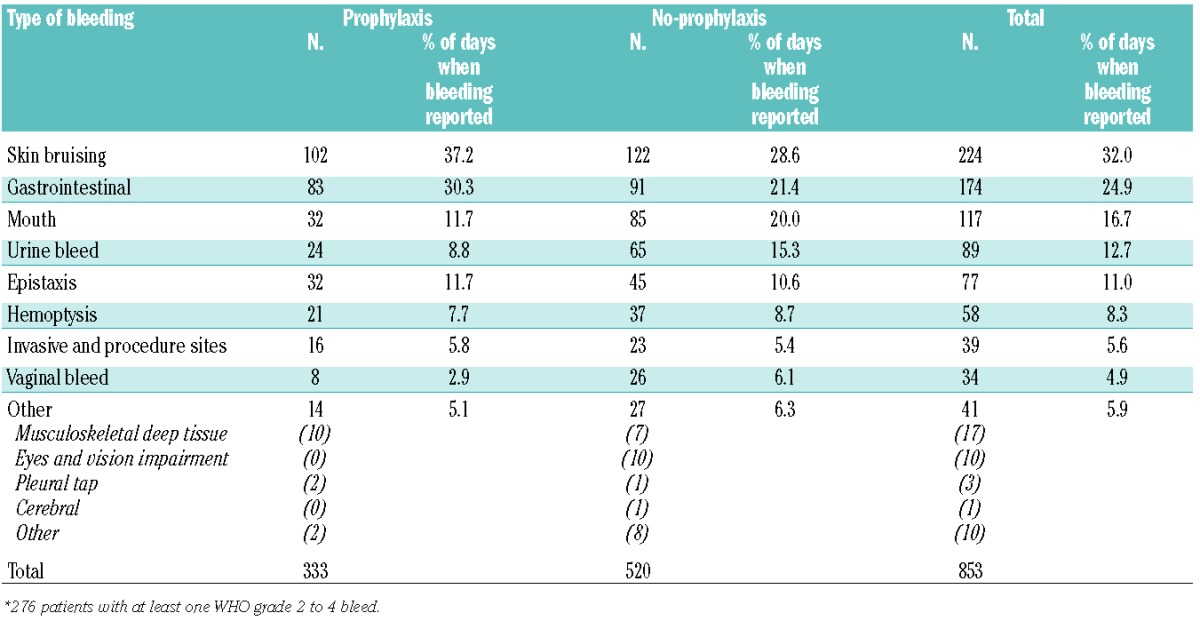
Bleeding types were grouped to describe characteristics of bleeding: skin bruising included petechiae, purpura, and bruising; gastrointestinal bleeding included blood in the stool, melena and hematemesis; mouth bleeding included oral blood blisters and oropharyngeal bleeding; and other included musculoskeletal or deep tissue bleeding, eyes and visual impairment, pleural tap, and cerebral bleeds.
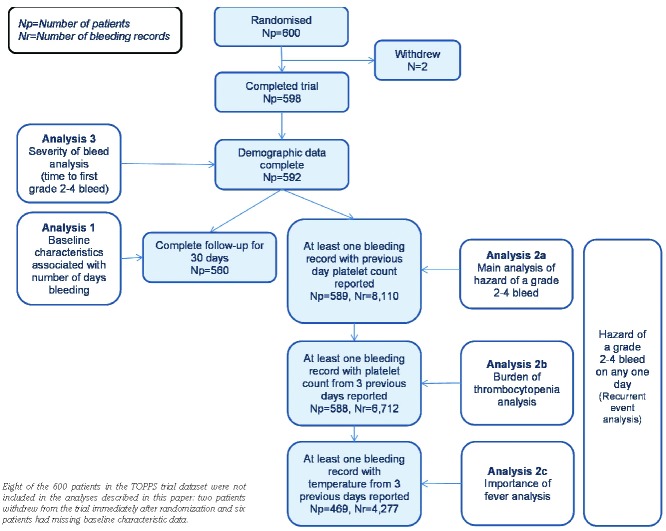
For the modeling (Figure 1), patients were grouped by treatment plan [autologous hematopoietic stem cell transplant (HSCT) and chemotherapy (induction and consolidation)/allogeneic HSCT (myeloablative and reduced intensity)] and diagnosis [acute myeloid leukemia (AML), myeloma, lymphoma and other diagnoses]. In contrast to the TOPPS analysis, only the first day of consecutive bruising or petechiae was counted as a bleed, unless bleeding on the subsequent day was worse.
Figure 1.
Shows the number of patients (Np) and number of bleeding records (Nr) included in each part of the analysis.
Modeling analysis
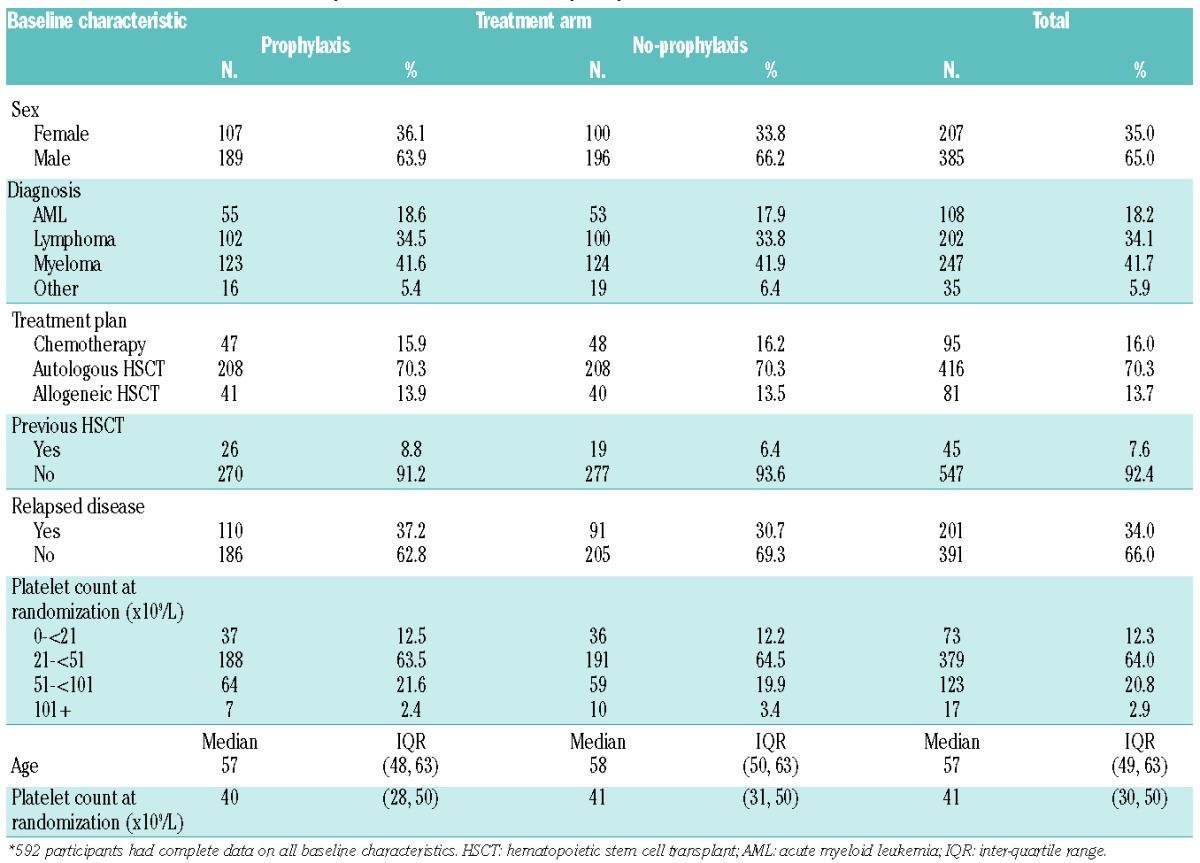
Negative binomial regression11 was used to model baseline characteristics associated with the number of days of grade 2–4 bleeding (analysis 1). The risk factors considered are shown in Table 1. Previous HSCT (defined as any prior transplant) and relapsed disease were also considered.
Table 1.
Baseline characteristics of the patients included in the analysis by treatment arm.*
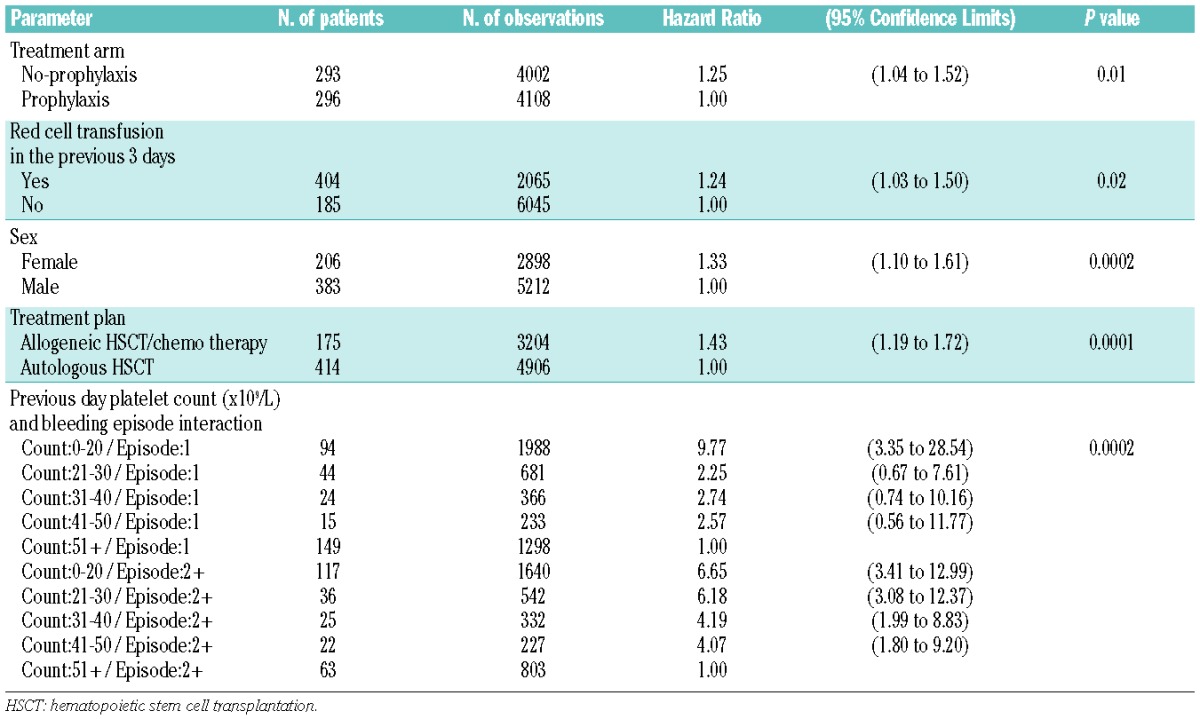
Recurrent event analysis12 was used to model the hazard of a grade 2–4 bleed on any one day, accounting for previous bleeds (analyses 2a, 2b and 2c). Robust sandwich variance estimates12 were used for the hazard confidence intervals. Analysis 2a was the main recurrent event analysis. Analysis 2b was performed on a subset of bleeding records in which the platelet count from all 3 previous days was reported. Analysis 2c was a further subset analysis in which patients’ temperature from all 3 previous days was investigated. Risk factors considered were baseline characteristics plus red cell transfusion in the preceding 1 or 3 days, previous day platelet count and previous day minor bleed.
Cox proportional hazards regression was used to investigate whether a minor bleed (grade 1) predicted a more severe bleed. The association between minor and major bleeds was also investigated as part of the recurrent event analysis.
Sensitivity analyses
Analyses were repeated excluding skin bleeds (the most common type of bleed, often considered of lesser clinical significance) and vaginal bleeds (due to the different results for sex) to explore whether these bleeding types were particularly influential on the models. Hazard ratios (HR) were compared across the cohorts to ensure consistency. Further analyses were performed to check the different assumptions on skin bleed duration.
Analyses were undertaken using SAS version 9 (SAS Institute Inc., Cary, NC, USA). A P-value less than 0.05 was considered statistically significant.
Results
Baseline characteristics
Demographic and other data for patients in the modeling dataset are shown in Table 1. The majority (66%) of patients did not have relapsed disease and this percentage was similar across the treatment arms. Forty-five patients (8%) had had a previous HSCT and 13 patients (2%) had had a previous fungal infection. The median number of days with a grade 2–4 bleed was 0 days (IQR 0 to 1).
Characteristics of bleeding
For the 592 patients whose baseline characteristics were reported, 16,982 daily bleeding records were analyzed. A grade 2–4 bleed was reported in 700 (4%) of the daily bleeding records. Six hundred ninety-one (98.7%) of the grade 2–4 bleeds were grade 2. In total, 276 of the 592 (47%) patients had at least one grade 2–4 bleed; 140 (51%) of the 276 patients who had a grade 2–4 bleed had more than one bleed and 94 (34%) had three or more bleeds.
The types of bleed reported are shown in Table 2. Skin bruising was the most common type of bleed, reported on 32% of days when bleeding was reported to have occurred. The next most common type was gastrointestinal bleeding.
Table 2.
Sites and characteristics of the grade 2 to 4 bleeds by treatment arm.*
Baseline characteristics associated with the number of days of bleeding (analysis 1)
Baseline data were complete for 592 patients and 560 of these patients had complete follow-up data for the 30 days of the study; 256 (46%) of the 560 patients had at least one grade 2–4 bleed in the 30 days. Of the grade 2–4 bleeds, 97.3% were grade 2 bleeds.
The results of the multivariate modeling of baseline characteristics associated with number of days of bleeding are shown in Table 3. Treatment plan, sex and treatment arm were all found to be significantly associated with the number of days of bleeding. Female patients, allograft or chemotherapy recipients and patients in the no-prophylaxis arm had the highest incidence of grade 2–4 bleeding. There was also some borderline significant evidence that patients who hah had a previous HSCT were more likely to have a significant bleed (P=0.06).
Table 3.
Results of the multivariate modeling of baseline characteristics associated with the number of days of bleeding over the 30 days.
The model in Table 3 was also run separately for subgroups of patients undergoing autologous HSCT or allogeneic HSCT/chemotherapy. The findings were not different from those of the overall model (results not shown).
Hazard of a grade 2–4 bleed on any given day (analysis 2a)
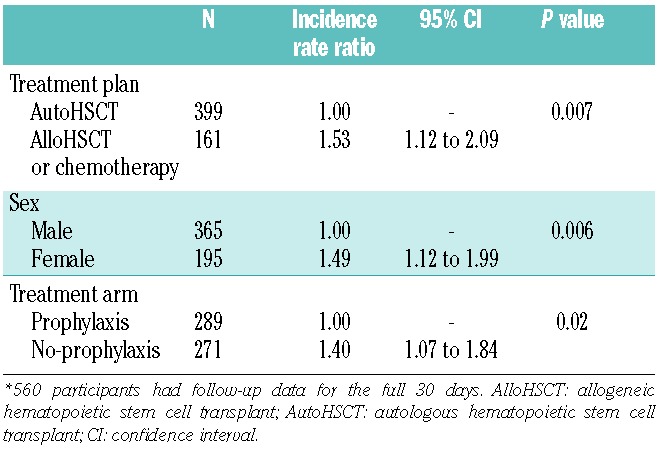
This analysis investigated factors associated with the hazard of a grade 2–4 bleed on any one day using a recurrent event analysis, which does not require follow-up data for the full 30 days. The dataset was restricted to 8,110 bleeds occurring in the 589 patients whose platelet count from the preceding day and any red cell transfusions from the preceding 3 days were recorded.
Patients in the no-prophylaxis arm of the study, patients who had received a red cell transfusion in the preceding 3 days, female patients and allogeneic HSCT/ chemotherapy recipients had the highest hazards of a grade 2–4 bleed. A significant association between platelet count on the previous day and bleeding episode (first bleed or subsequent bleed) was also found; patients with lower platelet counts on the preceding day had a higher hazard of a grade 2–4 bleed, particularly for subsequent bleeds (Table 4).
Table 4.
Multivariate recurrent event analysis (n = 598 patients).
The duration of severe thrombocytopenia (analysis 2b)
The relevance to grade 2–4 bleeding of a low platelet count over the preceding 3 days was investigated (n=6,712 bleeding records, patients=588). There were 442 observations for which the platelet count was less than 10×109/L on each of the preceding 3 days, 1,820 observations for which the platelet counts were all less than 20×109/L and 5,227 observations for which the platelet counts were all less than 50×109/L.
After adjusting for platelet count on the previous day and the other significant factors (treatment arm, red cell transfusion in the preceding 3 days, sex and treatment plan), there was evidence of an association with bleeding and a platelet count below 10×109/L for each of the preceding 3 days (hazard ratio 1.5, 95% CI: 1.1 to 2.0, P=0.009). No interaction between treatment arm and platelet count below 10×109/L was found, indicating that this result was not due to the treatment arm.
To investigate this further, the number of days (out of the previous three) on which the platelet count was below 10×109/L was considered in the modeling. After adjusting for the significant factors, the number of days with a count below 10×109/L was found to be significantly associated with a grade 2–4 bleed (P<0.0001) and there was a significant interaction with treatment plan (P=0.005). The hazard of a grade 2–4 bleed increased with the number of days with a platelet count <10×109/L for allogeneic HSCT or chemotherapy patients. In contrast, the hazard did not change over time for autologous HSCT patients, or even showed a (non-significant) tendency to decrease over the 3 days (Figure 2).
Figure 2.
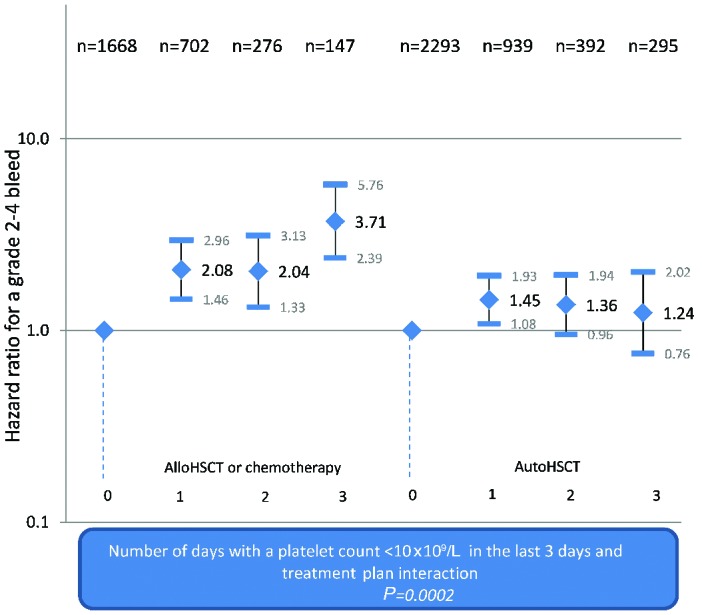
Risk-adjusted hazard ratios for grade 2–4 bleeds according to the number of days with a platelet count <10×109/L by treatment plan.
There was no significant association between bleeding and a platelet count below 20×109/L or 50×109/L in the preceding 3 days.
The relevance of fever to grade 2–4 bleeding (analysis 2c)
This analysis investigated the relevance of the patient’s highest temperature in the preceding 3 days (n=4,277 bleeding records, patients=469) to the risk of a grade 2–4 bleed on any given day. After adjusting for the factors shown in Table 4, a significant association between patient’s highest temperature and the hazard of grade 2–4 bleeding was found (P=0.03): patients with a temperature of at least 38°C had the highest hazard of a grade 2–4 bleed (HR: 1.7, 95% CI: 1.3 to 2.4, compared to those with a temperature <37.5°C).
Severity of bleeding (analysis 3)
Of the 592 patients, 492 (83%) had at least one minor (grade 1) bleed at some point in the 30 days. Two hundred seventy-six patients had a grade 2–4 bleed and 155 (56%) of these had at least one minor bleed first. If minor bleeds that occurred after a major bleed were ignored (n=93), 39% (155/399) of the patients who had a minor bleed had a subsequent major bleed compared to 63% (122/193) of patients who had no minor bleed.
There was no evidence that minor bleeding predicted a grade 2–4 bleed in either the Cox proportional hazards model (analysis 3) which modeled time to first grade 2–4 bleed, or the risk-adjusted (adjusted for the factors shown in Table 4) recurrent event analysis (analysis 2a) which modeled whether a minor bleed on the previous day predicted a more severe bleed on the following day. In fact, the Cox model showed that patients who did not experience a minor bleed had a higher hazard of a grade 2–4 bleed [P<0.0001, HR: 2.4 (1.9–3.0)].
Sensitivity analyses
All models were re-run after removing vaginal-only bleeds (n=24 bleeding records) and also after removing skin-only bleeds (n=137 in the baseline characteristics model and n=152 in other models). The models were similar after excluding these bleeds. However, there was some evidence of an association with previous HSCT and bleeding after removing the skin-only bleeds in the baseline characteristics model (analysis 1), in the main recurrent event analysis (analysis 2a) and in the “importance of fever” (analysis 2c) model; patients who had had a previous HSCT had a higher incidence of grade 2–4 bleeds in analysis 1 and a higher hazard of a grade 2–4 bleed in analyses 2a and 2c (P=0.04, P=0.02 and P=0.002, respectively). Previous HSCT was not significant in the “duration of severe thrombocytopenia” (analysis 2b) model (P=0.3).
The severity of bleeding analyses (analysis 2a and 3) were also repeated after re-classifying the 152 skin-only bleeds as grade 1 and the results did not change: there was no evidence of minor (grade 1) bleeding predicting grade 2–4 bleeding. This analysis was undertaken to be consistent with the methodology of Webert et al. (see discussion).5
The hazard ratios for the main recurrent event analysis model were comparable to those for the “burden of thrombocytopenia” (analysis 2b) and “importance of fever” (analysis 2c) datasets. Counting the first of consecutive days of skin bleeding produced comparable results to counting each day of skin bleeding separately.
Discussion
There is increasing interest in developing risk-adapted policies for prophylactic platelet transfusions, and a better understanding of risk factors could be used to identify patients at greater or lesser risk of bleeding, and hence more or less likely to derive benefit from platelet transfusions. Statistical models were developed and applied in this study to characterize risk factors for bleeding as well as the importance of fever. The source dataset was the TOPPS trial which showed a small but significant benefit from prophylactic platelet transfusions, which may vary in different subgroups of patients.2,10 A recurrent event analysis model was developed to present information on the overall burden of bleeding.12
The results of the analyses described here confirmed the relevance of baseline characteristics in predicting subsequent bleeding. There were differences between autologous HSCT versus allogeneic HSCT/chemotherapy patients. In addition to treatment plan, treatment arm (prophylaxis versus no prophylaxis) and sex were also found to be significantly associated with the numbers of days of bleeding. The reasons why female patients consistently appeared to have a higher risk of bleeding is not clear. In our models, female sex remained statistically significant after removing vaginal-only bleeds. Women have higher levels of factor VIII, von Willebrand factor and fibrinogen than men but there is no evidence of a difference in the amount of fibrinolytic activity.13–15 Of interest, gender differences have been described in other clinical settings.16
The severity of thrombocytopenia was identified as a risk factor for bleeding. This analysis also investigated the burden or duration of severe thrombocytopenia, and patients with a lower platelet count on the previous day had a higher hazard of a grade 2 to 4 bleed. Of note, number of days with a platelet count below 10×109/L was significantly associated with a grade 2 to 4 bleed, suggesting a difference in the clinical implications between isolated days with severe thrombocytopenia and the cumulative effect of more prolonged periods with severe thrombocytopenia. In our analysis, there was no evidence of minor (grade 1) bleeding predicting a grade 2 to 4 bleed. Our result appears in contrast to the findings of Webert et al.5 However, these authors applied a different classification of bleeding, in which grade 1 bleeding included all skin bleeds. We, therefore, undertook sensitivity analyses in our models when excluding skin-only bleeds and also when classifying all skin-only bleeds in the TOPPS dataset as grade 1. The results were unchanged, indicating no association between a minor bleed on one day and a major bleed on the next.
In our model, a significant association between highest temperature and a hazard of a grade 2 to 4 bleed was seen.5 Many national guidelines have indicated that higher platelet count thresholds for transfusion may be appropriate in patients with sepsis or fever, although this recommendation has rarely been systematically evaluated.17,18 In support of the recommendation, recent studies using animal models have suggested that inflammation in conjunction with severe thrombocytopenia is a key determinant of bleeding risk.19
There are a number of limitations to the results presented here, as well as those common to post hoc analyses. The largest group of enrolled patients were those receiving autografts and therefore the focus of any implications of this study should primarily apply to this subgroup. The modeling analysis was restricted to factors collected as part of the main trial, and therefore the contributions of other factors such as those related to treatment intensity or supportive care could not be explored. There is uncertainty regarding the clinical significance of all grades of skin bleeding (which was the most common type of bleeding in the trial), although the main results for statistical models when re-run after removing skin-only bleeds were similar. Additionally, a significant association was found between previous HSCT and bleeding when the skin-only bleeds were removed, in line with the findings of Freidmann et al., whose analysis included only WHO grade 3 and 4 bleeding.4
With the limited resources available, data on fever were only collected on a large but not full subset of in-patients involved in the TOPPS trial. For the TOPPS trial as a whole, data completeness, including that for the main bleeding outcomes, was very high and sensitivity analyses found that the analysis subsets were similar to the main dataset. Data were not available on documented infection for our analysis, and the impact of sepsis on risk of bleeding (and role of platelets) may differ from that of fever alone.20 In the subsequent recurrent event analyses (analyses 2b and 2c), not all factors from analysis 2a reached statistical significance (treatment arm and sex were not significant in either model, and the interaction between previous day platelet count and bleeding episode was not significant in analysis 2c.) However, the effects of each factor were similar across all models (the hazard ratios for each model are shown in the Online Supplementary Appendix). Because of the identified limitations, our findings should be considered as hypothesis-generating.
No interaction was identified between treatment arm (prophylaxis versus no prophylaxis) and platelet count <10×109/L, highlighting how factors other than those addressed by platelet transfusions are important in determining bleeding risk. Similarly the lack of interaction between treatment arm and fever might suggest that risk related to fever cannot be addressed by platelet transfusions. These findings should be considered alongside those of the PLADO trial,1 in which the bleeding incidence was similar despite prophylactic platelet transfusion at differing doses, including ’double’ doses of platelets; in addition in this study any small increase in bleeding risk at very low platelet counts was not reduced by the use of platelet transfusions.
Platelet transfusions are biological products with risks, and ongoing measures to define appropriateness of use in different groups of patients continue to be required.21 Clinically stable patients undergoing autologous HSCT had the lowest risk of bleeding and benefited least from prophylactic platelet transfusions.8 Further research might address how any risk reduction policy could be modified by measures such as red cell transfusion, which was identified as a prognostic factor in this analysis. An increased risk of bleeding associated with a recent red cell transfusion may indicate that patients with significant anemia (requiring a red cell transfusion) are at a higher risk of bleeding.22,23 It should be noted that hemoglobin levels were not adjusted for in this analysis because this information had not been recorded for all patients within the trial.
In summary, identification of patients at lower risk of bleeding who would not benefit from prophylactic platelets may be a more cost-effective approach in transfusion practice. This approach should, however, be coupled with research to evaluate alternative strategies to reduce bleeding risk (such as use of anti-fibrinolytics), as correction of thrombocytopenia by platelet transfusion appears a relatively inefficient means to reduce bleeding risk.
Acknowledgments
The authors thank Charlotte Llewelyn and Mike Murphy for feedback on the manuscript. Sources of support: SS is supported, in part, by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre at Oxford University Hospitals NHS Trust and University of Oxford. The TOPPS study was supported by a grant from the National Health Service Blood and Transplant Research and Development Committee (PG04-5) and the Australian Red Cross Blood Service.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Slichter SJ, Kaufman RM, Assmann SF, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. New Engl J Med. 2010;362(7):600–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanworth SJ, Estcourt LJ, Powter G, et al. A no-prophylaxis platelet transfusion strategy for hematologic cancers. New Engl J Med. 2013;368(19):1771–1780. [DOI] [PubMed] [Google Scholar]
- 3.Wandt H, Schaefer-Eckart K, Wendelin K, et al. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomised study. Lancet. 2012;380(9850): 1309–1316. [DOI] [PubMed] [Google Scholar]
- 4.Friedmann AM, Sengul H, Lehmann H, Schwartz C, Goodman S. Do basic laboratory tests or clinical observations predict bleeding in thrombocytopenic oncology patients? A reevaluation of prophylactic platelet transfusions. Transfus Med Rev. 2002;16(1):34–45. [DOI] [PubMed] [Google Scholar]
- 5.Webert K, Cook RJ, Sigouin CS, Rebulla P, Hedde NM. The risk of bleeding in thrombocytopenic patients with acute myeloid leukemia. Haematologica. 2006;91(11): 1530–1537. [PubMed] [Google Scholar]
- 6.Rebulla P, Finazzi G, Marangoni F, et al. The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto. N Engl J Med. 1997;337(26):1870–1875. [DOI] [PubMed] [Google Scholar]
- 7.Estcourt LJ, Heddle N, Kaufman R, et al. The challenges of measuring bleeding outcomes in clinical trials of platelet transfusions. Transfusion 2013;53(7):1531–1543. [DOI] [PubMed] [Google Scholar]
- 8.Webert KE, Arnold DM, Lui Y, Carruthers J, Arnold E, Hedde NM. A new tool to assess bleeding severity in patients with chemotherapy-induced thrombocytopenia. Transfusion. 2012;52(11):2466–2474. [DOI] [PubMed] [Google Scholar]
- 9.Cook RJ, Heddle NM, Rebulla P, et al. Methods for the analysis of bleeding outcomes in randomized trials of PLT transfusion triggers. Transfusion. 2004;44(8):1135–1142. [DOI] [PubMed] [Google Scholar]
- 10.Stanworth S, Estcourt LJ, Llewelyn C, et al. for the TOPPS study investigators. Impact of prophylactic platelet transfusions on bleeding events in patients with hematologic malignancies: a sub-group analysis of a randomized trial. Transfusion. 2014;54(10):2385–2393. [DOI] [PubMed] [Google Scholar]
- 11.McCullagh P, Nelder JA. Generalized Linear Models. 2nd ed. London: Chapman & Hall/CRC; 1989. [Google Scholar]
- 12.Therneau TM, Grambsch PM. Modelling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 13.Kamath S, Lip GYH. Fibrinogen: biochemistry, epidemiology and determinants. QJM 2003;96(10):711–729. [DOI] [PubMed] [Google Scholar]
- 14.Kim CX, Bailey KR, Klee GG, et al. Sex and ethnic differences in 47 candidate proteomic markers of cardiovascular disease: the Mayo Clinic proteomic markers of arteriosclerosis study. PLoS One 2010;5(2): e9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eliasson M, Evrin PE, Lundblad D, Asplund K, Rånby M. Influence of gender, age and sampling time on plasma fibrinolytic variables and fibrinogen: a population study. Fibrinolysis 1993;7(5):316–323. [Google Scholar]
- 16.Trentzsch H, Nienaber U, Behnke M, Lefering R, Piltz S. Female sex protects from organ failure and sepsis after major trauma hemorrhage. Injury. 2014;45(Suppl 3):S20–28. [DOI] [PubMed] [Google Scholar]
- 17.BCSH. British Committee for Standards in Haematology: guidelines for the use of platelet transfusions. Br J Haematol. 2003;122(1):10–23. [DOI] [PubMed] [Google Scholar]
- 18.BCSH. British Committee for Standards in Haematology: transfusion guidelines for neonates and older children. Br J Haematol. 2004;124(4):433–453. [DOI] [PubMed] [Google Scholar]
- 19.Goerge T, Ho-Tin-Noe B, Carbo C, et al. Inflammation induces hemorrhage in thrombocytopenia. Blood. 2008;111(10): 4958–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho-Tin-Noé B, Demers M, Wagner DD. How platelets safeguard vascular integrity. J Thromb Haemost. 2011;9(Suppl 1):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estcourt LJ, Birchall J, Lowe D, Grant-Casey J, Rowley M, Murphy MF. Platelet transfusions in haematology patients: are we using them appropriately? Vox Sang. 2012;103(4):284–293. [DOI] [PubMed] [Google Scholar]
- 22.Valeri CR, Cassidy G, Pivacek LE, et al. Anemia-induced increase in the bleeding time: implications for treatment of nonsurgical blood loss. Transfusion. 2001;41(8): 977–983. [DOI] [PubMed] [Google Scholar]
- 23.Valeri CR, Khuri S, Ragno G. Role of the Hct in the treatment of thrombocytopenic patients. Transfusion. 2003;43(12):1761–1762. [DOI] [PubMed] [Google Scholar]