Abstract
Empagliflozin (Jardiance): a novel SGLT2 inhibitor for the treatment of type-2 diabetes
INTRODUCTION
Diabetes mellitus is a lifelong condition requiring continuous medical care. Type-2 diabetes mellitus (T2DM), which accounts for approximately 90% to 95% of all diagnosed diabetes, is a progressive disease resulting from an insulin secretory defect characterized by insulin resistance and some degree of insulin deficiency.1 Chronic long-term hyper glycemia associated with diabetes causes serious complications, including blindness, kidney failure, amputations, and death. The incidence of diabetes has increased dramatically in the United States over the last three decades, from 5.6 million cases to 21 million diagnosed cases in 2012. If this trend continues, one in three Americans will be diagnosed with diabetes by 2050.2,3
The prevalence of T2DM has a tremendous impact on obesity, which is a growing problem for America’s health care system. Because of the rise in obesity, there is a heightened demand for researchers to develop drug therapies that are effective in treating hyperglycemia as well as promoting weight loss.2 Despite current available therapies, about 50% of U.S. patients are unable to achieve their goals for glycosylated hemoglobin (HbA1c).4 Metformin, a biguanide, is the preferred oral hypoglycemic agent for initial therapy in patients with T2DM. A majority of patients will progress to combination therapy involving other oral agents or insulin to be taken with metformin.5 Metformin is the preferred medication because it has high efficacy in reducing HbA1c levels by 1.5 to 2 percentage points and fasting plasma glucose (FPG) levels by 60 to 80 mg/dL, as well as reducing plasma triglyceride levels and low-density lipoprotein-cholesterol levels by 8% to 15%.6
Due to declining beta-cell function, the majority of those who have initial success with metformin will eventually require one or more additional agents to achieve their treatment goals. The American Diabetes Association recommends that if patients do not achieve the goal of an HbA1c of less than 7% after maximal metformin and lifestyle changes for three months, additional therapy is indicated.7 Several options for further oral therapy exist. Agents that can be added to metformin include: sulfonylureas, thiazolidinediones, glucagon-like peptide-1 receptor agonists, dipeptidyl peptidase-4 inhibitors, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and insulin. Patient preference determines the second-line treatment choice of medications after metformin; the decision should be individualized depending on the degree of hyperglycemia present, the patient’s risk for hypoglycemia, the patient’s body mass index, and the risk for further weight gain.6
Empagliflozin (Jardiance, Boehringer Ingelheim), an SGLT2 inhibitor, is part of the newest class of oral hypoglycemic agents, which includes canagliflozin (Invokana, Janssen) and dapagliflozin (Farxiga, AstraZeneca/Bristol-Myers Squibb). In August 2014, empagliflozin became the most recent medication in its class to be approved by the Food and Drug Administration. Empagliflozin has a low side-effect profile when used in combination with other anti diabetic medications.8 There is little risk of hypoglycemia with empagliflozin because the mechanism of action is independent of beta-cell function and insulin pathway.5
Empagliflozin is indicated for the improvement of glycemic control in conjunction with diet and exercise in adults with type-2 diabetes mellitus.8
PHARMACOLOGY
The kidney plays an important role in glucose homeostasis via its production, utilization, and most importantly reabsorption of glucose from glomerular filtrate, which is mediated via SGLT2.9 SGLT2 facilitates an estimated 90% of renal glucose reabsorption. Inhibition of SGLT2 increases urinary glucose excretion (UGE) by the kidney, resulting in a reduction of plasma glucose levels in an insulin-independent manner. Empagliflozin is a highly potent, selective, competitive inhibitor of SGLT2 approved as a treatment for T2DM in patients with normal kidney function. Empagliflozin is an orally active tablet referred to chemically as D-Glucitol,1,5-anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3furanyl] oxy]phenyl]methyl]phenyl]-, (1S). The chemical structure is C23H27ClO7, with a molecular weight of 450.91 g/mol (Figure 1). In a preclinical study conducted by Grempler et al., empagliflozin had the highest selectivity for SGLT2 over SGLT1 (more than 2,500-fold), compared to dapagliflozin (more than 1,200 fold) and canagliflozin (more than 250-fold).10
Figure 1.
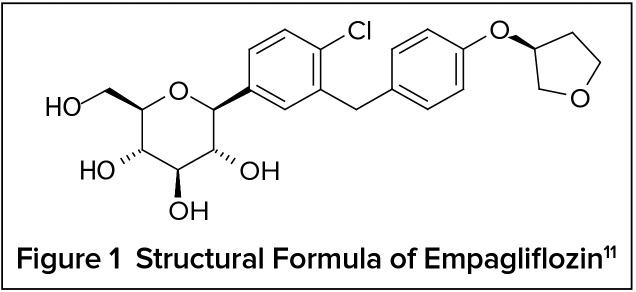
Structural Formula of Empagliflozin11
In patients with T2DM, empagliflozin lowered fasting and postprandial glucose levels by: 1) increasing total glucose excretion; 2) improving beta-cell function; and 3) shifting substrate utilization from glucose to lipid, despite a compensatory increase in endogenous glucose production. In patients with T2DM, increases in urinary glucose excretion have been observed after a single dose of empagliflozin, with total glucose excretion increasing 11-fold with the 10-mg dose, 18-fold with the 25-mg dose, and 14-fold with the 100-mg dose compared with placebo. Empagliflozin produced a 36% to 45% inhibition of glucose reabsorption after a single dose and maintained 36% to 48% inhibition after 27 days of daily administration.12
Empagliflozin inhibited reabsorption of up to 40% of filtered glucose at single daily doses of 0.5 mg to 10 mg, rising to 40% to 60% inhibition of filtered glucose at higher doses and reaching a plateau at around the 100-mg dose. When empagliflozin was administered with food, there were no relevant effects on UGE. The mean cumulative UGE was 71.7 g in a fasting state compared with 75.9 g in a fed state with the 50-mg empagliflozin dose over 24 hours following oral administration. All doses of empagliflozin exhibited higher amounts of glucose excretion when compared with placebo in the single rising-dose study.
PHARMACOKINETICS
Empagliflozin is an orally active, selective inhibitor of SGLT2 with a bioavailability of 78%, no active metabolites, and very limited drug–drug interactions.11
Absorption11
After consumption of a high-fat and high-calorie meal, oral administration of empagliflozin 25 mg resulted in a 16% decrease in the area under the curve and a 37% decrease in the peak concentration (Cmax) compared with administration in the fasted state, although these changes are not considered to be clinically important. After oral administration, empagliflozin exhibits rapid absorption, reaching peak levels 1.5 hours after a single dose.
Distribution11
Empagliflozin is 86.2% protein-bound in healthy volunteers and distributed to tissues and fluids. Approximately 36.8% of empagliflozin is partitioned into red blood cells in healthy volunteers. Empagliflozin has a population-based volume of distribution of approximately 73.8 L at steady state.
Metabolism11
Empagliflozin is primarily metabolized by glucuronidation by UGT2B7, UGT1A3, UGT1A8, and UGT1A9. There are no major metabolites of empagliflozin in the plasma; however, three conjugates were found with each metabolite, consisting of less than 10% of the total drug in circulation.
Excretion11
Empagliflozin exerts renal excretion; approximately 54.4% of a radio-labeled dose of oral empagliflozin was retrieved in the urine, half of which was identified as unchanged drug. Approximately 41.2% of the radio-labeled dose of oral empagliflozin was recovered in the feces, most of which was identified as unchanged drug. The total body clearance of empagliflozin is 10.6 L per hour and the expected half-life of empagliflozin is around 12.4 hours. The long half-life of empagliflozin permits once-daily dosing.
CLINICAL TRIALS
Empagliflozin as Monotherapy13
To evaluate the efficacy and safety of empagliflozin monotherapy, 986 patients with T2DM participated in a double-blind, placebo-controlled study. Patients who were treatment-naïve with inadequately controlled T2DM entered an open-label placebo run-in period for two weeks. Patients who remained poorly controlled at the end of the run-in period and had an HbA1c between 7% and 10% were randomized to placebo, empagliflozin 10 mg, empagliflozin 25 mg, or a reference comparator. At week 24, patients treated with a daily dose of empagliflozin 10 mg or 25 mg had significant reductions in HbA1c, body weight, and systolic blood pressure compared with placebo. Results of the primary endpoint showed placebo-adjusted reductions in HbA1c from baseline to week 24 of 0.74 percentage points (P < 0.001) and 0.85 percentage points (P < 0.001) for the empagliflozin 10-mg and 25-mg doses, respectively. Patients treated with empagliflozin 10 mg and 25 mg showed significant placebo-adjusted decreases in body weight of 1.93 kg (P < 0.001) and 2.15 kg (P < 0.001). Results also showed placebo-adjusted reductions in systolic blood pressure of 2.6 mm Hg (P = 0.023) for empagliflozin 10 mg and 3.4 mm Hg (P = 0.003) for empagliflozin 25 mg. The incidence of genital infections was higher in patients treated with empagliflozin compared with placebo—4.2% with empagliflozin 10 mg and 3.6% with empagliflozin 25 mg compared with 0.7% with placebo.
Empagliflozin With Metformin14
A randomized, double-blind, placebo-controlled, 12-week trial was conducted involving 495 participants between 18 and 80 years of age from 16 countries who were inadequately controlled on metformin alone or metformin in combination with another oral antidiabetic (OAD) agent. Metformin use in these patients required a maintenance dose of 1,500 mg per day or greater, not exceeding the maximum tolerated dose. HbA1c requirements were as follows: for patients on metformin monotherapy, an HbA1c of 6.5% to 9%; for patients who were on metformin and another OAD medication prior to the study and for all other patients at the beginning of the placebo period, an HbA1c of 7% to less than 10%. A four-week washout period was required for patients who were taking another antidiabetic medication with their metformin. Metformin was continued during the washout phase of the other OAD agent. For the study, patients were given either a placebo, empagliflozin 1, 5, 10, 25, or 50 mg, or sitagliptin 100 mg once daily with or without food. Sitagliptin was added to provide a clinical perspective in comparison to other OAD agents and to assess the sensitivity of the trial. The primary endpoint evaluated was a change in HbA1c from baseline to week 12. The secondary endpoints evaluated were a change in HbA1c over time, the change in FPG and body weight from baseline to week 12, and the proportion of participants who achieved an HbA1c of 7% or less or whose HbA1c decreased at least 0.5 percentage points at week 12.
Overall, the study revealed that empagliflozin administered once daily as addon therapy with metformin resulted in clinically significant decreases in HbA1c, FPG, and body weight compared to the placebo, with the largest reductions seen in the 10-, 25-, and 50-mg formulations. The 10-, 25-, and 50-mg doses of empagliflozin achieved reductions in HbA1c levels of −0.56, −0.55, and −0.9, respectively (P < 0.0001); this was better than or comparable to 100 mg of sitagliptin, which had a reduction of only −0.45 (P < 0.0001). Empagliflozin was well tolerated overall with minimal side effects, such as urinary tract infections in 14 individuals (4% of the study population), mainly in females. Other genital infections, also occurring in 14 individuals, were self-reported and considered to be mild or moderate. Although this side effect is seen in other SGLT2 inhibitors, the genital infections in this study occurred at a lower frequency compared with other SGLT2 inhibitors and were equivalent between male and female patients. One case of nausea due to the study drug was reported in the 10-mg empagliflozin group. The study was discontinued early in nine patients taking empagliflozin because of unspecified adverse events: two in the 1-mg group, four in the 10-mg group, and three in the 50-mg group.
Empagliflozin With Insulin11,15
Empagliflozin was investigated as an add-on treatment in adults with T2DM on basal insulin during a 78-week, randomized, double-blind, placebo-controlled trial. Patients were randomized to receive a placebo (n = 170), empagliflozin 10 mg (n = 169), or empagliflozin 25 mg (n = 155). The study included an initial 18-week fixed insulin dose period, after which the dose was adjusted at investigator discretion. The primary endpoint evaluated at week 18 was a change from baseline in HbA1c. Secondary endpoints assessed with empagliflozin at week 78 included a statistically significant decrease in HbA1c, insulin sparing, and reductions in body weight compared with placebo. At week 18, placebo-adjusted reductions in HbA1c for empagliflozin 10 mg and 25 mg were 0.6 and 0.7 percentage points, respectively (P < 0.001). At week 78, the empagliflozin 10-mg and 25-mg arms had HbA1c reductions of 0.4 and 0.6 percentage points (P < 0.001). In addition to reductions in HbA1c at week 78, the placebo-adjusted required daily insulin dose was decreased by 6.7 IU and 6.0 IU for empagliflozin 10 mg (P = 0.002) and 25 mg (P = 0.009), respectively. The placebo arm showed an increase of 5.5 IU from baseline. Results also showed a reduction in body weight of 2.4 kg for both empagliflozin 10 mg and 25 mg, compared with an increase of 0.7 kg for placebo. Further analysis showed reductions in FPG and systolic blood pressure. Reports of hypoglycemia were similar: 36.1% for patients on empagliflozin 10 mg and 25 mg and 35.3% for placebo. Adverse events, such as urinary tract infections, were reported in 14.8%, 11.6%, and 8.8% of patients receiving empagliflozin 10 mg, empagliflozin 25 mg, and placebo, respectively.
SAFETY AND TOLERABILITY
Adverse Drug Events
Severe hypoglycemia is limited when empagliflozin is administered alone or in combination with metformin alone, metformin with a sulfonylurea, or pioglitazone with or without metformin. However, the risk of hypoglycemia is increased when empagliflozin is used in combination with insulin secretagogues or insulin.11 Chances of genital mycotic infections and urinary tract infections (Table 1) rise with consumption of this drug; therefore, patients should be monitored closely if they are at risk or have a history of any of these adverse drug events.11,14,15
Table 1.
Key Features of SGLT2 Inhibitors
| Product | Empagliflozin11 | Canagliflozin17 | Dapagliflozin18 |
|---|---|---|---|
| Cost of a 30-day supply16* | 10-mg and 25-mg tablets: $411 | 100-mg and 300-mg tablets: $411 | 5-mg and 10-mg tablets: $412 |
| Usual daily dose | 10 mg daily in the morning; may increase to 25 mg once daily | 100 mg daily before the first meal; may increase to 300 mg once daily if eGFR > 60 | 5 mg daily in the morning; may increase to 10 mg once daily |
| Pregnancy category | C | C | C |
| Renal impairment | Dosage adjustment required for eGFR of 45 to < 60 mL/min/1.73 m2; use not recommended for eGFR of < 45 mL/min/1.73 m2 | Dosage adjustment required for eGFR of 45 to < 60 mL/min/1.73 m2; use not recommended for eGFR of < 45 mL/min/1.73 m2 | Use not recommended for eGFR of < 60 mL/min/1.73 m2 |
| Hepatic impairment | Severe: not recommended | Severe: not recommended | Severe: not recommended |
| Warnings/precautions |
|
|
|
| Common adverse events |
|
|
|
| Drug interactions | No significant clinical interactions noted |
|
No significant clinical interactions noted |
| Combination products | None | Canagliflozin/metformin (Invokamet, Janssen) | Dapagliflozin/metformin (Xigduo, AstraZeneca) |
Based on average wholesale price of the usual daily dose rounded to the nearest dollar
eGFR = estimated glomerular filtration rate; RAS = renin-angiotensin system
Contraindications
Empagliflozin is contraindicated in patients with serious hypersensitivity to any components of the formulation, severe renal impairment (estimated glomerular filtration rate [eGFR] less than 30 mL/min/1.73 m2), end-stage renal disease, or dialysis. Empagliflozin should not be initiated if eGFR is less than 45 mL/min/1.73 m2.11
Drug Interactions
Drug–drug interactions were evaluated in an open-label, randomized, crossover study of healthy patients with T2DM. Empagliflozin was administered with medications commonly used by T2DM patients. Clinically relevant interactions were not observed between empagliflozin and digoxin, linagliptin, metformin, ramipril, sitagliptin, verapamil, warfarin, or a combined oral contraceptive (ethinyl estradiol/levonorgestrel). Dose adjustments were not necessary for any drug combinations studied.11
DOSAGE AND ADMINISTRATION
Empagliflozin is administered at an initial dose of 10 mg orally in the morning, with a gradual increase to a maximum dose of 25 mg once daily if necessary.11
COST
The average wholesale price (AWP) for a 30-day supply of empagliflozin in 10-mg or 25-mg dosages is $411. As of May 2015, the AWPs for canagliflozin and dapagliflozin were virtually the same as that of empagliflozin. AWPs for SGLT2 inhibitors available in the United States are shown in Table 1.16
P&T COMMITTEE CONSIDERATIONS
Empagliflozin is the latest SGLT2 inhibitor to be approved by the FDA for use in adults with type-2 diabetes as an adjunct to diet and exercise. Clinical trials have evaluated empagliflozin as monotherapy as well as in combination with metformin, pioglitazone, glyburide, and insulin. Patients newly diagnosed with diabetes as well as those with inadequate glycemic control on multiple-drug therapy have experienced reductions of HbA1c with empagliflozin therapy. Mean baseline HbA1c values of patients receiving empagliflozin in clinical trials have ranged from 7.9% to 9.3%, and mean HbA1c reductions with therapy have ranged from 0.4 to 0.9 percentage points.4 While no direct comparative clinical trials have been conducted with empagliflozin and other SGLT2 inhibitors, a review of clinical trials suggests that all have comparable efficacy and tolerability. There does not appear to be a therapeutic advantage of using one SGLT2 inhibitor over another. However, empagliflozin shows a decreased incidence in genital mycotic infections in men and women compared with other SGLT2 inhibitors. In addition, the precautions associated with the other SGLT2 inhibitors, such as dapagliflozin’s increased risk of bladder cancer and canagliflozin’s higher risk of genital infections (more than 10%), may make empagliflozin preferable in specific situations.
Similar to the two SGLT2 inhibitors that were already available (dapagliflozin and canagliflozin),10 empagliflozin has favorable pharmacokinetic properties, such as a long half-life allowing once-daily dosing, no active metabolites, and limited drug–drug interactions. Similar to the other SGLT2 inhibitors, empagliflozin is dosed once daily without regard to meals, decreases weight, and has a low incidence of hypoglycemia.10 Empagliflozin is predominately eliminated renally.
CONCLUSION
Clinical trials have demonstrated the efficacy and safety of empagliflozin as monotherapy and combination therapy in patients with T2DM. Empagliflozin lowers HbA1c approximately 0.8 percentage points and is generally well tolerated. Its side-effect profile is similar to that of other SGLT2 inhibitors. The cost of empagliflozin is comparable to other SGLT2 inhibitors but higher than metformin or sulfonylureas. Empagliflozin provides another oral option for diabetes patients inadequately controlled on or unable to take metformin. Empagliflozin may not be the most effective monotherapy for patients with uncontrolled diabetes and is on a par with the other SGLT2 inhibitors for efficacy and safety. The long-term effects of this class of drugs on the urinary tract remain to be seen, since such medications have been on the market only since March 2013. The FDA is requiring four post-marketing studies for empagliflozin to obtain data from long-term, clinical-outcomes-based studies and further clarify the safety of empagliflozin in patients with T2DM.8
REFERENCES
- 1.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, Georgia: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 4.Zinman B, Inzucchi S, Broedl U, et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME) Cardiovasc Diabetol. 2014;13(1):1–19. doi: 10.1186/1475-2840-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metformin prescribing information. Baltimore, Maryland: Lupin Pharmaceuticals, Inc.; 2011. [Google Scholar]
- 6.Triplitt CL, Reasner CA, II, Isley WL. Diabetes mellitus. In: DiPiro JT, Talbert RL, Yee GC, et al., editors. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York, New York: McGraw-Hill; 2008. pp. 1205–1241. [Google Scholar]
- 7.American Diabetes Association Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1–S90. [PubMed] [Google Scholar]
- 8.Food and Drug Administration FDA approves Jardiance to treat type-2 diabetes. Aug 1, 2014. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm407637.htm. Accessed August 17, 2014.
- 9.Bakris GL, Fonseca VA, Sharma K, et al. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75(12):1272–1277. doi: 10.1038/ki.2009.87. [DOI] [PubMed] [Google Scholar]
- 10.Grempler R, Thomas L, Eckhardt M, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14(1):83–90. doi: 10.1111/j.1463-1326.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 11.Jardiance (empagliflozin) prescribing information. Ridgefield, Connecticut: Boehringer Ingelheim Pharmaceuticals; 2014. [Google Scholar]
- 12.Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type-2 diabetes. Diabetes Obes Metab. 2013;15(7):613–621. doi: 10.1111/dom.12073. [DOI] [PubMed] [Google Scholar]
- 13.Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose co-transporter 2 inhibition in type-2 diabetic patients. J Clin Invest. 2014;124(2):499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenstock J, Seman LJ, Jelaska A, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type-2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15(12):1154–1160. doi: 10.1111/dom.12185. [DOI] [PubMed] [Google Scholar]
- 15.Rosenstock J, Jelaska A, Wang F, et al. Empagliflozin as add-on to basal insulin for 78 weeks improves glycemic control with weight loss in insulin-treated type-2 diabetes (T2DM) Diabetes. 2013;62(suppl 1):A285. [Google Scholar]
- 16.Red Book Online. Ann Arbor, Michigan: Truven Health Analytics; Accessed May 8, 2015. [Google Scholar]
- 17.Invokana (canagliflozin) prescribing information. Titusville, New Jersey: Janssen Pharmaceuticals, Inc.; 2013. [Google Scholar]
- 18.Farxiga (dapagliflozin) prescribing information. Princeton, New Jersey: Bristol-Myers Squibb Company; 2014. [Google Scholar]


