Abstract
How blood samples are processed and stored before being analyzed for alcohol levels is of concern in the forensic and toxicological fields, and is important in the standardization of research methods. This experiment explored for systematic differences in ethanol levels among several methods of processing and storing blood samples. Five adults consumed a standard alcoholic drink (0.7 g/kg) over a 15-min period, and blood samples were taken 5 times during a 3-h period following drinking onset. Samples for plasma and whole blood were drawn into Vacutainers® containing either an anticoagulant or an anticoagulant plus preservative. Samples for serum were drawn into Vacutainers containing no additives or a preservative only. Separate sets of samples were analyzed on the day of the study, after storage at room temperature (25°C) for 24 h, after storage at room temperature for 10 days, or after 10 days of refrigerated storage. Neither processing condition (i.e., type of additive) nor storage condition significantly affected ethanol levels. Consistent with the literature, plasma and serum samples had significantly higher concentrations of ethanol than whole blood. This study shows that blood samples containing ethanol at levels ranging from 60 to 90 mg/dL (0.06 to 0.09 mg%) are not significantly altered by the type of collection tube used or storage condition during a 10-day period.
Introduction
The accurate determination of alcohol concentration levels in human blood samples is important for valid results in research studies and often has critical medical and legal ramifications in forensic and toxicological reports (1,2). Standardization of techniques and the most complete understanding of differences among techniques and sample types are crucial to be able to state with confidence that a reported amount of alcohol was, in fact, present.
Ethyl alcohol (ethanol) levels in plasma, serum, and whole blood are not equivalent. It is generally recognized that whole blood ethanol levels are less than that of either the plasma or serum samples from the same person. This relationship exists because serum and plasma samples contain more water than whole blood (3) and alcohol is distributed uniformly throughout body water (4,5). The ratio of ethanol in serum or plasma to whole blood has been reported to be in the range of 1.09 to 1.18 (6–10), and serum and plasma ethanol levels have been found to be equivalent (9).
Biological samples can be collected in tubes that have a wide range of options for preservatives, anticoagulants, and other additives and subsequently stored at room temperature, refrigerated, or frozen. The purpose obviously is to maintain the sample and the compound of interest in a state that will not degrade from the in vivo value at the time of collection. Several studies have addressed this problem assessing ethanol in blood samples. With regard to storage conditions and times, Dubowski et al. (11) showed that ethanol levels in whole blood samples stored up to 1 year (refrigerated at 4°C) without preservative declined slightly (less than 5%), but this decrease was not statistically significant. Samples stored with the preservative and biocide sodium azide did not show any ethanol degradation over the 12-month storage period. Jones (12), however, did find slight decreases in blood ethanol concentration in samples stored with preservatives (sodium fluoride and potassium oxalate) and refrigerated for one year. He reports that decreases in the range of 1.8 to 2.6% (depending on starting concentrations of the sample) are analytically significant. Sodium fluoride, a common preservative for blood samples, has been recommended for blood alcohol samples (13,14). Kaye’s report (13), however, states that samples without this preservative have yielded reliable results for samples stored at room temperature (25°C) for 2 days. At least one additional report (15) has shown that the addition of sodium fluoride preservative did not significantly alter whole blood samples whether the samples were stored at room temperature or refrigerated for up to 14 days. Storage of samples at temperatures higher than room temperature has been studied. Winek et al. (16) analyzed ethanol levels in serum and whole blood in samples stored for 35 days at temperatures ranging from 26.7 to 37.8°C. They found that ethanol levels in serum (either with or without a preservative) did not degrade while levels in whole blood did significantly decline by as much as 19%, but the relationship did not seem to be directly dependent on temperature. The addition of other types of preservatives or anticoagulants has not been studied. The present study was undertaken to systematically examine the effects of a preservative with and without an anticoagulant on blood alcohol levels processed immediately or stored for up to 10 days.
Methods
Subjects
Five physically and psychologically healthy Caucasian adults (3 women, 2 men) having an average age of 24 ± 2.8 years and of normal body weight (average BMI of 22.5 ± 2.9) signed informed consent to participate in the study. They all consumed alcoholic drinks on a regular basis (range of 3 to 18 drinks per week), but none met criteria for past or present alcohol abuse or dependence. None were cigarette smokers. All participants had hematocrits within the normal range (average 41 ± 2.9, range 38–45). Participants were recruited through advertisements in local newspapers and on the internet and paid for their participation. The study and informed consent forms were approved by the McLean Hospital Institutional Review Board.
Procedures
The experiment took place as part of a larger study on the effects of alcohol on standing stability and cognitive performance (to be reported elsewhere). Participants arrived at the laboratory following an overnight fast and abstaining from alcohol for at least 24 h. They were given a small breakfast of 1 piece of toast with butter or jam, plus 12 oz of water. Twenty minutes before the first blood sample, a physician inserted an indwelling intravenous catheter (BD Saf-T-Intima™, Becton Dickinson) in an antecubital vein to which a stopcock was attached to facilitate the repeated blood draws. At 60 min before alcohol drink administration and at 20, 40, 60, 120, and 180 min after drinking, the physician withdrew blood samples in 10-mL syringes. Blood was immediately transferred to 10-mL red-, 7-mL lavender-, or 7-mL gray-top Vacutainer tubes (Becton Dickinson) with a blood transfer device (holder with pre-attached multiple sample female luer adapter; Becton Dickinson) for sample preparation and storage. Red-top tubes contained no additives; lavender-top tubes contained 12 mg potassium EDTA as an anticoagulant; gray-top–1 tubes contained 30 mg sodium fluoride as a preservative; and gray-top–2 tubes contained 15 mg sodium fluoride plus 12 mg of potassium oxalate as an anticoagulant (Table I). Samples for plasma (gray top–2 and lavender top) were immediately centrifuged for 15 min at 3500 rpm; the supernate was pipetted into polystyrene tubes, capped, and processed or stored according to one of four conditions that will be described. Samples for serum (red and gray top–1) remained at room temperature for 45 min to clot before centrifugation (15 min at 3500 rpm). Samples for whole blood (gray top–2 and lavender top) were inverted gently 8–10 times before being pipetted into polystyrene tubes, capped, and processed or stored. The four storage conditions were analysis on the day of collection within 6 h [as soon as possible (ASAP)], analysis after storage at room temperature (25°C) for 24 h (RT, 24 h), analysis after storage at room temperature for 10 days (RT, 10 days), and analysis after being refrigerated for 10 days (4°C, 10 days). All 4 tube types (6 Vacutainers) were collected at each of the 5 post-drinking time points for a total of 30 tubes per subject for each of the 4 storage conditions; an additional 6 tubes per subject were also collected for a baseline (pre-drinking) sample for each condition.
Table I.
Samples and Tube Types*
| Anticoagulant | No Preservative | Preservative |
|---|---|---|
| No | Serum (red-top tube) | Serum (gray top–1) |
| Yes | Plasma (lavender-top tube) | Plasma (gray top–2) |
| Whole blood (lavender-top tube) | Whole blood (gray top–2) |
Collection tubes: red did not contain additives; lavender contained K3EDTA (12 mg) as anticoagulant; gray–1 contained sodium fluoride (30 mg) as preservative; and gray–2 contained potassium oxalate (12 mg) as anticoagulant and sodium fluoride (15 mg) as preservative.
Alcohol administration
The alcoholic drink was prepared using a name-brand 40 proof vodka. The vodka was mixed with chilled orange juice to a volume of 400 mL and adjusted to deliver a dose of 0.7 g/kg of body weight. The mixture was divided equally into three plastic cups and placed on ice until administration. At the appropriate time, participants were instructed to consume one cup of drink at a time over a 15-min period. They had 5 min to consume the contents of each cup.
Analytical procedure
The following reagents were used in the ethanol analysis: ethyl alcohol (200 proof, absolute, anhydrous; Pharmco Products, Brookfield, CT); N-propyl alcohol and sodium tungstate (Sigma-Aldrich Chemical, St. Louis, MO); sulfuric acid (Fisher Scientific, Pittsburgh, PA); and aqueous ethanol standards (1.0 mg, 2.0 mg, and 3.0 mg ethanol in 1 mL, EMD Chemicals, Gibbstown, NJ). All reagents were certified ACS grade. Lyophilized serum-based and whole blood controls were purchased from Utak Laboratories (Valencia, CA).
Working ethanol standards were prepared at concentrations of 80, 160, and 320 mg/dL from 1 mL ethanol q.s. to 1000, 500, and 250 mL deionized water, respectively (17). The working internal standard consisted of 1 mL n-propyl alcohol q.s. to 500 mL with 0.66 N sulfuric acid (1.85 mL concentrated H2SO4 to 100 mL deionized water) to yield a concentration of 160 mg/dL and a 10% (w/v) aqueous solution of sodium tungstate was employed to precipitate out the proteins (18,19).
Refrigerated samples were allowed to come to room temperature before processing. Serum and plasma samples were vortex mixed thoroughly (20 s) and centrifuged for 5 min at 3500 rpm at room temperature (25°C) prior to extraction. Whole blood samples were inverted gently at least 8–10 times to insure homogeneity. Standards, controls and subject samples were extracted using 200-µL aliquots to which 200 µL each of working internal standard and 10% sodium tungstate were added. Extractions were covered with parafilm and mixed thoroughly with a vortex mixer for 20–30 s and centrifuged at 4200 rpm for 7 min at 25°C. The clear supernatant was transferred (100 µL) into autosampler vials with crimp caps for sequential direct injection (0.5 µL) onto the gas chromatograph (GC).
Instrumentation
Ethanol concentrations were analyzed using a Hewlett-Packard (Palo Alto, CA) model 5890 series II GC equipped with a flame-ionization detector and model 18593B autosampler. Ultra high-purity (99.999%) helium supplied by Airgas (Hingham, MA) served as the carrier gas at a column flow of 5.0 mL/min and a split flow of 25 mL/min. The capillary column was a J&W Scientific DB-WAX (15 m × 0.320-mm i.d., 0.50-µm film thickness, Agilent Technologies, Santa Clara, CA). An initial oven temperature of 40°C was maintained for 3.0 min and then ramped at 10°C/min to a final temperature of 100°C. The purge valve was kept on for a split injection, and the injector and detector temperatures were set at 225°C and 275°C, respectively.
Data analysis and quality control
Standards (80, 160, and 320 mg/dL) were analyzed in duplicate and averaged to construct the standard curve using linear regression. Quality control samples (whole blood and lyophilized serum-based) were included with each analytical run. Interassay coefficients of variation (CVs) were 6.9% and 7.7%, with target values of 100 and 150 mg/dL, respectively. Intraassay CVs were 5.0% for serum-based control 150 mg/dL and 2.4% for whole blood control. In addition, commercially prepared standards were extracted to assure accuracy of the aqueous based standards that were prepared in-house. Interassay CVs were 5.9%, 2.9%, and 4.5% at levels of 100, 200, and 300 mg/dL, respectively. Intraassay CV for the 100 mg/dL standard was 4.0%.
Statistical analysis
Linear mixed model analysis of variance was used for a main effects analysis and to assess interactions (SPSS 13.0 for Mac OS X, SPSS). Main effects for time did not include the pre-drinking values (all zeros) as entering means with no variance could affect interpretations of interactions among the main factors. Post hoc comparisons were performed with the least significant difference test after a significant main effect or interaction was found. Significance was set at p ≤ 0.05.
Results
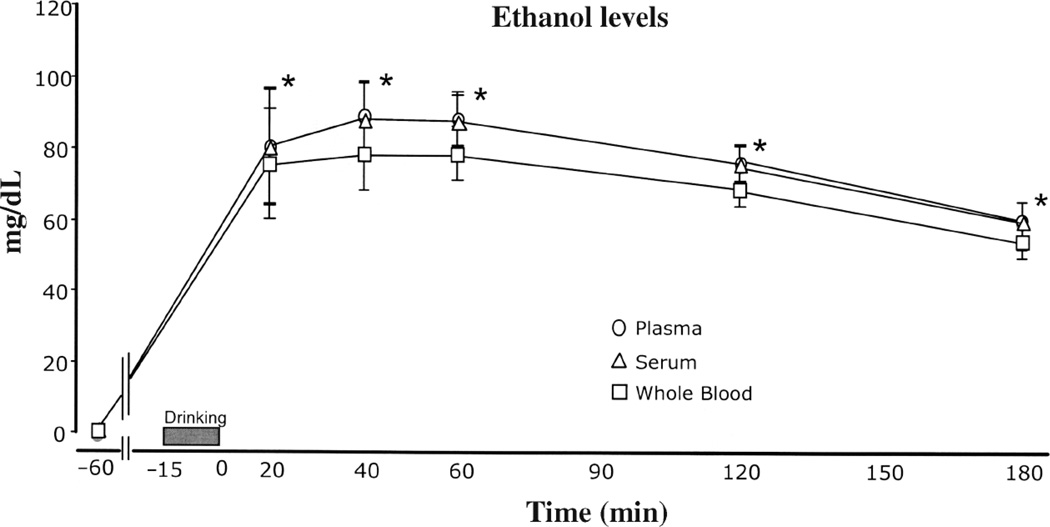
Compared to whole blood samples, ethanol levels were higher for plasma and serum samples [F(2,475) = 6.643, p = 0.001] (Figure 1). Ethanol concentration in the plasma and serum samples were, on the average, 11% higher than whole blood samples overall across all time points, collection tube, and processing condition (Table II). Plasma- and serum-to-whole blood ratios of ethanol values (means ± SD) averaged 1.11 ± 0.04 (range 1.04 to 1.16) across processing conditions and collection time points. Secondly, for each time point and processing condition, ethanol levels in plasma were compared to ethanol levels in serum and it was found that there were no significant differences between plasma and serum ethanol levels. Plasma-to-serum ratios were 1.0 ± .03 (range: 0.95 to 1.05) for all time points and processing conditions.
Figure 1.
Ethanol levels in three sample types of blood by time of collection. Values are means (± sem). All samples at the −60 min time point were negative for ethanol. * At each of the time points after drinking, plasma and serum levels were significantly higher than whole blood levels (p < 0.05).
Table II.
Ethanol Levels (mg/dL) by Processing Condition
| Time Point (min) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Processing Condition* | Sample Type | Tube Type† | −60 | 20 | 40 | 60 | 120 | 180 |
| ASAP (N = 5) | Plasma | Gray–2 | 0 | 80.1 | 90.8 | 94.6 | 80.1 | 61.8 |
| Lavender | 0 | 82.7 | 87.0 | 89.8 | 75.9 | 61.6 | ||
| Plasma average | 0 | 81.5 | 88.9 | 92.2 | 78.0 | 61.7 | ||
| Serum | Gray–1 | 0 | 86.2 | 93.6 | 86.8 | 77.4 | 61.1 | |
| Red | 0 | 80.3 | 85.0 | 88.2 | 76.6 | 61.5 | ||
| Serum average | 0 | 83.2 | 89.3 | 87.5 | 77.0 | 61.3 | ||
| Whole blood | Gray–2 | 0 | 80.9 | 82.3 | 81.3 | 72.7 | 56.6 | |
| Lavender | 0 | 75.8 | 72.8 | 78.3 | 69.8 | 55.7 | ||
| Whole blood average | 0 | 78.3 | 78.1 | 79.8 | 71.3 | 56.2 | ||
| All ASAP samples average | 0 | 81.0 | 85.7 | 86.5 | 75.4 | 59.7 | ||
| RT, 24 h (N = 5) | Plasma | Gray–2 | 0 | 87.8 | 96.6 | 90.4 | 76.7 | 59.5 |
| Lavender | 0 | 84.4 | 89.1 | 90.5 | 74.9 | 64.9 | ||
| Plasma average | 0 | 86.1 | 92.9 | 90.4 | 75.8 | 62.2 | ||
| Serum | Gray–1 | 0 | 87.6 | 94.2 | 90.3 | 76.6 | 60.2 | |
| Red | 0 | 81.5 | 89.0 | 85.0 | 78.5 | 61.3 | ||
| Serum average | 0 | 84.6 | 91.6 | 87.7 | 77.6 | 60.8 | ||
| Whole blood | Gray–2 | 0 | 83.1 | 87.2 | 79.7 | 71.6 | 58.6 | |
| Lavender | 0 | 78.0 | 78.1 | 74.7 | 67.1 | 52.0 | ||
| Whole blood average | 0 | 80.5 | 82.6 | 77.2 | 69.4 | 55.3 | ||
| All RT, 24 h samples average | 0 | 83.7 | 89.0 | 85.1 | 74.3 | 59.4 | ||
| RT, 10 days (N = 4) | Plasma | Gray–2 | 0 | 78.3 | 89.4 | 86.3 | 71.5 | 56.0 |
| Lavender | 0 | 70.2 | 84.5 | 83.3 | 74.7 | 59.2 | ||
| Plasma average | 0 | 74.3 | 86.9 | 84.8 | 73.3 | 57.6 | ||
| Serum | Gray–1 | 0 | 78.1 | 85.8 | 86.2 | 75.3 | 59.3 | |
| Red | 0 | 72.4 | 81.9 | 87.1 | 71.8 | 56.1 | ||
| Serum average | 0 | 75.2 | 83.8 | 86.6 | 73.5 | 57.9 | ||
| Whole blood | Gray–2 | 0 | 74.2 | 79.2 | 83.0 | 70.0 | 52.3 | |
| Lavender | 0 | 61.6 | 66.5 | 75.5 | 61.4 | 49.3 | ||
| Whole blood average | 0 | 67.9 | 72.8 | 79.2 | 65.7 | 50.8 | ||
| All RT, 10 days samples average | 0 | 72.4 | 81.2 | 83.5 | 70.7 | 55.3 | ||
| 4°C, 10 days (N = 4) | Plasma | Gray–2 | 0 | 81.5 | 87.7 | 82.9 | 76.0 | 55.8 |
| Lavender | 0 | 70.0 | 81.1 | 81.5 | 73.1 | 58.3 | ||
| Plasma average | 0 | 75.8 | 84.4 | 82.2 | 74.5 | 57.0 | ||
| Serum | Gray–1 | 0 | 78.3 | 88.6 | 87.2 | 72.9 | 58.4 | |
| Red | 0 | 70.4 | 79.4 | 85.3 | 72.2 | 59.7 | ||
| Serum average | 0 | 74.3 | 84.0 | 86.2 | 72.5 | 59.0 | ||
| Whole blood | Gray–2 | 0 | 72.2 | 80.0 | 76.0 | 64.6 | 49.1 | |
| Lavender | 0 | 71.0 | 74.9 | 75.3 | 67.1 | 53.8 | ||
| Whole blood average | 0 | 71.6 | 77.5 | 75.6 | 65.8 | 51.4 | ||
| All 4°C, 10 days samples average | 0 | 73.9 | 82.0 | 81.3 | 70.9 | 55.8 | ||
Processing conditions: ASAP samples were analyzed as soon as possible on day of collection; RT, 24 h samples were analyzed after storage for 24 h at room temperature (25°C); RT, 10 days samples were analyzed after storage for 10 days at room temperature (25°C); and 4°C, 10 days samples were refrigerated for 10 days and then analyzed.
Collection tubes: red did not contain additives; lavender contained K3EDTA (12 mg) as anticoagulant; gray–1 contained sodium fluoride (30 mg) as preservative; and gray–2 contained potassium oxalate (12 mg) as anticoagulant and sodium fluoride (15 mg) as preservative.
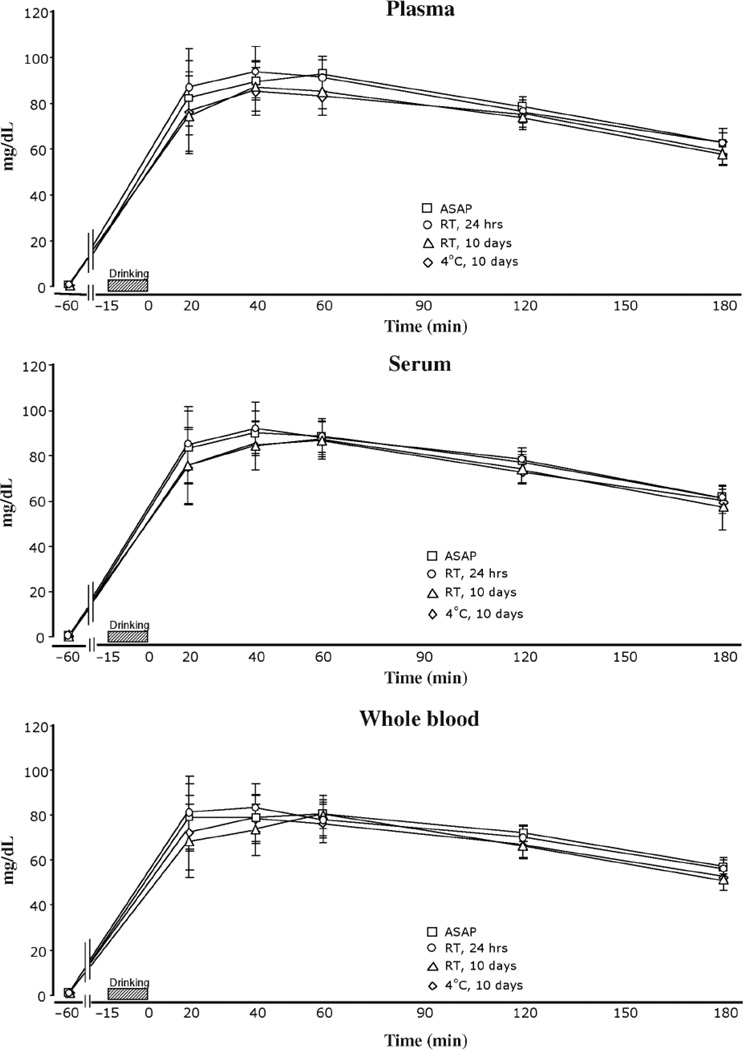
Processing condition, that is, how the samples were stored before analysis, did not significantly affect ethanol levels (Figure 2). However, there was a statistical trend for samples stored for 10 days to have slightly lower values than those analyzed immediately or within 24 h [F(3,475) = 2.432, p = 0.064]. Collapsed across sample type, collection tube type, and time (not including pre-drinking zeros), the means (± sem) for the ASAP and RT, 24 h samples were 77.62 ± 1.8 and 78.31 ± 1.8 mg/dL, respectively, versus 72.69 ± 2.1 and 72.79 ± 2.1 mg/dL for RT, 10 days and 4°C, 10 days samples, respectively.
Figure 2.
Ethanol levels in plasma, serum, and whole blood by time of collection. Plasma was obtained by centrifuging the samples immediately after collection for 15 min and pipetting the supernate. Serum was obtained by allowing the sample to sit for 45 min (allowing the sample to clot) before centrifuging and pipetting the supernate. Whole blood samples were not centrifuged before pipetting into analysis tubes. ASAP samples were analyzed as soon as possible on study day (within 6 h of collection); RT, 24 h samples were stored at room temperature (25°C) for 24 h and then analyzed; RT, 10 days samples were stored at room temperature (25°C) for 10 days and then analyzed; and 4°C, 10 days samples were refrigerated for 10 days and then analyzed. All samples at the −60 min time point were negative for ethanol. Values are means (± sem). Values for the 10 day samples were slightly lower, but not significantly different than ASAP or 24 h samples for plasma, serum, and whole blood.
The type of collection tube used did not significantly affect ethanol levels. There were no significant differences among levels from samples collected in tubes containing or not containing anticoagulants and/or preservatives (Table II).
A significant main effect for time was found with ethanol levels peaking within the 40 to 60 min timeframe and showing a decline afterwards [F(4,475) = 25.75, p < 0.001] (Figures 1, 2). Peak levels were highest at the 40 and 60 min time points. Average levels at 40 min were 84.4 ± 2.2 mg/dL; average levels at 60 min were 84.1 ± 2.2 mg/dL. Levels at 20, 120, and 180 min were significantly lower.
No significant interactions between or among the main factors were found indicating that differences in ethanol levels between whole blood, plasma, and serum samples were not augmented or decreased by influences of time, storage condition, or collection tube type.
Discussion
The main effects analyses revealed that our results are consistent with previous reports in the analytical and forensic literature. Our serum-to-whole blood and plasma-to-whole blood ratios of 1.11, indicating that serum and plasma ethanol levels were greater than whole blood ethanol levels, agree with those reported over the past 20 years (6–10). Barnhill et al. (6) analyzed the ratios of serum to whole blood for a large range of ethanol concentrations (from < 50 mg/dL to > 400 mg/dL) and found that the ratio was concentration-dependent. They report ratios ranging from 1.12 to 1.18. For the serum ethanol levels observed in the present study (~70–90 mg/dL), Barnhill and co-workers’ (6) ratio of 1.14 is in close agreement with our ratio. Charlebois et al. (7) reported an average serum to whole blood ratio of 1.14 (range of 1.04–1.26). Hodgson and Shajani (8) reported an averaged plasma to whole blood ratio of 1.11 for two time points after drinking, and Jones et al. (10) reported a plasma to whole blood average of 1.10 following intravenous administration of ethanol. Furthermore, Jones and colleagues’ study found that the plasma to whole blood ratio was consistent over a 6-h sampling period. Winek and Carfagna (9) reported ratios of 1.14 for both serum to whole blood and plasma to whole blood. Thus, our results replicate this well-observed phenomenon and extend this finding across different types of collection tubes and for a multiple set of storage conditions.
Of concern in the analysis of ethanol levels in blood samples is the effect of storage and processing condition. Jones (12) showed that ethanol losses in samples are positively correlated with the length of storage and the original ethanol concentration in the blood. Furthermore, Jones (12) concluded that even though the absolute amounts of ethanol lost from the sample is greater the higher the starting concentration, a greater percentage of loss needs to occur from lower concentration samples in order to be analytically significant. He reports that for samples with starting ethanol concentrations of 52 and 158 mg/dL, a decrease of 1.37 (2.6%) and 2.95 mg/dL (1.9%), respectively, are analytically significant. Whole blood samples spiked with specified amounts of alcohol to produce fixed blood-alcohol concentrations and then refrigerated were found to be remarkably stable over a 12-month period, losing only 0.2 to 0.3% monthly, for a total loss of less than 5% (11). Refrigerated samples with an initial concentration of 86 mg/dL lost 4 mg/dL over a 12-month period (4.7%), which was not statistically significant. Several reports (1,13) state that reliable levels of alcohol can be obtained from whole blood samples stored at room temperature (25°C) for two days and those refrigerated (4–5°C) or frozen (−10°C) for two weeks, but they report greater losses than Dubowski et al. (11). Overall, our results indicate that there appears to be a slight but non-significant decline in alcohol levels when the blood is stored in tubes over a 10-day period. Samples processed the day of collection or within 24 h were, on the average, 5 to 9% higher in alcohol concentration than samples processed 10 days later, indicating a loss of alcohol from the sample. This was observed regardless of whether the samples were refrigerated or kept at room temperature. Although these declines were not statistically significant, based on Jones’ analysis (12), these declines may be analytically significant and await further experimentation.
Collecting blood samples in different types of tubes did not affect the ethanol levels as there were no systematic differences among plasma, serum, or whole blood ethanol levels in tubes with or without either preservatives and/or anticoagulants. Theoretically, the addition of sodium fluoride preservative in the gray-top collection tubes would prevent degradation of the ethanol levels over time, but any differences observed in our study were not statistically significant. This would indicate that such additives are not important in ethanol analyses that take place within 1–10 days of collection, even in samples that are stored at room temperature. Temperatures higher than normal room temperature (26.7–37.8°C) have been shown to degrade alcohol levels in whole blood, but not serum, by as much as 19% over 35 days (16). Our samples were not subjected to temperatures in this range, so the generalizability of their results to our findings is unknown. Winek and coworkers’ (16) results also indicate that the addition of a preservative (sodium fluoride) is not necessary when analyzing serum samples. Similarly, our serum samples collected in gray-top tubes with preservative were not significantly different from samples collected in no additive (red-top) tubes. The Winek et al. (16) study was not able to evaluate the role of a preservative in whole blood samples, but our results would indicate that ethanol levels are not dependent on the presence or absence of a preservative in samples refrigerated or stored at room temperature for 10 days.
The absorption and elimination of alcohol in the human body has been well characterized (20,21). The present study was not designed to explore pharmacokinetic properties of ethanol absorption and elimination, but as to be expected, ethanol levels here increased rapidly following drinking and then showed a gradual decline. The levels of ethanol observed in our samples at five time points following drinking were not systematically or significantly altered by the type of tube used to collect the sample nor by how it was stored before analysis. The present study extends previous research on the influence of collection and storage conditions on ethanol levels by systematically evaluating several types of conditions not previously studied. Because the obtained values for a particular sample type (plasma, serum, or whole blood) were nearly identical regardless of processing condition or tube collection type, it appears that many of the standard laboratory collection and processing methods will produce results that are both reliable and valid.
Acknowledgments
Supported by Grant AA010536 from the National Institute on Alcohol Abuse and Alcoholism and KO5DA00345 from the National Institute on Drug Abuse. The authors thank Mary C. Hunt, Brian Wideman, and the technical support group of Agilent Technologies.
References
- 1.Ferrari LA, Triszcz JM, Giannuzzi L. Kinetics of ethanol degradation in forensic blood samples. Forensic Sci. Int. 2006;161:144–150. doi: 10.1016/j.forsciint.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 2.Harding P. Alcohol Toxicology for Prosecutors: Targeting Hardcore Impaired Drivers. Alexandria, VA: American Prosecutors Research Institute; 2003. Interpretation of alcohol results; pp. 5–28. [Google Scholar]
- 3.Porter WH, Moyer TP. Clinical toxicology. In: Burtis CA, Ashwood ER, editors. Tietz Fundamentals of Clinical Chemistry. W.B. Saunders, Philadelphia, PA: 2001. pp. 636–679. [Google Scholar]
- 4.Hahn RG, Norberg A, Jones AW. Rate of distribution of ethanol into the total body water. Am. J. Ther. 1995;2:50–56. doi: 10.1097/00045391-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Norberg A, Sandhagen B, Bratteby LE, Gabrielsson J, Jones AW, Fan H, Hahn RG. Do ethanol and deuterium oxide distribute into the same water space in healthy volunteers? Alcohol Clin. Exp. Res. 2001;25:1423–1430. doi: 10.1097/00000374-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Barnhill MT, Jr, Herbert D, Wells DJ., Jr Comparison of hospital laboratory serum alcohol levels obtained by an enzymatic method with whole blood levels forensically determined by gas chromatography. J. Anal. Toxicol. 2007;31:23–30. doi: 10.1093/jat/31.1.23. [DOI] [PubMed] [Google Scholar]
- 7.Charlebois RC, Corbett MR, Wigmore JG. Comparison of ethanol concentrations in blood, serum, and blood cells for forensic application. J. Anal. Toxicol. 1996;20:171–178. doi: 10.1093/jat/20.3.171. [DOI] [PubMed] [Google Scholar]
- 8.Hodgson BT, Shajani NK. Distribution of ethanol: plasma to whole blood ratios. Can. Soc. Forensic Sci. J. 1985;18:73–77. [Google Scholar]
- 9.Winek CL, Carfagna M. Comparison of plasma, serum, and whole blood ethanol concentrations. J. Anal. Toxicol. 1987;11:267–268. doi: 10.1093/jat/11.6.267. [DOI] [PubMed] [Google Scholar]
- 10.Jones AW, Hahn RG, Stalberg HP. Distribution of ethanol and water between plasma and whole blood; inter- and intra-individual variations after administration of ethanol by intravenous infusion. Scand. J. Clin. Lab. Invest. 1990;50:775–780. doi: 10.1080/00365519009091072. [DOI] [PubMed] [Google Scholar]
- 11.Dubowski KM, Gadsden RH, Sr, Poklis A. The stability of ethanol in human whole blood controls: an interlaboratory evaluation. J. Anal. Toxicol. 1997;21:486–491. doi: 10.1093/jat/21.6.486. [DOI] [PubMed] [Google Scholar]
- 12.Jones AW. Are changes in blood-ethanol concentration during storage analytically significant? Importance of method imprecision. Clin. Chem. Lab. Med. 2007;45:1299–1304. doi: 10.1515/CCLM.2007.289. [DOI] [PubMed] [Google Scholar]
- 13.Kaye S. The collection and handling of the blood alcohol specimen. Am. J. Clin. Pathol. 1980;74:743–746. doi: 10.1093/ajcp/74.5.743. [DOI] [PubMed] [Google Scholar]
- 14.NCCLS. Blood alcohol testing in the clinical laboratory: Approved guideline. National Committee on Clinical Laboratory Standards. 1997 [Google Scholar]
- 15.Winek CL, Paul LJ. Effect of short-term storage conditions on alcohol concentrations in blood from living human subjects. Clin. Chem. 1983;29:1959–1960. [PubMed] [Google Scholar]
- 16.Winek T, Winek CL, Wahba WW. The effect of storage at various temperatures on blood alcohol concentration. Forensic Sci. Int. 1996;78:179–185. doi: 10.1016/0379-0738(95)01884-0. [DOI] [PubMed] [Google Scholar]
- 17.Widdop B. Hospital toxicology and drug abuse screening. In: Moffat AC, Jackson JV, Moss MS, Widdop B, editors. Clarke’s Isolation and Identification of Drugs. London, U.K.: The Pharmaceutical Press; 1986. pp. 3–34. [Google Scholar]
- 18.Berkman S, Henry RJ, Golub OJ, Segalove M. Tungstic acid precipitation of blood proteins. J. Biol. Chem. 1954;206:937–943. [PubMed] [Google Scholar]
- 19.Folin O, Wu H. A system of blood analysis. J. Biol. Chem. 1919;38:81–87. [Google Scholar]
- 20.Norberg A, Gabrielsson J, Jones AW, Hahn RG. Within- and between-subject variations in pharmacokinetic parameters of ethanol by analysis of breath, venous blood and urine. Br. J. Clin. Pharmacol. 2000;49:399–408. doi: 10.1046/j.1365-2125.2000.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mumenthaler MS, Taylor JL, Yesavage JA. Ethanol pharmacokinetics in white women: nonlinear model fitting versus zero-order elimination analyses. Alcohol Clin. Exp. Res. 2000;24:1353–1362. [PubMed] [Google Scholar]