Abstract
We compared the health-related quality-of-life of patients with newly diagnosed multiple myeloma aged over 65 years or transplant-ineligible in the pivotal, phase III FIRST trial. Patients received: i) continuous lenalidomide and low-dose dexamethasone until disease progression; ii) fixed cycles of lenalidomide and low-dose dexamethasone for 18 months; or iii) fixed cycles of melphalan, prednisone, thalidomide for 18 months. Data were collected using the validated questionnaires (QLQ-MY20, QLQ-C30, and EQ-5D). The analysis focused on the EQ-5D utility value and six domains pre-selected for their perceived clinical relevance. Lenalidomide and low-dose dexamethasone, and melphalan, prednisone, thalidomide improved patients’ health-related quality-of-life from baseline over the duration of the study across all pre-selected domains of the QLQ-C30 and EQ-5D. In the QLQ-MY20, lenalidomide and low-dose dexamethasone demonstrated a significantly greater reduction in the Disease Symptoms domain compared with melphalan, prednisone, thalidomide at Month 3, and significantly lower scores for QLQ-MY20 Side Effects of Treatment at all post-baseline assessments except Month 18. Linear mixed-model repeated-measures analyses confirmed the results observed in the cross-sectional analysis. Continuous lenalidomide and low-dose dexamethasone delays disease progression versus melphalan, prednisone, thalidomide and has been associated with a clinically meaningful improvement in health-related quality-of-life. These results further establish continuous lenalidomide and low-dose dexamethasone as a new standard of care for initial therapy of myeloma by demonstrating superior health-related quality-of-life during treatment, compared with melphalan, prednisone, thalidomide.
Introduction
Multiple myeloma (MM) is an incurable hematologic malignancy that mainly affects elderly individuals, and is characterized by the proliferation of plasma cells.1 Five-year relative survival rates have been shown to diminish with increasing patient age; however, improved survival rates have been reported in newly diagnosed MM (NDMM) patients in recent years since the introduction of novel agents such as thalidomide, lenalidomide, and bortezomib.2–4
Melphalan and prednisone (MP) combined with thalidomide (MPT) or bortezomib (MPV) are considered standard first-line treatment options in elderly NDMM patients who are ineligible for autologous stem cell transplantation.5–13 Although lenalidomide in combination with low-dose dexamethasone (Rd) is an established standard treatment option in patients with relapsed/refractory MM, recent data have emerged on the efficacy of this combination in patients with NDMM.1,5,10,11,14–18
The randomized pivotal phase III FIRST trial (Frontline Investigation of lenalidomide/dexamethasone [Rev/Dex] versus Standard Thalidomide; IFM 2007-01/MM-020; clinicaltrials.gov identifier: 00689936; EudraCT No. 2007-004823-39) compared the efficacy and safety of Rd, administered continuously until progressive disease (PD) or for a fixed 18-month duration (Rd18), with MPT given for 18 months in NDMM patients who were aged 65 years or over, or who were aged under 65 years and ineligible for stem cell transplantation. Continuous Rd showed improved progression-free survival (PFS) and overall survival (OS) benefit at interim analysis compared with MPT.19
Although extending survival is clearly the ultimate treatment goal of myeloma therapy, reducing disease-related symptoms and improving quality-of-life (QoL) are equally important. MM patients have a high symptom burden, including pain and fatigue, which are associated with reduced overall health-related QoL (HRQoL), particularly in relation to physical functioning.20–23 In this population, treatment objectives should be to improve disease management by delaying disease progression, optimizing response, and prolonging survival. In addition, it is particularly important in elderly patients to maintain QoL and minimize treatment-related toxicity and discomfort.24,25 Therefore, we incorporated patient-reported measures in the FIRST trial to determine if the choice of initial therapy regimen resulted in differences in symptom burden and HRQoL over time.
Methods
Trial design
The FIRST trial was a pivotal phase III, randomized, open-label, 3-arm, international study. Study details have been published previously.18 Briefly, transplant-ineligible NDMM patients were randomized to continuous Rd in 28-day cycles until PD (n=535), to Rd18 (Rd in 28-day cycles for 18 cycles; 72 weeks) (n=541), or to MPT in 42-day cycles for 12 cycles (72 weeks) (n=547). HRQoL was a secondary end point, and data were collected for a maximum of 18 months or until PD using three validated questionnaires. For the purpose of this HRQoL analysis, and because the continuous Rd and Rd18 regimens were identical over the 18 months, the two lenalidomide arms were collated into one “Rd” arm in a post hoc fashion. The trial protocol was approved by each study site’s Independent Ethics Committee or Institutional Review Board, and the study was conducted in accordance with the Declaration of Helsinki.
HRQoL assessments
HRQoL was evaluated with the myeloma-specific QLQ-MY20 questionnaire as well as with the general oncology-related QLQ-C30 and the generic EuroQoL EQ-5D surveys.26–35 These questionnaires were administered in paper format at the hospital. Patients completed the questionnaires at several time points: baseline; at the end of Cycle 1 (after 4 weeks of treatment with Rd and after 6 weeks of treatment with MPT); after 3, 6, 12, and 18 months of treatment; and at study discontinuation. These questionnaires are among the most extensively validated in MM36 and can be easily completed with minimal patient burden.37,38 The analysis focused on the EQ-5D utility value and six pre-selected and clinically relevant HRQoL domains: two from the QLQ-MY20 (Disease Symptoms and Side Effects of Treatment); and four from the QLQ-C30 (Global Health Status, Physical Functioning, Fatigue, and Pain). These domains were chosen before data analysis following a workshop discussion with hematologists, and were based on perceived clinical relevance. Full results from all domains are presented in the Online Supplementary Appendix and are generally in line with the results presented.
EQ-5D, QLQ-MY20, and QLQ-C30 domains were scored in accordance with their published guidelines.27,28,32,37,38 Results were transformed into scales ranging from 0 to 100 for QLQ-MY20 and QLQ-C30. For the functional scales (Global Health Status and Physical Functioning), higher scores indicate better HRQoL, whereas for the symptom scales (Fatigue, Pain, Disease Symptoms, and Side Effects of Treatment), lower scores indicate a better health state. Health utility values were derived from the EQ-5D using the UK general population weights algorithm,28 which provides a range of scores from worst imaginable health state (−0.594) to best imaginable health state (1.000).
Statistical analyses
Compliance rates at each scheduled assessment were calculated as the number of compliant patients divided by the number of patients with clinical data at that assessment. Analyses were performed on intention-to-treat patients; data cut off was May 24, 2013, corresponding to a median follow up of 37 months. In accordance with scoring requirements, patients were considered compliant if half of the questions from the QLQ-MY20 and QLQ-C30 were completed, and if all the questions from the EQ-5D were answered. Cross-sectional and longitudinal HRQoL analyses and estimation of overall treatment effects were performed and are described in detail in the Online Supplementary Appendix.
In order to assess if statistical differences translated into clinically meaningful differences/improvements, the minimal important difference (MID) associated with each domain was considered. MM-specific MIDs were applied to the QLQ-C30 and QLQ-MY20 domains: Global Health Status (MID=7); Physical Functioning (MID=9); Pain (MID=12); Fatigue (MID=10); Disease Symptoms (MID=10); and Side Effects of Treatment (MID=6).30,39,40 The Walters and Brazier41 MID of 0.07 was applied to the EQ-5D utility. Rigorous MID methods, in which the mean and 95% confidence intervals (CI) of change must meet the MID, were applied and are reported where relevant.
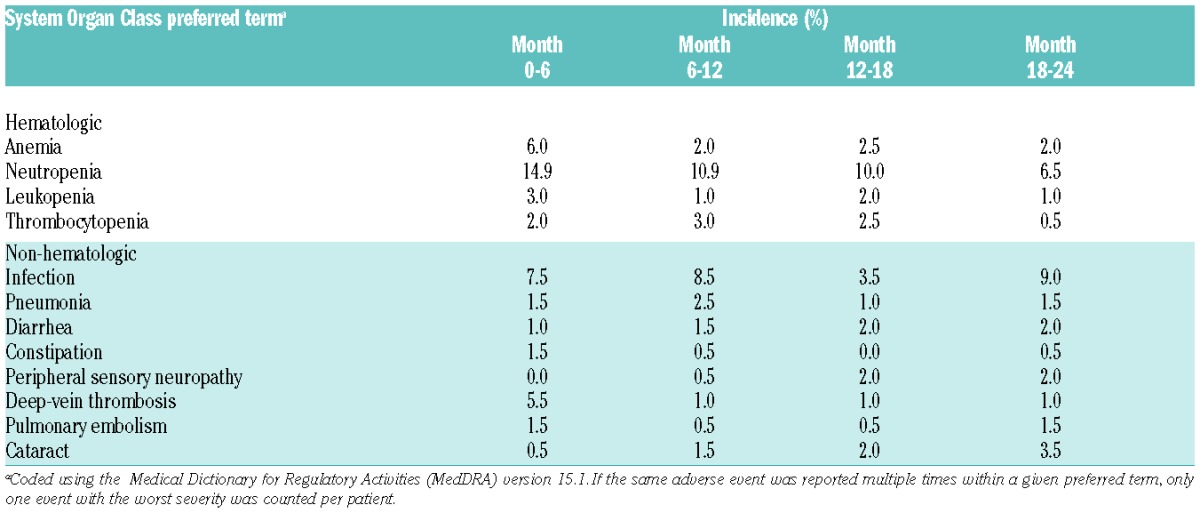
To complement the patient-reported Side Effects of Treatment data (QLQ-MY20 domain), a selection of the most predominant and clinically meaningful hematologic and non-hematologic grade 3–4 adverse events (AEs) is presented up to 24 months for patients receiving continuous Rd. The selected AEs presented here reflect closely those in previous reports of the FIRST study.18,19
Results
Base-line characteristics
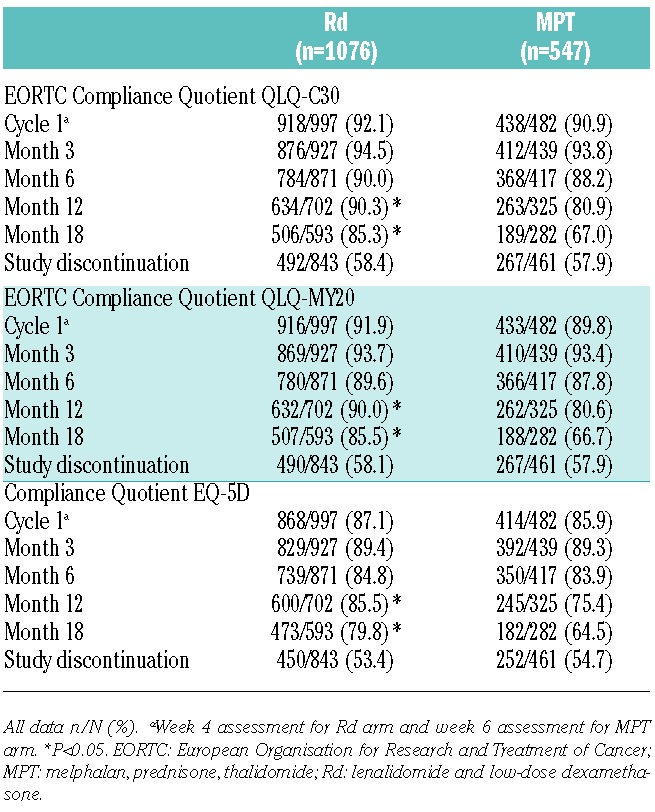
The FIRST study randomized a total of 1623 patients to Rd (n=1,076) versus MPT (n=547). Base-line patients’ characteristics were well balanced between treatment arms (Table 1), and no statistically significant differences in HRQoL scores between groups were observed at baseline (Table 2).
Table 1.
Base-line characteristics of patients included in the health-related quality-of-life analysis.
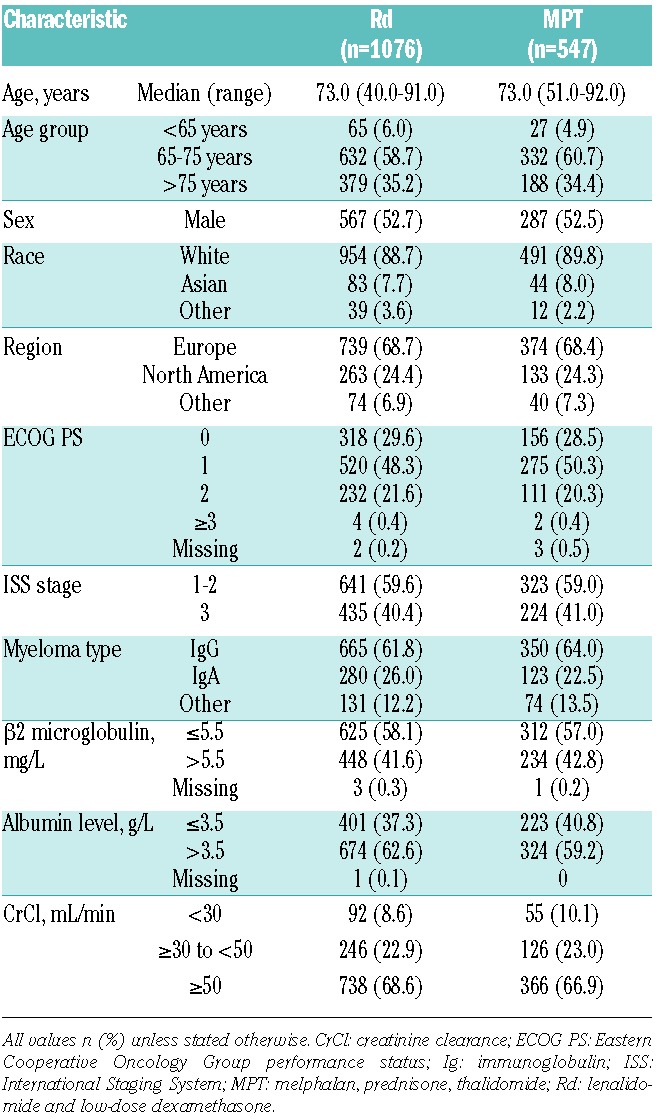
Table 2.
Mean scores of the selected HRQoL domains at baseline. HRQoL domains.
Compliance
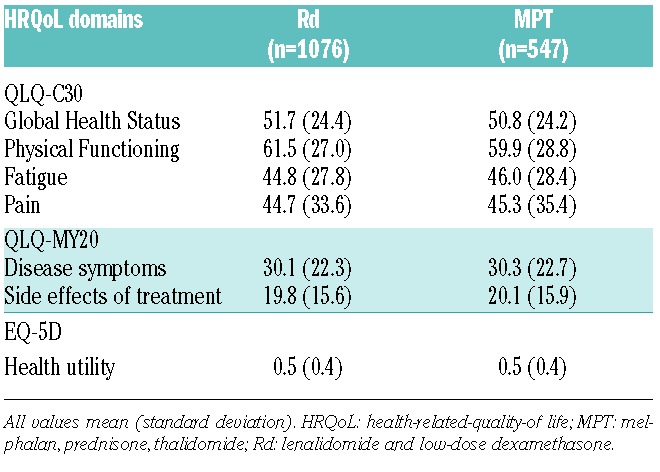
Rates with each of the questionnaires were high, particularly at the end of Cycle 1, and after 3 and 6 months (≥84%), and were similar between treatment arms. However, at Month 12 and Month 18, compliance rates were significantly lower in the MPT arm versus the Rd arm for all questionnaires (Table 3). Compliance at the study discontinuation visit was the lowest (approx. 53%-59%) of all the assessment visits in both treatment arms, but was not statistically significantly different between groups (Table 3).
Table 3.
Health-related quality-of-life reporting compliance.
HRQoL results
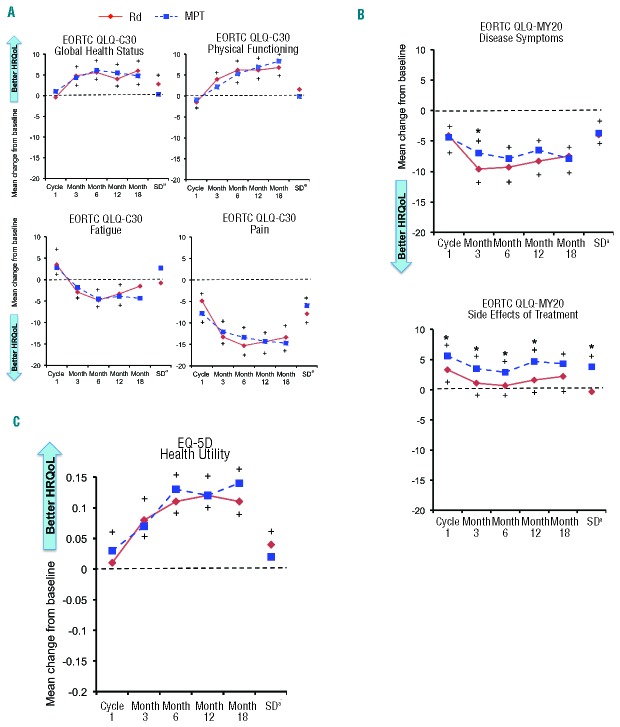
Statistically significant symptom relief, as measured by the QLQ-C30 Pain and Fatigue domains and QLQ-MY20 Disease Symptoms domain, was achieved in both arms. Both treatment arms showed statistically significant (P<0.05) reductions in pain (QLQ-C30 Pain and QLQ-MY20 Disease Symptoms domains) at all post-baseline assessments (Figure 1A and B). When an MID score of 12 for Pain (including the lower bound 95% CI) was applied, Rd demonstrated clinically meaningful improvement at Months 6 and 12, whereas MPT showed no clinically meaningful improvement. Rd demonstrated a significantly greater reduction in QLQ-MY20 Disease Symptoms compared with MPT at Month 3 (P=0.04), and an overall lower symptom score across all assessments (Figure 1B). The Rd arm also showed significant improvement in Fatigue from baseline at Month 3, Month 6, and Month 12 (Figure 1A). Although both arms showed worsening in the Side Effects of Treatment domain, as measured by the QLQ-MY20, the Rd arm showed consistently lower scores (fewer/less severe side-effects) across all post-baseline assessments, with all but Month 18 being statistically significantly (P<0.05) lower than the MPT arm (Figure 1B). The Side Effects of Treatment MID of 6 was not reached by any regimen; for Rd, the maximum score was 3.3 and for MPT it was 5.6.
Figure 1.
Cross-sectional analysis of mean HRQoL score changes from baseline per assessment visit and at study discontinuation in the Rd and MPT arms (EORTC QLQ-C30 and QLQ-MY20 total score range 1 to 100; EQ-5D total score range −0.594 to 1.000). (A) EORTC QLQ-C30. (B) EORTC QLQ-MY20. (C) EQ-5D. +Significant within-group change from baseline (P<0.05, 1-sample t-test). *Significant between-group difference in change from baseline (P<0.05, 2-sample t-test). aSD can occur at any time point. EORTC: European Organisation for Research and Treatment of Cancer; HRQoL: health-related quality-of-life; MPT: melphalan, prednisone, thalidomide; Rd: lenalidomide and low-dose dexamethasone; SD: study discontinuation.
To assess the tolerability of prolonged administration of lenalidomide and low-dose dexamethasone (continuous Rd arm), we analyzed selected grade 3–4 AEs by time of onset in the continuous Rd arm for patients who received lenalidomide for more than 24 months; the selected events occurred in 201 of 532 patients (Table 4). During the overall study period, neutropenia was the only grade 3–4 AE reported in 10% or over of patients receiving prolonged treatment, and was reported most frequently during the first six months. The incidence of anemia was highest during the first six months and decreased over time.
Table 4.
Selected grade 3–4 adverse events reported in more than 2% of patients by onset period for patients in the lenalidomide and low-dose dexamethasone arm.
Both Rd regimens and MPT improved patients’ HRQoL from baseline over the duration of the study across all preselected domains of the QLQ-C30 and EQ-5D questionnaires, but HRQoL dropped at progression. Statistically significant improvement (P<0.05) from baseline was observed in both treatment arms for functional scales Global Health Status, Physical Functioning, and EQ-5D Health Utility (Figure 1A and C) at all time points after Cycle 1. No statistically significant differences between treatment arms were reported for Global Health Status, Physical Functioning, and EQ-5D Health Utility. However, the Rd treatment arm demonstrated consistent clinically meaningful improvement in HRQoL as measured by EQ-5D at all post-baseline assessments except at Month 1. The MPT treatment arm demonstrated clinically meaningful improvement only at Month 3.
Mixed-model analysis
Results of the linear mixed-model repeated-measures analyses confirmed those observed in the cross-sectional analysis. Significant within-treatment improvements over time were observed in both treatment arms in all domains except Fatigue and Side Effects of Treatment (Figure 2A). A significant (P<0.0001) between-group difference in mean change from baseline was observed for the Side Effects of Treatment domain in favor of the Rd arm, indicating fewer severe side effects than the MPT arm (Figure 2B).
Figure 2.
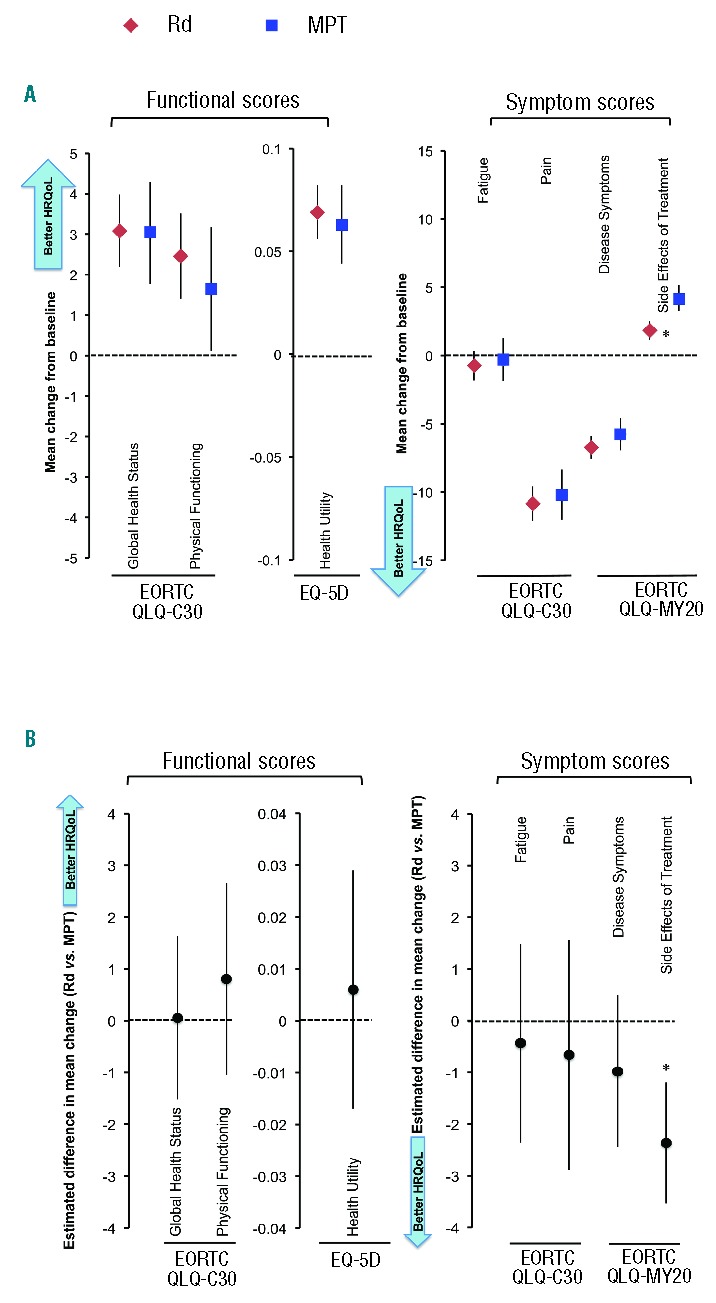
Linear mixed-model repeated-measures analysis of mean change from baseline and mean difference in change from baseline at 18 months in the Rd and MPT arms. (A) Mean change from baseline at 18 months. (B) Least-squares mean difference in change from baseline for Rd versus MPT at 18 months. Error bars represent 95% confidence intervals. *P<0.05. EORTC: European Organisation for Research and Treatment of Cancer; HRQoL: health-related quality-of-life; MPT: melphalan, prednisone, thalidomide; Rd: lenalidomide and low-dose dexamethasone.
Effect of age on HRQoL outcomes
We compared the difference in mean change from baseline between the Rd and MPT arms for patients aged 75 years or under (Rd, n=697; MPT, n=359) and over 75 years (Rd, n=379; MPT, n=188) in a post hoc analysis (Figure 3). Overall, within- and between-treatment HRQoL outcomes in patients aged 75 years or under were consistent with those observed for the overall study population, except Fatigue, which significantly improved from baseline in the Rd arm (P=0.0002), but not in the MPT arm. Changes in Side Effects of Treatment on the MM-specific QLQ-MY20 questionnaire showed a statistically significant benefit (fewer/less severe side effects) for Rd over the MPT arm in both age groups (P<0.05) (Figure 3A). In patients aged over 75 years, Pain and Disease Symptoms both showed significant improvement across treatment arms. Rd demonstrated statistically significant improvement for Health Utility. Fatigue significantly declined in both treatment arms. Physical Functioning scores declined significantly in the MPT arm, but not in the Rd arm, and were significantly (P<0.05) better in the Rd arm versus the MPT arm (Figure 3B).
Figure 3.
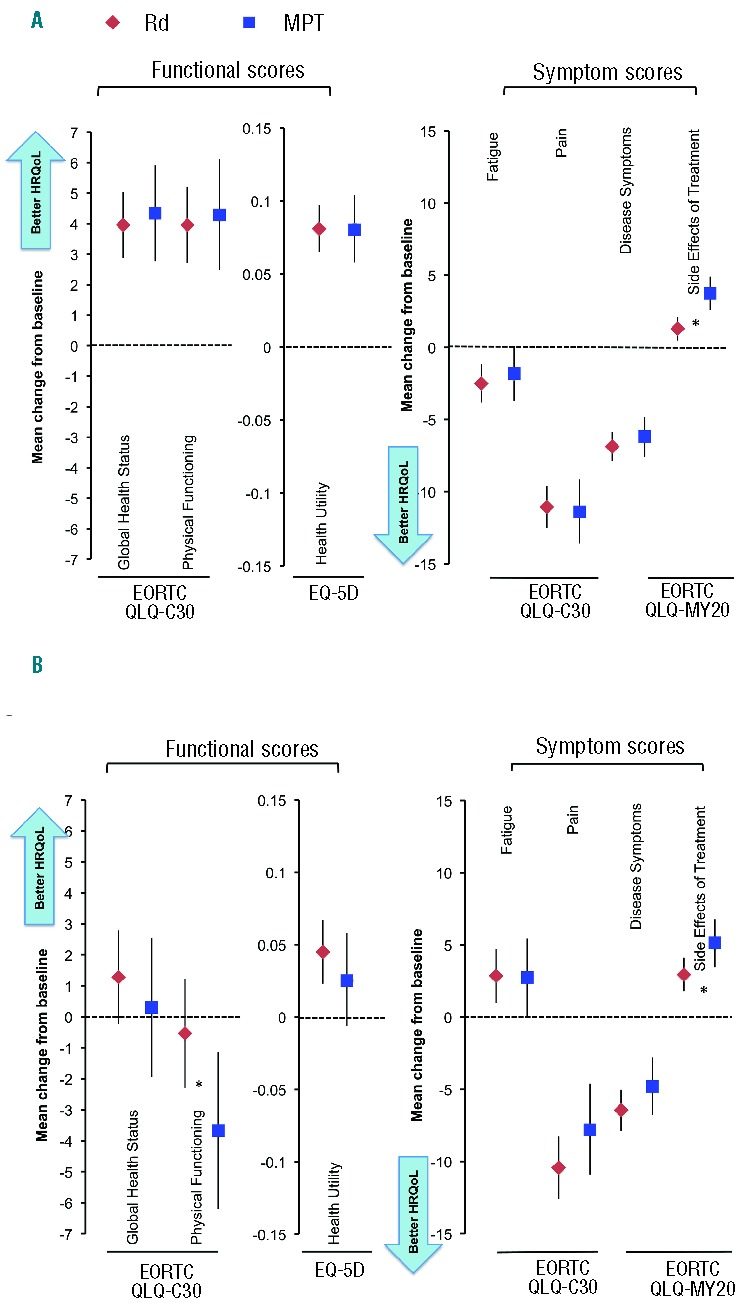
Linear mixed-model repeated-measures analysis: change from baseline at 18 months in patients aged ≤75 years in the Rd and MPT arms. (A) Patients aged ≤75 years. (B) Patients aged >75 years. *P<0.05 for between-group estimated difference in mean change from baseline. EORTC: European Organisation for Research and Treatment of Cancer; HRQoL: health-related quality-of-life; MPT: melphalan, prednisone, thalidomide; Rd: lenalidomide and low-dose dexamethasone.
Discussion
We show that the improved PFS with Rd is accompanied by an improvement in HRQoL, showing both statistically significant and clinically meaningful changes from baseline. Rd showed statistical superiority to MPT in Side Effects of Treatment, and no evidence of inferiority to MPT in any of the pre-selected HRQoL domains. This finding is of importance because it demonstrates that the improved PFS in this study does not come at a cost of increased symptoms or decreased HRQoL.
The FIRST study is the largest phase III trial conducted in transplant-ineligible elderly NDMM patients to date, and the first to evaluate HRQoL in NDMM patients receiving Rd. Primary efficacy results from this study indicated that continuous Rd administration significantly improved PFS and OS at the interim analysis compared with the MPT regimen,19 highlighting a double paradigm change in a setting in which alkylating agents and fixed-duration chemotherapy have long been considered treatment standards. Indeed, non-MP regimens are increasingly challenging traditional melphalan-based approaches in NDMM patients, as seen from randomized phase III trial data, including E4A0314 and UPFRONT.42 The observed differences in HRQoL between continuous Rd and MPT likely reflect both the good tolerability of the Rd regimen (leading to fewer side effects of treatment) and prolonged time to progression with continuous Rd. Improvements in HRQoL have previously been reported during treatment with melphalan, prednisone, lenalidomide followed by lenalidomide maintenance (MPR-R) in patients with NDMM,39,43 and the addition of thalidomide to MP did not negatively affect HRQoL.44 However, our results contrast with the HRQoL trade-off reported with bortezomib in the VISTA trial, which compared MPV versus MP in NDMM patients and in which MPV had superior PFS and OS efficacy, but where patients’ HRQoL was significantly compromised, especially in the first four cycles of treatment.45,46
In the FIRST trial, although HRQoL was improved and maintained during the progression-free state, it deteriorated when disease progression occurred, regardless of the treatment received. This finding augments the clinical importance/benefit of the prolonged PFS, and time to progression observed with Rd versus MPT,19 as a longer PFS in this comparison is associated with better HRQoL. Rd treatment demonstrated significant improvement in the Disease Symptoms domain and significantly lower scores for the MM-specific QLQ-MY20 Side Effects of Treatment domain at specific time points versus MPT (although not to the level of MID). Improvements corresponded with a lower incidence of neurotoxicity and hematologic AEs with Rd, a higher discontinuation rate with MPT, and improved response rates with continuous Rd and Rd18 (75.1% and 73.4%, respectively, versus 62.3% with MPT), as seen in the primary efficacy data.19 Treatment with MPT was associated with greater HRQoL worsening in the Side Effects of Treatment domain compared with Rd treatment. Indeed, higher rates of grade 3–4 neutropenia, leukopenia, constipation, and peripheral neuropathy were observed in the MPT arm,19 which may explain the lower rate of HRQoL compliance among patients randomized to MPT. Indeed, previous studies have reported that the addition of thalidomide to MP results in increased grade 3–4 toxicity.6,47 In our study, the Rd doublet regimen was better tolerated than MPT over time. Patients who received MPT discontinued treatment sooner and experienced AEs leading to study drug discontinuation prior to PD more frequently than those randomized to Rd.19 We have now shown that HRQoL improved after treatment initiation and was generally maintained while patients were progression-free, but deteriorated with PD. The HRQoL decrease upon PD captured quantitatively here is also consistent with qualitative results on the burden of relapse on MM patients’ emotional and physical well-being.48 This adds additional support to the validity of the HRQoL measures included in the FIRST trial; further evidence of their reliability was also seen in the similarity in base-line scores between the FIRST, VISTA,45 and HOVON 4949 studies.
Analysis by age showed that, compared with MPT, Rd treatment did not negatively affect general HRQoL in patients aged over 75 years. Indeed, only Rd showed a statistically significant improvement in EQ-5D Health Utility in this age group, and also showed significantly less worsening of Physical Functioning scores compared with MPT. Both treatment arms showed improvement in the pain scales.
The sustained improvement in HRQoL reported in the present study is indicative of good tolerability. One caveat, however, is that we did not collect HRQoL data beyond 18 months of treatment; therefore, we are unable to draw conclusions on the effects of long-term continuous Rd treatment on patients’ HRQoL. This is of particular significance as, in addition to the FIRST trial, several clinical studies support the value of continuous versus fixed-duration treatment, making it important that the improved clinical outcomes do not come at the cost of reduced HRQoL. To try to answer this question, we carried out an exploratory analysis to look for trends in AEs during up to two years of treatment. The analysis did not show an increase in incidence of AEs during Months 18–24, suggesting that, from the perspective of side effects, HRQoL does not deteriorate beyond 18 months of treatment. Furthermore, during the MM-015 phase III trial, with a median follow up of 30 months, more patients treated with MPR-R achieved minimal important improvements in HRQoL outcomes compared with those on MP, and there were no significant differences in HRQoL outcomes between patients on continuous treatment versus placebo.39,43
Although not all the statistically significant changes reached the MID threshold, Rd showed evidence of clinically meaningful improvement in Pain and in HRQoL as measured by EQ-5D, whereas MPT failed to demonstrate a clinically meaningful improvement in any domain (with the exception of 1 time point in EQ-5D). Neither treatment showed a clinically significant worsening of HRQoL in any domain. With regard to the Side Effects of Treatment domain, MID analysis showed that patients in the MPT arm approached the negative MID threshold of 6, whereas those in the Rd arm did not.
Future analyses should investigate MID at the individual patient level to understand what proportion of patients experienced a clinically meaningful improvement in each treatment group and whether there is a difference between groups.
In summary, efforts to improve progression-free intervals and survival are essential in the case of an incurable disease such as MM. However, it is just as important to ensure that a patient’s HRQoL is maintained and not compromised during the extended survival period and that the side-effects of treatment are not worse than the disease symptoms. Continuous lenalidomide treatment has been shown to further improve PFS and disease outcomes, and improved survival rates have been reported in NDMM patients in recent years. The manageable and favorable toxicity and HRQoL profiles of Rd versus MPT may facilitate patient adherence, positioning the alkylator-free oral Rd regimen as a very efficient and well-tolerated treatment in a first-line setting. Our results further establish the combination of lenalidomide and low-dose dexamethasone as a new standard of care for initial therapy of myeloma by demonstrating superior QoL during treatment compared with MPT, although longer-term follow up data (>18 months) on cumulative toxicity and HRQoL are needed.50
Acknowledgments
We thank the patients who participated in this study, the staff members at the study sites who cared for them, and the representatives of the sponsors who were involved in data gathering and analyses. We thank Vanessa Gray-Schopfer (PhD) and Ron Hogg (PhD), OmniScience SA, Adam Hutchings, Dolon Ltd, and Keisha Peters, Excerpta Medica, who provided medical writing services and assistance funded by Celgene Corporation. The authors were fully responsible for all content and editorial decisions for this manuscript.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work was supported by Celgene Corporation.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111(5):2521–2526. [DOI] [PubMed] [Google Scholar]
- 3.Kristinsson SY, Landgren O, Dickman PW, Derolf AR, Bjorkholm M. Patterns of survival in multiple myeloma: a population-based study of patients diagnosed in Sweden from 1973 to 2003. J Clin Oncol. 2007;25(15):1993–1999. [DOI] [PubMed] [Google Scholar]
- 4.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2013;28(5):1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Multiple myeloma. Version 1. 2014. Available at: http://www.nccn.org. [DOI] [PubMed]
- 6.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209–1218. [DOI] [PubMed] [Google Scholar]
- 7.Fayers PM, Palumbo A, Hulin C, et al. Thalidomide for previously untreated elderly patients with multiple myeloma: metaanalysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011;118(5):1239–1247. [DOI] [PubMed] [Google Scholar]
- 8.Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664–3670. [DOI] [PubMed] [Google Scholar]
- 9.Kapoor P, Rajkumar SV, Dispenzieri A, et al. Melphalan and prednisone versus melphalan, prednisone and thalidomide for elderly and/or transplant ineligible patients with multiple myeloma: a meta-analysis. Leukemia. 2011;25(4):689–696. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig H, Miguel JS, Dimopoulos MA, et al. International Myeloma Working Group recommendations for global myeloma care. Leukemia. 2014;28(5):981–992. [DOI] [PubMed] [Google Scholar]
- 11.Moreau P, San Miguel J, Ludwig H, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi133–vi137. [DOI] [PubMed] [Google Scholar]
- 12.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. [DOI] [PubMed] [Google Scholar]
- 13.Palumbo A, Attal M, Roussel M. Shifts in the therapeutic paradigm for patients newly diagnosed with multiple myeloma: maintenance therapy and overall survival. Clin Cancer Res. 2011;17(6):1253–1263. [DOI] [PubMed] [Google Scholar]
- 14.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. [DOI] [PubMed] [Google Scholar]
- 16.Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23(11):2147–2152. [DOI] [PubMed] [Google Scholar]
- 17.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357(21):2133–2142. [DOI] [PubMed] [Google Scholar]
- 18.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906–917. [DOI] [PubMed] [Google Scholar]
- 19.Facon T, Dimopoulos MA, Dispenzieri A, et al. Initial phase 3 results of the FIRST (Frontline Investigation of Lenalidomide + Dexamethasone versus Standard Thalidomide) trial (MM-020/IFM 07 01) in newly diagnosed multiple myeloma (NDMM) patients (pts) ineligible for stem cell transplantation (SCT). Blood. 2013;122(21):Abstract 2. [Google Scholar]
- 20.Molassiotis A, Wilson B, Blair S, Howe T, Cavet J. Unmet supportive care needs, psychological well-being and quality of life in patients living with multiple myeloma and their partners. Psychooncology. 2011;20(1): 88–97. [DOI] [PubMed] [Google Scholar]
- 21.Mols F, Oerlemans S, Vos AH, et al. Health-related quality of life and disease-specific complaints among multiple myeloma patients up to 10 yr after diagnosis: results from a population-based study using the PROFILES registry. Eur J Haematol. 2012;89(4):311–319. [DOI] [PubMed] [Google Scholar]
- 22.Poulos AR, Gertz MA, Pankratz VS, Post-White J. Pain, mood disturbance, and quality of life in patients with multiple myeloma. Oncol Nurs Forum. 2001;28(7):1163–1171. [PubMed] [Google Scholar]
- 23.Johnsen AT, Tholstrup D, Petersen MA, Pedersen L, Groenvold M. Health related quality of life in a nationally representative sample of haematological patients. Eur J Haematol. 2009;83(2):139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palumbo A, Mina R. Part II: role of maintenance therapy in transplant-ineligible patients. J Natl Compr Canc Netw. 2013;11(1):43–49. [DOI] [PubMed] [Google Scholar]
- 25.San Miguel JF, Mateos MV. Advances in the treatment for newly diagnosed multiple myeloma patients ineligible for autologous stem cell transplantation. Leuk Suppl. 2013;2(S1):S21–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 27.Cocks K, Cohen D, Wisloff F, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur J Cancer. 2007;43(11):1670–1678. [DOI] [PubMed] [Google Scholar]
- 28.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–1108. [DOI] [PubMed] [Google Scholar]
- 29.Gulbrandsen N, Wisloff F, Brinch L, et al. Health-related quality of life in multiple myeloma patients receiving high-dose chemotherapy with autologous blood stem-cell support. Med Oncol. 2001;18(1): 65–77. [DOI] [PubMed] [Google Scholar]
- 30.Kvam AK, Fayers PM, Wisloff F. Responsiveness and minimal important score differences in quality-of-life questionnaires: a comparison of the EORTC QLQ-C30 cancer-specific questionnaire to the generic utility questionnaires EQ-5D and 15D in patients with multiple myeloma. Eur J Haematol. 2011;87(4):330–337. [DOI] [PubMed] [Google Scholar]
- 31.Pickard AS, Wilke CT, Lin HW, Lloyd A. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics. 2007;25(5): 365–384. [DOI] [PubMed] [Google Scholar]
- 32.Stead ML, Brown JM, Velikova G, et al. Development of an EORTC questionnaire module to be used in health-related quality-of-life assessment for patients with multiple myeloma. European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Br J Haematol. 1999;104(3):605–611. [DOI] [PubMed] [Google Scholar]
- 33.Uyl-de Groot CA, Buijt I, Gloudemans IJ, Ossenkoppele GJ, Berg HP, Huijgens PC. Health related quality of life in patients with multiple myeloma undergoing a double transplantation. Eur J Haematol. 2005;74(2):136–143. [DOI] [PubMed] [Google Scholar]
- 34.Wisloff F, Eika S, Hippe E, et al. Measurement of health-related quality of life in multiple myeloma. Nordic Myeloma Study Group. Br J Haematol. 1996;92(3): 604–613. [DOI] [PubMed] [Google Scholar]
- 35.Wisloff F, Hjorth M. Health-related quality of life assessed before and during chemotherapy predicts for survival in multiple myeloma. Nordic Myeloma Study Group. Br J Haematol. 1997;97(1):29–37. [DOI] [PubMed] [Google Scholar]
- 36.Osborne TR, Ramsenthaler C, Siegert RJ, Edmonds PM, Schey SA, Higginson IJ. What issues matter most to people with multiple myeloma and how well are we measuring them? A systematic review of quality of life tools. Eur J Haematol. 2012;89(6):437–457. [DOI] [PubMed] [Google Scholar]
- 37.EORTC QLQ-C30 Scoring Manual. EORTC, Brussels. 3rd ed., 2001. Available from: http://groups.eortc.be/qol/manuals.
- 38.Cheung K, Oemar M, Oppe M, Rabin R. On behalf of the EuroQol Group. EQ-5D User Guide. Basic information on how to use EQ-5D. version 2.0., March 2009. Available from: http://www.euroqol.org.
- 39.Dimopoulos MA, Delforge M, Hajek R, et al. Lenalidomide, melphalan, and prednisone, followed by lenalidomide maintenance, improves health-related quality of life in newly diagnosed multiple myeloma patients aged 65 years or older: results of a randomized phase III trial. Haematologica. 2013;98(5):784–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kvam AK, Fayers P, Wisloff F. What changes in health-related quality of life matter to multiple myeloma patients? A prospective study. Eur J Haematol. 2010; 84(4):345–353. [DOI] [PubMed] [Google Scholar]
- 41.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14(6):1523–1532. [DOI] [PubMed] [Google Scholar]
- 42.Niesvizky R, Flinn IW, Rifkin R, et al. Patient-reported quality of life (QoL) in elderly, newly diagnosed multiple myeloma (MM) patients receiving bortezomib-based combinations: results from all randomized patients in the community-based, phase 3b UPFRONT study. Blood. 2011;118(21): Abstract 1864. [Google Scholar]
- 43.Dimopoulos MA, Palumbo A, Hajek R, et al. Factors that influence health-related quality of life in newly diagnosed patients with multiple myeloma aged ≥ 65 years treated with melphalan, prednisone and lenalidomide followed by lenalidomide maintenance: results of a randomized trial. Leuk Lymphoma. 2014;55(7):1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verelst SG, Termorshuizen F, Uyl-de Groot CA, et al. Effect of thalidomide with melphalan and prednisone on health-related quality of life (HRQoL) in elderly patients with newly diagnosed multiple myeloma: a prospective analysis in a randomized trial. Ann Hematol. 2011;90(12):1427–1439. [DOI] [PubMed] [Google Scholar]
- 45.Delforge M, Dhawan R, Robinson D, Jr, et al. Health-related quality of life in elderly, newly diagnosed multiple myeloma patients treated with VMP vs. MP: results from the VISTA trial. Eur J Haematol. 2012;89(1):16–27. [DOI] [PubMed] [Google Scholar]
- 46.Sonneveld P, Verelst SG, Lewis P, et al. Review of health-related quality of life data in multiple myeloma patients treated with novel agents. Leukemia. 2013;27(10):1959–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006; 367(9513):825–831. [DOI] [PubMed] [Google Scholar]
- 48.Hulin C, Hansen T, Laurenson S, Lawrence J, Streetly M. A European study of the emotional and physical impact of relapse on patients with multiple myeloma. Haematologica. 2014;99(Suppl 1):Abstract P635. [Google Scholar]
- 49.Wijermans P, Schaafsma M, Termorshuizen F, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol. 2010;28(19):3160–3166. [DOI] [PubMed] [Google Scholar]
- 50.Van de Donk NW, Gorgun G, Groen RW, et al. Lenalidomide for the treatment of relapsed and refractory multiple myeloma. Cancer Manag Res. 2012;4:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]