Abstract
Developing optimal radiation-free central nervous system prophylaxis is a desirable goal in acute lymphoblastic leukemia, to avoid the long-term toxicity associated with cranial irradiation. In a randomized, phase II trial enrolling 145 adult patients, we compared intrathecal liposomal cytarabine (50 mg: 6/8 injections in B-/T-cell subsets, respectively) with intrathecal triple therapy (methotrexate/cytarabine/prednisone: 12 injections). Systemic therapy included methotrexate plus cytarabine or L-asparaginase courses, with methotrexate augmented to 2.5 and 5 g/m2 in Philadelphia-negative B- and T-cell disease, respectively. The primary study objective was the comparative assessment of the risk/benefit ratio, combining the analysis of feasibility, toxicity and efficacy. In the liposomal cytarabine arm 17/71 patients (24%) developed grade 3–4 neurotoxicity compared to 2/74 (3%) in the triple therapy arm (P=0.0002), the median number of episodes of neurotoxicity of any grade was one per patient compared to zero, respectively (P=0.0001), and even though no permanent disabilities or deaths were registered, four patients (6%) discontinued intrathecal prophylaxis on account of these toxic side effects (P=0.06). Neurotoxicity worsened with liposomal cytarabine every 14 days (T-cell disease), and was improved by the adjunct of intrathecal dexamethasone. Two patients in the liposomal cytarabine arm suffered from a meningeal relapse (none with T-cell disease, only one after high-dose chemotherapy) compared to four in the triple therapy arm (1 with T-cell disease). While intrathecal liposomal cytarabine could contribute to improved, radiation-free central nervous system prophylaxis, the toxicity reported in this trial does not support its use at 50 mg and prompts the investigation of a lower dosage. (clinicaltrials.gov identifier: NCT-00795756).
Introduction
Since the landmark study of Omura et al.,1 prophylactic cranial irradiation has become a useful adjunct to intrathecal methotrexate for the prevention of central nervous system (CNS) recurrence in adult patients with acute lymphoblastic leukemia (ALL). Given that the risk of CNS relapse is less than 5% with modern ALL therapy, due to the concurrent use of CNS-penetrating agents such as high-dose methotrexate/cytarabine (HD-M/A),2–4 attempts to omit cranial irradiation were initiated in an effort to minimize chemotherapy delay and risks of direct or cumulative CNS toxicity. The issue of irreversible neurotoxicity caused by radiotherapy has become prominent in long-term survivors of childhood ALL. A high incidence of secondary cancers,5 cognitive dysfunction6,7 and organic brain damage detectable by magnetic resonance imaging and favoring early onset dementia8 have been described. Because the outcome of adolescents and younger adults with ALL has greatly improved using pediatric-type protocols,9–11 long-term radiation-related hazards can become of great concern for many patients cured by these programs.
The cure rate of childhood ALL can be outstanding, and the omission of cranial irradiation does not affect survival or risk of CNS relapse.12 With total therapy program XV, Pui et al. obtained a 85.6% 5-year event-free survival rate.13 In this study CNS prophylaxis was radiation-free and consisted of triple intrathecal therapy (ITT), which proved superior to standard intrathecal methotrexate in a randomized clinical trial,14 along with systemic CNS-active therapy. The risk of CNS progression was low (3.9%) and comparable to that observed in other pediatric trials, with or without cranial irradiation. Because in this study only the risk of marrow relapse but not that of CNS relapse was decreased, further intensification of intrathecal prophylaxis was planned.
Intrathecal liposome-encapsulated cytarabine (DepoCyte®, ITD) might be advantageous in this setting, the slow cytarabine release allowing therapeutic drug concentrations in the cerebrospinal fluid for up to 14 days.15,16 In adult ALL, ITD was highly effective at the time of CNS recurrence. Goekbuget et al. obtained 12 complete remissions with one or two ITD injections in 14 adult patients with CNS relapse (86%);17 however, 21% of them developed severe neurotoxicity. In primary CNS prophylaxis, three to six ITD injections were used together with the HyperCVAD regimen, which included four HD-M/A courses. Jabbour et al. at M.D. Anderson Cancer Center (MDACC) reported prohibitive neurotoxicity in five out of 31 of these patients (16%).18 However, the intervals between HD-M/A and ITD were rather short (ITD on day 10 of HD-M/A) and no patient suffered from CNS relapse.
The Northern Italy Leukemia Group (NILG) performed a randomized phase II trial of radiation-free CNS prophylaxis comparing standard ITT (methotrexate, cytarabine, corticosteroids) with ITD. The treatment included three HD-M/A courses, with longer intervals between any ITD and HD-M/A courses (21 days) and higher methotrexate and lower cytarabine dosages than in the MDACC study (methotrexate 1.5–5 g/m2 and cytarabine 2 g/m2 instead of 1 g/m2 and 3 g/m2, respectively). The primary study endpoint was to assess the risk/benefit ratio of this strategy, in view of the safety issue raised by the MDACC study and the paucity of comparative clinical data in primary CNS prophylaxis of adult ALL.
Methods
Diagnosis and treatment
Diagnostic criteria and the treatment program with minimal residual disease/risk-oriented hematopoietic stem cell transplantation (HSCT) are detailed in the Online Supplement. The study was approved by the Institutional Review Boards of all participating institutions. Eligible patients signed an informed consent form in accordance with the Helsinki declaration of 1975, as revised in 2008.
Central nervous system prophylaxis trial
Pre-random stratification was by B- or T-cell cell lineage and risk class [standard risk: white blood cell count <30×109/L, pre-B/”common” phenotype, BCR-ABL1-negative (B-ALL) or <100×109/L and cortical-T CD1a+ phenotype (T-ALL)]. Patients were randomized to ITT (methotrexate 12.5 mg, cytarabine 50 mg, prednisone 40 mg) or ITD 50 mg as shown in Figure 1. Although intrathecal prophylaxis was discontinued at resistance, recurrence or HSCT, all the patients were evaluable until they received the allocated intrathecal prophylaxis. Other CNS-active therapy included lineage-targeted methotrexate19,20 at 2.5 g/m2 in Philadelphia chromosome/BCR-ABL1-negative (Ph–) B-ALL and 5 g/m2 in T-ALL (age ≤55 years), together with high-dose cytarabine 2 g/m2 (8 doses, 2 courses) or L-asparaginase 10,000 IU/m2 (2 doses, 1 course). No specific recommendation for intrathecal prophylaxis at/after HSCT was made in this study.
Figure 1.
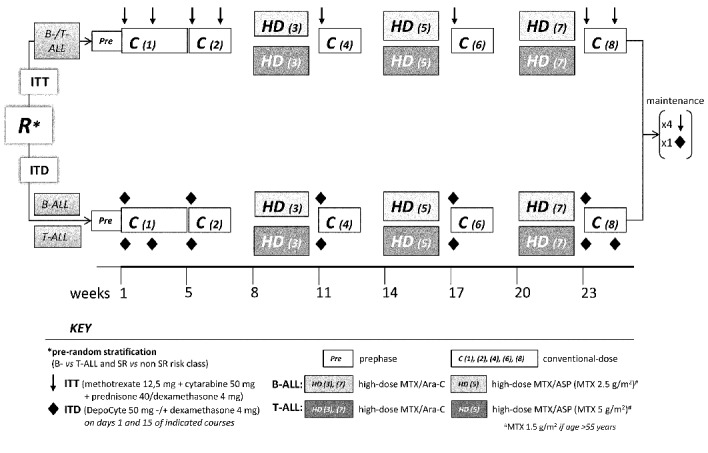
Study design. ITT was administered on days 1 and 15 of courses 1, 2 and 8; on day 1 of courses 4, 6 and maintenance months 2, 3, 4 and 5 (×12). ITD was administered on day 1 of courses 1, 2, 4, 5, 7, 8 and maintenance month 2 (×6, BALL); plus day 15 of courses 1 and 8 in T-ALL (×8). Except for courses 1–2 and 8 in T-ALL, an interval of 21 days was maintained between any ITD and/or high-dose course. Concomitant systemic dexamethasone 5 mg/m2/bd was given for 5 days with each intrathecal injection.
Study design
The study was sponsored by “Papa Giovanni XXIII” Hospital (Bergamo, Italy), on behalf of the NILG. The primary endpoint was the comparative evaluation of the risk/benefit profile of the two CNS prophylaxis strategies. Because of the low number of expected CNS recurrences and the planned number of study patients (n=150) no formal sample size estimate was felt appropriate. Instead, close monitoring and validation of occurring events under the supervision of a Data Safety Monitoring Board was planned to spot any suspected safety risk promptly.
Neurotoxicity was evaluated according to the National Cancer Institute Common Toxicity Criteria (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf) and feasibility according to the number of total intrathecal injections administered versus planned number. The benefit was assessed comparing rates of isolated and combined CNS recurrence. The protocol was amended in December, 2010, when intrathecal dexamethasone 4 mg was added to ITD in an attempt to decrease the incidence of severe backache and headache (also substituting for prednisone in the ITT arm).
Definitions and statistics
Standard definitions of complete remission, early death, resistance, isolated or combined bone marrow relapse, overall survival and disease-free survival were adopted.21,22 A CNS relapse was defined by the morphological and immunophenotypic detection of B- or T-lymphoblasts in the cerebrospinal fluid of patients who had been in complete remission and who were developing signs of neuromeningeal disease, with or without abnormal findings on computed tomography or magnetic resonance imaging. Serial immunophenotypic evaluation of cerebrospinal fluid from patients in complete remission was not planned.
The patients’ baseline characteristics and number of intrathecal injections and subjects experiencing endpoint events were compared with the χ2 test or Fisher exact test for categorical variables, and the t-test or non-parametric test for continuous variables. Survival graphs were drawn using the Kaplan-Meier method and compared by the log-rank test using censored data. CNS relapse graphs were determined using the competing risk method, in which death in remission and non-CNS relapse were competing events, and compared with the Gray non-parametric test. P values are two-sided.
Results
Study patients
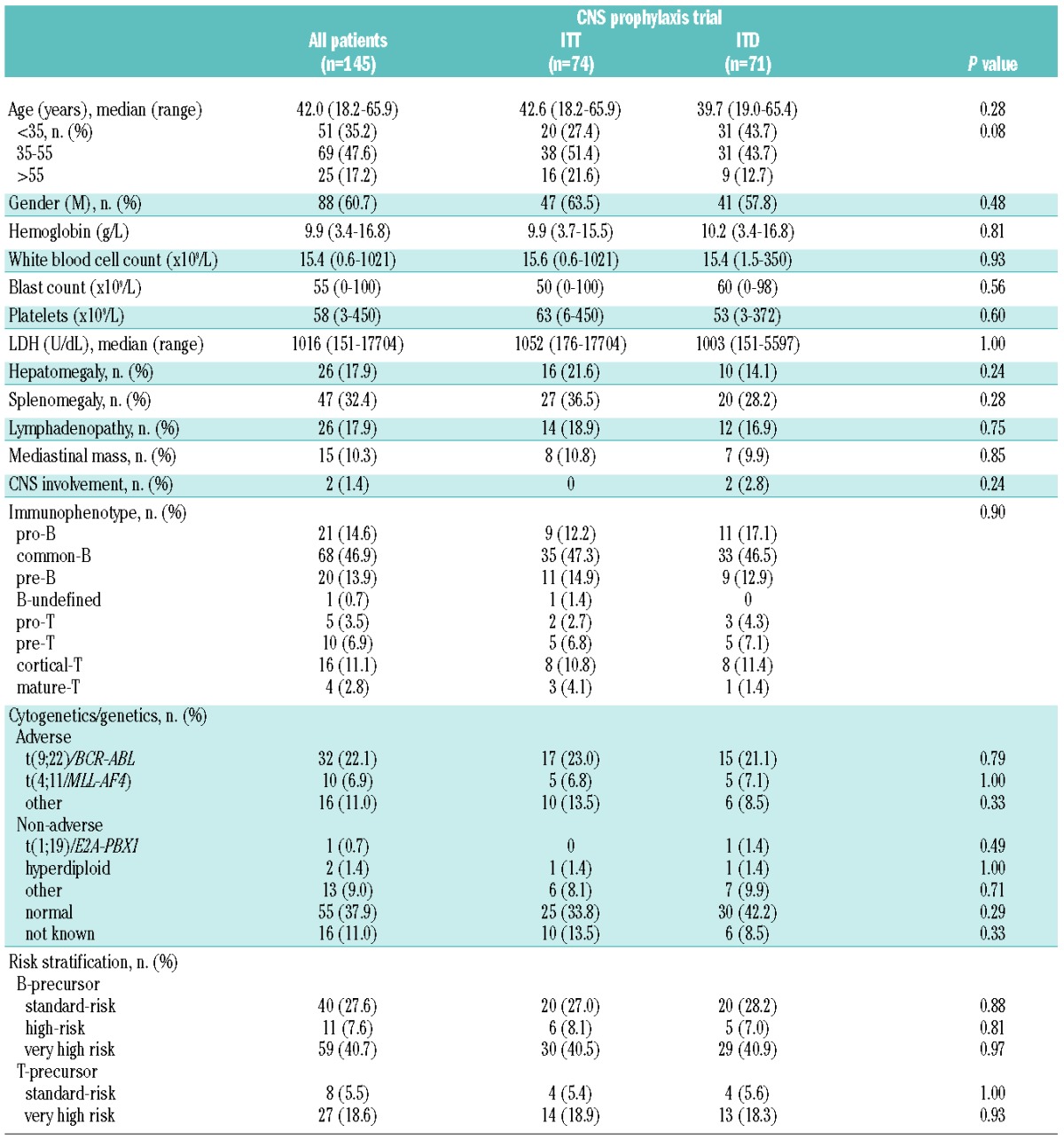
The study was conducted between January, 2008 and August, 2012, when it was closed, after the randomization of 145 out of 150 planned patients, due to a safety issue raised by the European Medicine Agency, concerning a risk of microbial contamination of DepoCyte® vials (EMA/555991/2012). Out of 151 eligible patients, six refused randomization and therefore received standard ITT prophylaxis (Figure 2). Table 1 shows the diagnostic characteristics of the patients, comparing those in the standard ITT arm (n=74) and the experimental ITD arm (n=71). The median age of the patients was 42 years (range, 18.2–65.9 years) and no difference between the patients in the two study arms was detected. Overall the complete remission rate was 89.3% (n=183) with identical rates across CNS trial and non-trial subsets, leaving 66 and 64 evaluable patients with complete remission in the two trial arms.
Figure 2.
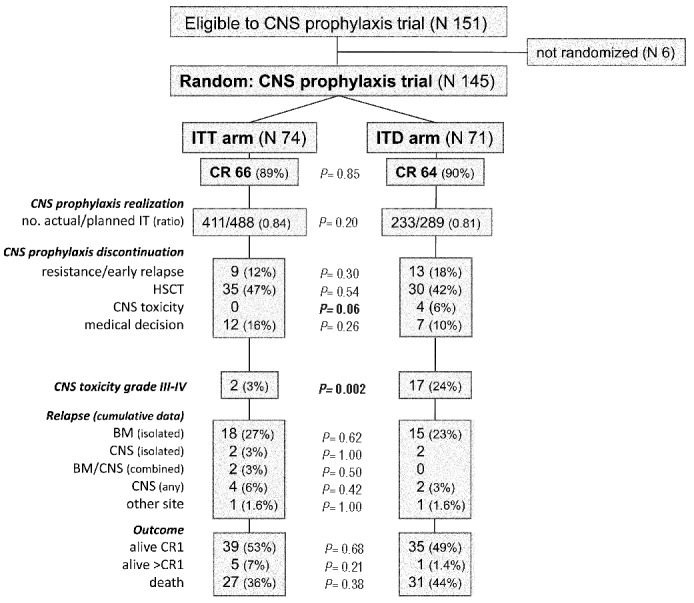
CONSORT diagram showing study flow, primary endpoint results (feasibility and toxicity of ITD compared to ITT) and clinical outcomes [rates of complete remission (CRI), early study drop-out, relapse and survival].
Table 1.
Demographic and clinical characteristics of the study patients.
Feasibility and toxicity of central nervous system prophylaxis
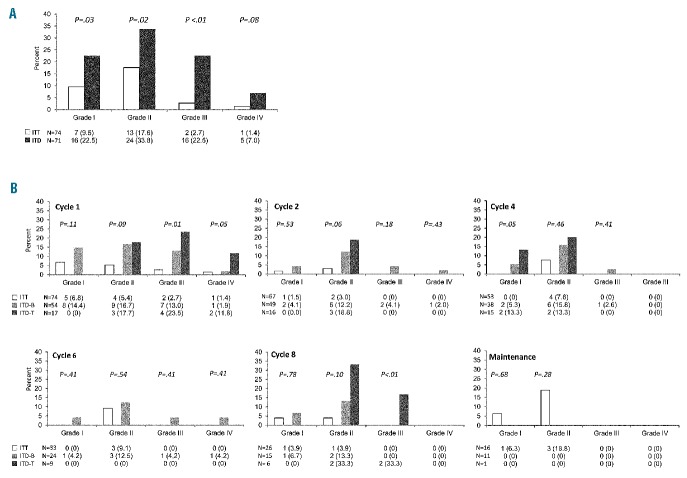
After exclusion of early study losses caused by resistance, relapse, HSCT or death in remission, the feasibility of intrathecal prophylaxis did not differ significantly between the ITT and ITD arms, yielding an intrathecal prophylaxis realization ratio of 0.84 and 0.82, respectively (Figure 2). Discontinuation for reasons other than resistance/relapse, death and HSCT included persistent hematotoxicity (n=3), other toxicity (n=6), technical failure (n=2) and patient’s refusal (n=1) in the ITT arm; and persistent hematotoxicity (n=1), other toxicity (n=5) and patient’s wish for concurrent seminoma therapy (n=1) in the ITD arm. However, four patients randomized to ITD were forced to discontinue intrathecal prophylaxis on account of serious neurotoxicity whereas none had to do so in the ITT arm, a difference which was close to being statistically significant (P=0.06). The overall assessment of neurotoxicity by ITD was a major study endpoint. Altogether ITD proved more toxic than ITT, across all toxicity levels (Figures 2 and 3A). In the ITD arm 17 patients (24%) suffered from CTC grade 3–4 neurotoxicity compared with only two (3%) in the ITT arm (P=0.0002), and significantly more patients developed multiple and/or recurrent adverse events (Table 2A). The median number of episodes of neurotoxicity of any grade per patient was one (range, 0–8), compared to zero (range, 0–15) in the ITT arm (P=0.001). The most unfavourable subset comprised ten patients reporting more than three episodes of neurotoxicity (14% of randomized patients), for a total of 19 grade 3–4 symptoms, in comparison with only two similar patients in the ITT arm (2.7% of randomized patients), none of whom had grade 3–4 neurotoxicity (P=0.02). Types of neurological adverse events are detailed in the Online Supplement. The prevailing symptoms were pain and headache caused by meningeal/radicular irritation. Other grade 3–4 toxicities in the ITD arm consisted of altered consciousness (n=4 distinct episodes), syncope (n=2), seizure (n=2), loss of vision (n=1), cranial neuropathy (n=1), motor neuropathy (n=3) and dizziness (n=1). Although none of these complications proved irreversible or fatal, they caused temporary disability, hospital admission, treatment delay and/or permanent withdrawal of intrathecal prophylaxis in some cases (n=4).
Figure 3.
Comparative analysis of neurotoxicity according to (A) CTC grading and (B) treatment cycle and ALL immunophenotype in the ITD arm and the ITT arm.
Table 2.
Comparative incidence of isolated or multiple and recurrent neurotoxic adverse events due to intrathecal CNS prophylaxis in (A) the ITT and ITD study arms, and in (B) the ITD versus dexamethasone/ITD subgroups of the ITD study arm. GI-IV denotes increasing grades of neurotoxicity according to the CTC evaluation scale.
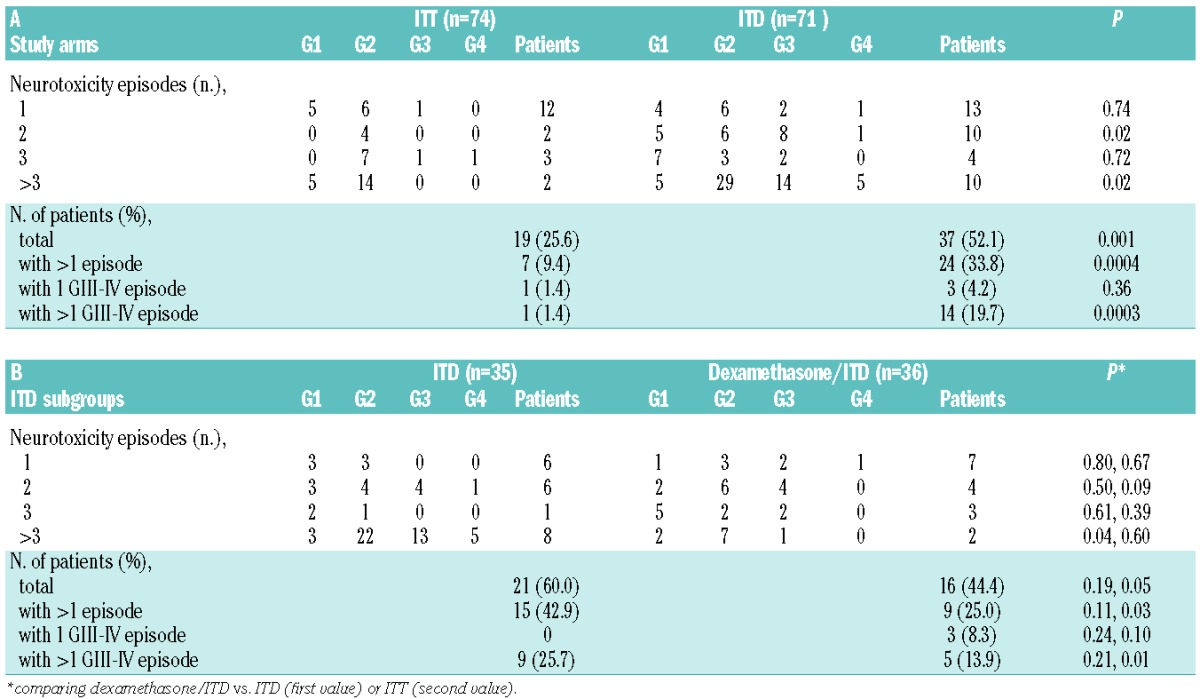
A secondary toxicity analysis concerned the different ITD schedule adopted in B- and T-ALL, and the adjunct of intrathecal dexamethasone decided at the interim analysis. Grade 3–4 neurotoxicity by ITD was more common in T-ALL [6/17 (35%) versus 11/54 (20%) with B-ALL] at treatment cycles 1–2 and 8 (Figure 3B), when ITD was administered every 2 weeks three and two consecutive times, respectively (Figure 1). The addition of intrathecal dexamethasone 4 mg to each ITD in the second part of the trial led to a slight reduction of overall neurotoxicity, with a more definite effect on the incidence of multiple adverse events, since the proportion of patients with more than three toxicities decreased from 22.8% (n=8/35) to 5.5% (n=2/36, P=0.04), and there was a decrease in grade 3–4 toxicities from 18 episodes in eight patients (2.25 per patient) to one episode in two patients (0.5 per patient) (Table 2B). The improved toxicity profile of the intrathecal dexamethasone/ITD combination was further underlined by the loss or marked reduction of the statistical significance concerning the incidence of neurotoxic complications in comparison with the ITT arm.
Efficacy of central nervous system prophylaxis and general outcome measures
In the ITD arm there were two delayed isolated CNS relapses (1 Ph− and 1 Ph+ B-ALL) at 603 and 704 days, compared to four total CNS relapses (1 T-ALL, 1 Ph− and 2 Ph+ B-ALL) at days 75, 281, 348 and 572 in the ITT arm (Figure 4A). All CNS relapses but one (in the ITT arm) were observed in patients who did not undergo HSCT. The single relapsed Ph+ patient in the ITD arm was older than 55 years and was not given any systemic high-dose chemotherapy because of a poor performance status. Thus, provided at least one high-dose course was given concomitantly, the incidence of any CNS recurrence was 1.5% in the ITD arm (0% in T-ALL), and 6% in the ITT arm (5.6% in T-ALL). Median and 4-year overall survival and disease-free survival estimates were similar in the ITT and ITD arms (Figure 4B,C).
Figure 4.
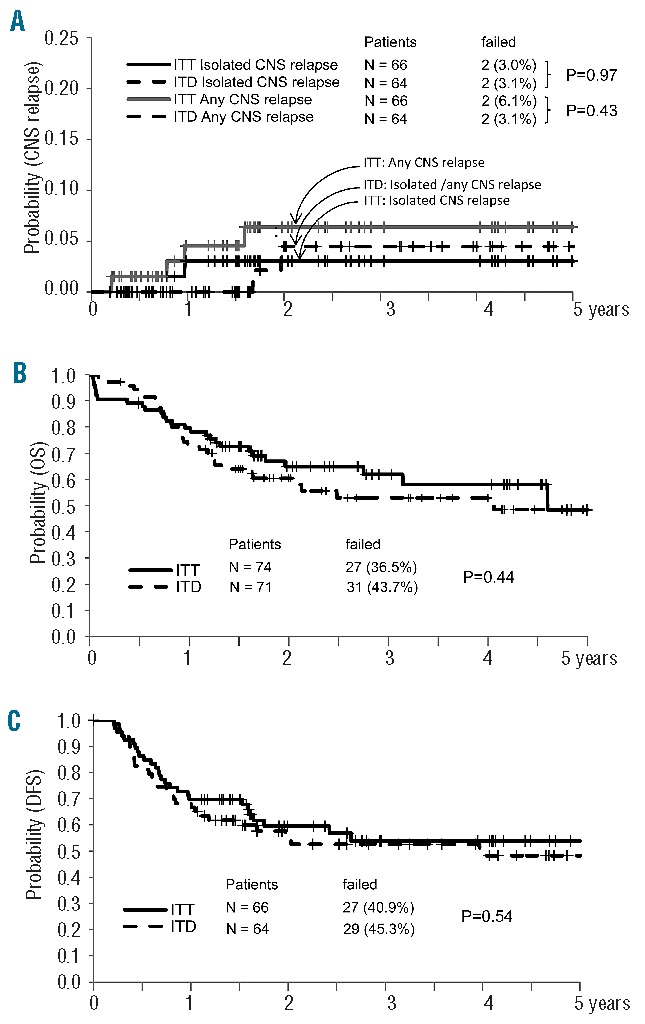
CNS relapse incidence and outcome estimates with 95% confidence intervals (CI) by randomization arm. (A) Three-year CNS relapse incidence: ITT (n=66), four CNS relapses (6.1%, 95% CI 2–14%), two isolated (3%, 95% CI 0.6–9%) and two combined (3.1%, 95% CI 0.6–9%); ITD (n=64), two isolated CNS relapses (3.1%, 95% CI 0.8–14%) (P=0.43). (B) Overall survival: ITT (n=74), median duration 4.5 years, 62% at 3 years (95% CI 49–74%) and 58% at 4 years (95% CI 44–72%); ITD (n=71), median duration 4.0 years, 53% at 3 and 4 years (95% CI 40–66%) (P=0.44). (C) Disease-free survival: ITT (n=66), median duration not reached, 54% at 3 and 4 years (95% CI 40–67%); ITD (n=64), median duration 3.9 years, 53% at 3 years (95% CI 39–66%) and 48% at 4 years (95% CI 34–63%) (P=0.54).
Discussion
This trial was undertaken to evaluate the overall risk/benefit profile of ITD in the prophylaxis of meningeal relapse in adult ALL, primarily to assess and monitor the feasibility and safety of intrathecal therapy. ITD is a slow-release liposome-encapsulated cytarabine formulation, considered here because of its marked activity in neoplastic meningitis caused by solid tumors, lymphoma and ALL.16,23,24 CNS relapse confers a very bad outlook and is still observed in some patients with ALL. ITD could help optimize the design of modern radiation-free prophylactic regimens and improve the compliance to intrathecal therapy of patients at high risk of complications, such as the elderly and those with Ph+ ALL,25–27 limiting the total number of lumbar punctures to six or fewer. In the German elderly Ph− ALL trial,25 the use of ITD instead of intrathecal methotrexate reduced induction mortality compared to that in a historical cohort of patients. Another clinical advantage of ITD-based, radiation-free CNS prophylaxis would be the lack of the chemotherapy delays typically associated with the application of cranial irradiation. It was, therefore, disappointing that five of 31 patients in the first ITD trial from MDACC developed unacceptable neurotoxicity,18 as reported in 10% or more of the patients enrolled in other ITD studies.28,29
In our randomized phase II trial with systemic HD-M/A, the dose of methotrexate was 1.5, 2.5 or 5 g/m2 (depending on the patients’ age and disease subset) and that of cytarabine was 2 g/m2, while intervals between any prior or subsequent ITD and/or HD-M/A were set at 21 days and not 10–14 days (MDACC study). This gave us the opportunity to compare directly the cumulative CNS toxicity of these drugs in conjunction with either ITT or ITD given at the stated intervals. The only exception to this design concerned patients with T-ALL, who received additional ITD at cycles 1 and 8, shortening this interval to 14 days for three and two consecutive ITD administrations, respectively.
As to study design, the expected CNS progression rate in the standard ITT arm was 5% or less, to be compared with a similar or even lower figure in the ITD arm. With this preliminary consideration, a formal sample size calculation according to classical statistical tenets would give rise to a disproportionately high number of patients to be recruited for an efficacy study, not met by the planned number of study subjects (n=150). However, because of the inconsistency of comparative neurotoxicity data in the CNS prophylaxis of adult ALL and the need to adequately screen ITD in combination with systemic chemotherapy, it was nevertheless decided to privilege the collection of standardized, methodologically sound data in the context of a phase II randomized clinical trial.30 In this regard, it is not easy to translate a comparison of CNS recurrence rate and drug-related neurotoxicity into an a priori calculation of expected adverse events, which were instead explored and described to provide a logical basis for discussion and/or larger studies.
The study results indicated a significantly higher neurotoxicity from ITD than from ITT. Overall, 17 patients in the ITD arm developed one or more episodes of grade 3–4 neurotoxicity (24%). Excluding the more easily manageable cases of high-grade, transient inflammatory/radicular pain or headache, eight of the 17 patients including six patients in complete remission (9.4%) suffered from serious, debilitating neurological symptoms, such as disturbed consciousness, cranial and/or peripheral neuropathy, seizure and loss of vision. This often led to hospital readmission and caused permanent discontinuation of CNS prophylaxis in four patients. Although none of these patients had a CNS relapse, this type and rate of clinical complications is hardly acceptable in a prophylactic regimen for adult ALL, because not previously reported with the traditional intrathecal methotrexate or ITT, as clearly confirmed by the results in the standard arm of the trial. However, ITD-related toxicity was eventually reversible and no patient developed a conus syndrome or died of these complications, which might suggest some benefit from increasing the intervals between ITD and HD-M/A courses and lowering the intravenous cytarabine dose compared to that used in the MDACC study (from 3 to 2 g/m2). Because the majority of the affected patients resumed ITD prophylaxis, albeit at an initially lower dosage (25 mg), the ITD program resulted statistically feasible in comparison with the ITT control arm.
Other study results deserve to be discussed, since neurotoxicity due to ITD is rather unpredictable and has not been associated with any specific disease subset or drug scheduling. In this trial the incidence of grade 3–4 neurotoxicity was both higher and worse in patients with TALL who received, by design, ITD every 14 days at cycles 1–2 and 8. Thus a longer than 21-day interval could lower the risk of neurotoxicity, as for ITD and HD-M/A courses. With regards to intrathecal dexamethasone added to ITD following the interim analysis, the risk of neurotoxicity was slightly lower and that of multiple grade 3–4 toxicities was significantly lower and closer to that observed in the ITT arm, independently of the concurrent use of intravenous dexamethasone as per protocol specifications. This could be explained by a greater dexamethasone concentration achieved in the cerebrospinal fluid immediately before ITD administration, reducing the risk of acute meningeal reactions.
With regards to the efficacy analysis, which remains basically undefined due to the very small number of expected events relative to the total number of study patients, the risk of CNS relapse was low in the ITD arm, especially in patients with T-ALL (0%) and also in patients with Ph− B-ALL provided at least one HD-M/A course was given concomitantly (1.5%). This, together with the MDACC study results (no CNS relapses), would suggest that ITD plus HD-M/A represents a powerful radiation-free combination for CNS prophylaxis, perhaps with an increased efficacy in TALL in relation to the higher methotrexate dose of 5 g/m2 used in our study. In contrast to the German trial in the elderly,25 the complete remission rate was not improved in the ITD arm; however, since this was already in a high range for this cohort of patients aged 18.2–65.9 years (89% in the ITT arm) we argue that it could not be increased significantly.
ITD 50 mg is the most effective drug for intrathecal injection or administration via an Ommaya reservoir for meningeal ALL. Its use as a prophylactic agent was prompted by the awareness of the poor outcome of patients following CNS relapse. So far, 102 patients have been enrolled and received ITD in two distinct prospective studies of primary CNS prophylaxis: 31 in the MDACC phase II trial and 71 in the NILG phase II randomized trial. Both studies confirmed a risk of serious CNS toxicity greater than 10%, related to the sustained drug release in the cerebrospinal fluid and potentiated by a temporally close exposure to systemic high-dose cytarabine; the risk seems too high to recommend this drug as standard intrathecal prophylaxis with ALL regimens combining ITD with HD-M/A courses. At the same time, because ITD is highly active against CNS leukemia, it would be wrong to discard this agent from the search for an optimal radiation-free prophylactic regimen. Especially in patients with ALL subsets at higher risk of CNS progression, this search could entail an evaluation of lower doses (15–25 mg), which may retain significant pharmacological activity15 while having a safer toxicity profile, the association with intrathecal dexamethasone, and avoidance of temporally too close administration of ITD and any prior or subsequent ITD or HD-M/A.
Acknowledgments
DepoCyte® was kindly provided by Mundipharma International (Dr Thomas Mehrling, Dr Kasia Hilgier, Dr Michele Elefanti). Dr Eros Di Bona (Vicenza), Dr Leonardo Campiotti (Varese), Dr Monica Tajana (Cremona), Prof Robin Foà (Rome), Prof Monica Bocchia (Siena), Dr Alessandro Levis (Alessandria), and Dr D Caracciolo (Turin), Italy, contributed patients to the study.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
The authors would like to thank the Associazione Italiana Ricerca sul Cancro (grant code: AIRC IG 2008 code 6105), Associazione Italiana Leucemie (AIL), Bergamo and Venezia, and Associazione “Paolo Belli”, Bergamo, Italy.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Omura GA, Moffitt S, Vogler WR, Salter MM. Combination chemotherapy of adult acute lymphoblastic leukemia with randomized central nervous system prophylaxis. Blood. 1980;55(2):199–204. [PubMed] [Google Scholar]
- 2.Cortes J, O’Brien SM, Pierce S, Keating MJ, Freireich EJ, Kantarjian HM. The value of high-dose systemic chemotherapy and intrathecal therapy for central nervous system prophylaxis in different risk groups of adult acute lymphoblastic leukemia. Blood. 1995;86(6):2091–2097. [PubMed] [Google Scholar]
- 3.Sancho JM, Ribera JM, Oriol A, et al. Central nervous system recurrence in adult patients with acute lymphoblastic leukemia: frequency and prognosis in 467 patients without cranial irradiation for prophylaxis. Cancer. 2006;106(12):2540–2546. [DOI] [PubMed] [Google Scholar]
- 4.Jabbour E, Thomas D, Cortes J, Kantarjian HM, O’Brien S. Central nervous system prophylaxis in adults with acute lymphoblastic leukemia: current and emerging therapies. Cancer. 2010;116(10):2290–2300. [DOI] [PubMed] [Google Scholar]
- 5.Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9(3):257–268. [DOI] [PubMed] [Google Scholar]
- 6.Krull KR, Zhang N, Santucci A, et al. Long-term decline in intelligence among adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiation. Blood. 2013;122(4):550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. J Clin Oncol. 2013;31(35): 4407–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuitema I, Deprez S, Van Hecke W, et al. Accelerated aging, decreased white matter integrity, and associated neuropsychological dysfunction 25 years after pediatric lymphoid malignancies. J Clin Oncol. 2013;31(27):3378–3388. [DOI] [PubMed] [Google Scholar]
- 9.Ribera JM, Oriol A, Sanz MA, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Espanol de Tratamiento en Hematologia pediatric-based protocol ALL-96. J Clin Oncol. 2008;26(11):1843–1849. [DOI] [PubMed] [Google Scholar]
- 10.Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27(6):911–918. [DOI] [PubMed] [Google Scholar]
- 11.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532–543. [DOI] [PubMed] [Google Scholar]
- 12.Richards S, Pui CH, Gayon P. Systematic review and meta-analysis of randomized trials of central nervous system directed therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60(2):185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matloub Y, Lindemulder S, Gaynon PS, et al. Intrathecal triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared with intrathecal methotrexate: results of the Children’s Cancer Group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia, reported by the Children’s Oncology Group. Blood. 2006;108(4):1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S, Chatelut E, Kim JC, et al. Extended CSF cytarabine exposure following intrathecal administration of DTC 101. J Clin Oncol. 1993;11(11):2186–2193. [DOI] [PubMed] [Google Scholar]
- 16.Phuphanich S, Maria B, Braeckman R, Chamberlain M. A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. J Neurooncol. 2007;81(2):201–208. [DOI] [PubMed] [Google Scholar]
- 17.Gokbuget N, Hartog CM, Bassan R, et al. Liposomal cytarabine is effective and tolerable in the treatment of central nervous system relapse of acute lymphoblastic leukemia and very aggressive lymphoma. Haematologica. 2011;96(2):238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jabbour E, O’Brien S, Kantarjian H, et al. Neurologic complications associated with intrathecal liposomal cytarabine given prophylactically in combination with high-dose methotrexate and cytarabine to patients with acute lymphocytic leukemia. Blood. 2007;109(8):3214–3218. [DOI] [PubMed] [Google Scholar]
- 19.Galpin AJ, Schuetz JD, Masson E, et al. Differences in folylpolyglutamate synthetase and dihydrofolate reductase expression in human B-lineage versus T-lineage leukemic lymphoblasts: mechanisms for lineage differences in methotrexate polyglutamylation and cytotoxicity. Mol Pharmacol. 1997;52(1):155–163. [DOI] [PubMed] [Google Scholar]
- 20.Masson E, Relling MV, Synold TW, et al. Accumulation of methotrexate polyglutamates in lymphoblasts is a determinant of antileukemic effects in vivo. A rationale for high-dose methotrexate. J Clin Invest. 1996;97(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113(18):4153–4162. [DOI] [PubMed] [Google Scholar]
- 22.Bassan R, Rossi G, Pogliani EM, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28(22):3644–3652. [DOI] [PubMed] [Google Scholar]
- 23.Glantz MJ, LaFollette S, Jaeckle KA, et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol. 1999;17(10):3110–3116. [DOI] [PubMed] [Google Scholar]
- 24.Glantz MJ, Jaeckle KA, Chamberlain MC, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5(11):3394–3402. [PubMed] [Google Scholar]
- 25.Goekbuget N, Beck J, Brueggemann M, et al. Moderate intensive chemotherapy including CNS-prophylaxis with liposomal cytarabine is feasible and effective in older patients with ph-negative acute lymphoblastic leukemia (ALL): results of a prospective trial from the German Multicenter Study Group for Adult ALL (GMALL). Blood. 2012; 120(21):abstract 1493. [Google Scholar]
- 26.Patel SB, Gojo I, Tidwell ML, Sausville EA, Baer MR. Subdural hematomas in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia receiving imatinib mesylate in conjunction with systemic and intrathecal chemotherapy. Leuk Lymphoma. 2011;52(7):1211–1214. [DOI] [PubMed] [Google Scholar]
- 27.Bassan R. Optimal central nervous system prophylaxis in Philadelphia chromosome-positive acute lymphoblastic leukemia: collateral damage in the imatinib era? Leuk Lymphoma. 2011;52(7):1164–1165. [DOI] [PubMed] [Google Scholar]
- 28.Chamberlain MC. Neurotoxicity of intra-CSF liposomal cytarabine (DepoCyt) administered for the treatment of leptomeningeal metastases: a retrospective case series. J Neurooncol. 2012;109(1):143–148. [DOI] [PubMed] [Google Scholar]
- 29.Corazzelli G, Frigeri F, Russo F, et al. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and ‘unclassifiable’ highly aggressive B-cell lymphoma. Br J Haematol. 2011;156(2):234–244. [DOI] [PubMed] [Google Scholar]
- 30.Rubinstein L, Crowley J, Ivy P, Leblanc M, Sargent D. Randomized phase II designs. Clin Cancer Res. 2009;15(6):1883–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]