Allogeneic stem cell transplantation (allo-SCT) has the potential to cure patients with various hematologic malignancies. Significant morbidity and mortality, however, occurs following allo-SCT due to complications such as graft-versus-host disease (GvHD), infections and conditioning-related toxicity. In addition to early morbidity, it is increasingly appreciated that long-term allo-SCT survivors have an increased incidence of cardiovascular risk factors and have a greater burden of cardiovascular morbidity1,2 with odds ratios ranging from 2.3 to 3.0 in recipients of allo-SCT compared to a matched general population.2
There is mounting evidence that many of the complications of allo-SCT are at least partially related to endothelial damage. Consequently, there is a high need for parameters to accurately assess allo-SCT conditioning regimen-related effects on the endothelium as well as the potential role of the endothelium in the untoward events accompanying allo-SCT. Circulating endothelial cells (CECs) are mature endothelial cells present in the peripheral circulation and are a surrogate marker for endothelial damage. In a previous study to investigate the impact of conditioning regimen-related endothelial damage following allo-SCT, it was demonstrated that patients who received reduced intensity conditioning (RIC) had significantly lower CEC numbers than patients who underwent myeloablative (MAB) conditioning.3 However, patients were only followed for 21 days post transplant and consequently the extent of long-term endothelial damage was not established.
Given the current trend in allo-SCT towards the use of more RIC regimens,4 we investigated the impact of RIC versus MAB conditioning on endothelial damage in greater detail. CECs were enumerated at fixed time points in a large group of adults undergoing allo-SCT for up to two years post transplant. We also explored the use of CECs as a putative marker for GvHD and infections.
Our retrospective study included 112 adult patients receiving allo-SCT in the Erasmus MC Cancer Institute. One patient was excluded because of the application of a unique, alternatively intensified conditioning regimen prior to double umbilical cord blood transplantation (dUCBT), which differed from other dUCBT recipients and also differed from RIC and MAB conditioned patients. Patients’ characteristics from the 111 remaining patients are presented in the Online Supplementary Table S1. All patients were transplanted between August 2009 and November 2011 in the context of two prospective trials. Sibling donor patients (n=37) and matched unrelated donor patients (n=56) were included in the context of the HOVON 96 study (Netherlands Trial Registry: NTR2252), while dUCBT (n=18) were included in the context of the HOVON 106 study (NTR1573).5,6 MAB conditioning was received by 24 patients, consisting mainly of myeloablative TBI (12 Gy) and cyclophosphamide. RIC was received by 69 patients, consisting mainly of 2 Gy TBI combined with fludarabine.7 Lastly, 18 patients received a RIC-UCB consisting of 2×2 Gy total body irradiation (TBI) combined with fludarabine and cyclophosphamide prior to UCBT. None of the patients received in vivo T-cell depletion. All patients received cyclosporine A (CsA; trough level 250–350 μg/L) for at least three months and mycophenolate mofetil (MMF; 2×16 mg/kg) for at least one month as additional post-transplant GvHD prophylaxis with gradual tapering of the drug thereafter. Further details of the conditioning regimen, supportive care, CEC enumeration and statistical analysis are provided in the Online Supplementary Appendix. The minimal follow-up time was one year, and the median follow-up time for patients still alive was 34 months.
A total of 357 peripheral blood samples were evaluated for the presence of CECs at baseline, one (dUCBT recipients only), two (dUCBT recipients only), three, six, 12 and 24 months post transplant. Based on the number of follow-up days, we expected 473 samples, while 357 were analyzed, indicating that 75% of the expected samples were analyzed. CECs were defined as CD34+, CD146+, DRAQ5+, CD45− events and enumerated according to our previously described flow cytometric approach.8 Absolute CD34 counts did not correlate with CEC counts (r=0.09) (Online Supplementary Figure S1).
The influence of RIC and MAB conditioning on CEC kinetics is presented in Figure 1 (left panel). While there was no difference in CEC numbers between RIC and the MAB conditioned patients pre-transplant (P=0.71), patients who received MAB conditioning had higher CEC numbers than RIC recipients for up to 12 months following allo-SCT (P=0.000, P=0.000 and P=0.002 at 3, 6 and 12 months post allo-SCT, respectively). At 24 months following allo-SCT, CEC numbers were similar in RIC and MAB conditioned patients (P=0.64). In the MAB group, CEC numbers were higher 12 months post transplant than before transplant (one-sided Wilcoxon signed-rank test P=0.04).
Figure 1.
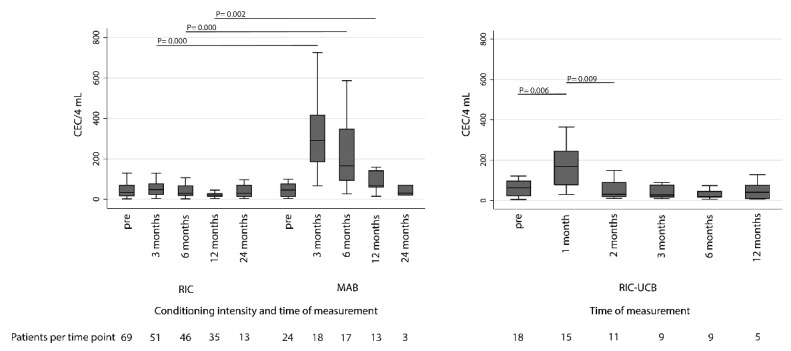
Box-and-whisker plots showing the influence of conditioning intensity on circulating endothelial cells (CEC). Left panel shows CEC kinetics in myeloablative (MAB) and reduced intensity conditioning (RIC) conditioned patients. Right panel shows CEC kinetics in dUCBT patients receiving RIC-UCB conditioning. (Boxes show 25th percentile, median and 75th percentile, whiskers show the lower and upper adjacent values, according to Tukey).
In patients receiving an RIC-UCB conditioning additional CEC numbers at one and two months post allo-SCT were available. A significant rise in CEC numbers was observed at one month following dUCBT (P=0.006), to decrease significantly towards base-line values from two months post allo-SCT onwards (P=0.009) (Figure 1, right panel).
We observed a CTC grade III-IV infection in 54%, 17% and 18% of the patients in the first three months, month 3 to month 6 and month 6 to month 12 post transplant, respectively. No significant differences in CEC numbers were observed at three months (P=0.12), 6 months (P=0.51) and 12 months (P=0.99) post transplant between those patients with versus those without a grade III-IV infection. Apart from CTC grade III-IV infections, cutomegalovirus (CMV) reactivations including those meeting CTC grade II criteria were separately scored in all patients. In 37 patients (33%), a CMV reactivation was observed. No significant differences in CEC numbers and the occurrence of CMV reactivation at three, six and 12 months post allo-SCT were observed.
In multivariable analysis at three, six and 12 months post transplant taking into account age, sex, HCT-CI score, donor source, conditioning intensity (MAB, RIC and UCB conditioning), occurrence of GvHD and occurrence of infections, MAB conditioning was associated with higher CEC numbers (P=0.000, P=0.000 and P=0.008, respectively) (Table 1). At three months following allo-SCT, the occurrence of aGvHD grade II-IV appeared associated with lower CEC numbers (P=0.003). The occurrence of cGvHD, limited and/or extensive was also associated with lower CEC numbers at six (P=0.019) and 12 (P=0.012) months post transplant.
Table 1.
Multivariable linear regression analysis on variables associated with the number of circulating endothelial cells.
We further explored the reasons underlying the differences in CEC numbers between patients experiencing GvHD versus those who had not. To exclude the possibility that our findings were due to the occurrence of donor-derived CECs in our assay, we evaluated CEC-chimerism one month after transplantation by using HLA class II mismatch-specific monoclonal antibodies in two dUCBT recipients with a class II mismatch with their donor graft (Figure 2A and B). We did not observe CEC-chimerism: all CECs appeared of recipient origin. We then hypothesized that the unexpected lower number of CEC in patients with overt GvHD could be due to a direct immune response of alloreactive donor lymphocytes towards recipient CEC. Unfortunately, it appeared technically impossible to visualize an immune response towards the small number of CECs that were detected by flow cytometry. Since an immune response of alloreactive donor T cells to CECs would require HLA-expression on CECs, we evaluated in peripheral blood mononuclear cell (PMBC) samples the percentage of CECs expressing a HLA class I antigen (8 samples) and HLA-class II antigens (13 samples). A large subset of CECs was found to express an HLA class I antigen (median CEC HLA class I positive 94%, range 81%–100%) as well as HLA class II antigens (median CEC HLA-DR positive 86%, range 80%–99%) (Figure 2C and D).
Figure 2.
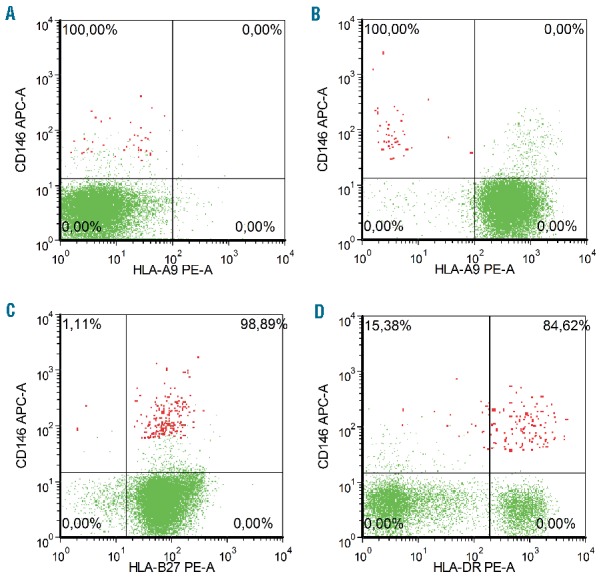
Presence of donor-specific HLA-A9 on lymphocytes (green) and CECs (red). (A) shows that 100% of all CECs did not express HLA-A9 prior to SCT. (B) shows that all CECs at one month following allo-SCT are of recipient origin, while virtually all lymphocytes are of donor origin and express HLA-A9. (C and D) show representative images of HLA class I and HLA class II expression on lymphocytes (green) and CECs (red). (C) Shows that HLA-B27 is expressed in 98.89% of all CECs at three months post-transplant (both donor and recipient harbored the HLA phenotype HLA-B27). (D) Shows HLA-DR expression in 84.62% of all CECs at three months post-transplant.
This study confirmed the previous observation that MAB induces more endothelial damage than RIC in the first month following allo-SCT.3,9 We now showed for the first time that in MAB-conditioned patients, endothelial damage is present for at least 12 months following transplantation. In contrast, in dUCBT patients receiving a 4 Gy TBI conditioning, a significant rise in CEC numbers as opposed to baseline was observed only at one month following allo-SCT. This suggests that endothelial damage following a relatively modest dose of TBI is only present for a short period of time. At 24 months post transplant, CEC numbers of RIC and MAB conditioned patients were similar. The prolonged endothelial damage in patients receiving MAB conditioning may possibly be associated with more long-term cardiovascular conditions, as compared to RIC.
This may be an important observation, especially since MAB conditioning is predominantly applied in younger patients, who will have more time to actually develop cardiovascular conditions. Given this, our data may support the suggestion to further examine the use of RIC regimens in subsets of younger patients,10 especially in younger patients who already have relevant cardiovascular risk factors or comorbidity. In contrast with some reports that suggested that GvHD is associated with increased endothelial damage,11–13 we observed significantly lower CEC numbers in patients who experienced GvHD.
It should, however, be noted that these previous studies were not all performed in humans, and different methods to assess endothelial damage were used. Following our observations that CECs strongly express HLA class I and class II antigens, we formulated the hypothesis that an alloreactive immune response may be exerted against CECs.
Because we did not observe CEC-chimerism in 2 patients at one month following SCT, it is unlikely that the lower CEC numbers in patients with overt GvHD were due to the occurrence of donor-derived CECs. Unfortunately, no suitable PBMC samples were available to test the occurrence of CEC-chimerism at later time points following transplantation. Prospective studies investigating whether or not CEC-chimerism occurs in the post-transplant period, and if so from what time point onwards, are needed.
We also hypothesized that GvHD-associated treatments, such as steroids and calcineurin inhibitors (CNI), which were routinely given to all patients, might account for the occurrence of less CECs in GvHD patients. However, since increased endothelial dysfunction has been linked to prednisone use14 and cortisol excess,15 and therefore likely leads to higher CEC numbers, it is unlikely that the lower CEC numbers in GvHD patients are due to steroid treatment. In addition, patients with and without GvHD in our study were fairly balanced regarding CNI treatment and had proper cyclosporine or tacrolimus trough levels at the time of CEC measurement, making it even more unlikely that our findings were due to differences in CNI treatment or CNI toxicity.
Another explanation for the lower CEC numbers in GvHD patients could be that vascular damage occurs to such a large extent that only endothelial fragments remain, which do not meet our criteria for intact endothelial cells and are, therefore, missed by the current flow cytometric approach.
There are several potential limitations of this study. Fixed time points were chosen to evaluate long-term changes related to the conditioning intensity, but are less suitable for the analysis of allo-SCT related complications such as GvHD. Clearly, these complications do not necessarily coincide with these fixed time points, and therefore rapid CEC kinetic changes might be missed by this approach. Other limitations include the relatively small number of patients for subgroup analyses, especially at 24 months post allo-SCT, and the relatively short follow up, which made it impossible to explore whether those patients with highest CEC numbers are indeed at increased risk of developing cardiovascular diseases.
In summary, we present the largest study to date evaluating the impact of conditioning regimens on CECs as parameter for vascular damage in allo-SCT. We found that patients receiving MAB conditioning have long-term endothelial damage as opposed to patients receiving RIC. Further studies are warranted to investigate the clinical relevance of the increased CEC numbers in MAB patients, especially regarding the possible association with long-term cardiovascular outcomes. In addition, we observed lower CEC numbers in GvHD patients, which may possibly be explained by a direct immune response against CECs. Future research should investigate whether such an immune response is indeed present.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Armenian SH, Sun CL, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120(23):4505–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow EJ, Baker KS, Lee SJ, et al. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32(3):191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woywodt A, Scheer J, Hambach L, et al. Circulating endothelial cells as a marker of endothelial damage in allogeneic hematopoietic stem cell transplantation. Blood. 2004;103(9):3603–3605. [DOI] [PubMed] [Google Scholar]
- 4.Passweg JR, Baldomero H, Bregni M, et al. Hematopoietic SCT in Europe: data and trends in 2011. Bone Marrow Transplant. 2013;48(9):1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somers JA, Braakman E, van der Holt B, et al. Rapid induction of single donor chimerism after double umbilical cord blood transplantation preceded by reduced intensity conditioning: results of the HOVON 106 phase II study. Haematologica. 2014;99(11):1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somers J, Brand A, van Hensbergen Y, et al. Double Umbilical Cord Blood Transplantation: A Study of Early Engraftment Kinetics in Leukocyte Subsets using HLA-Specific Monoclonal Antibodies. Biology Blood Marrow Transplant. 2013;19(2):266–273. [DOI] [PubMed] [Google Scholar]
- 7.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101(4):1620–1629. [DOI] [PubMed] [Google Scholar]
- 8.Kraan J, Strijbos MH, Sieuwerts AM, et al. A new approach for rapid and reliable enumeration of circulating endothelial cells in patients. J Thromb Haemost. 2012;10(5):931–939. [DOI] [PubMed] [Google Scholar]
- 9.Zeng L, Yan Z, Wang L, Du B, Pan X, Xu K. Irradiation is an early determinant of endothelial injury during hematopoietic stem cell transplantation. Transplant Proc. 2008;40(8):2661–2664. [DOI] [PubMed] [Google Scholar]
- 10.Estey EH. Intensity of conditioning for allogeneic haemopoetic cell transplantation. Lancet Oncol. 2012;13(10):966–968. [DOI] [PubMed] [Google Scholar]
- 11.Salat C, Holler E, Kolb HJ, Pihusch R, Reinhardt B, Hiller E. Endothelial cell markers in bone marrow transplant recipients with and without acute graft-versus-host disease. Bone Marrow Transplant. 1997;19(9):909–914. [DOI] [PubMed] [Google Scholar]
- 12.Yan Z, Zeng L, Jia L, Xu S, Ding S. Increased numbers of circulating ECs are associated with systemic GVHD. Int J Lab Hematol. 2011;33(5):507–515. [DOI] [PubMed] [Google Scholar]
- 13.Almici C, Skert C, Verardi R, et al. Changes in circulating endothelial cells count could become a valuable tool in the diagnostic definition of acute graft-versus-host disease. Transplantation. 2014;98(7):706–712. [DOI] [PubMed] [Google Scholar]
- 14.Turner E, Dishy V, Chung CP, et al. Endothelial function in systemic lupus erythematosus: relationship to disease activity, cardiovascular risk factors, corticosteroid therapy, and coronary calcification. Vasc Health Risk Manag. 2005;1(4):357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitworth JA, Williamson PM, Mangos G, Kelly JJ. Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag. 2005;1(4):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]


