Abstract
In the malaria endemic municipality of Miraflores in southeastern Amazonian Colombia, several aspects of the biology of local Anopheles species were investigated to supplement the limited entomological surveillance information available and to provide baseline data for malaria prevention and vector control. Anopheles darlingi Root, 1926 was the most abundant species (95.6%), followed by Anopheles braziliensis (Chagas) (3.6%) and Anopheles oswaldoi s.l. (Peryassu) (0.7%). During the dry season, exophagic activity was prevalent only between 1800–2100 hours; after this (2100–0600 hours) only endophagy was encountered. In contrast, during the rainy season, both endophagy and exophagy occurred throughout the collection period. The human biting rate for An. darlingi was 8.6. This species was positive for Plasmodium vivax VK210 with a sporozoite rate = 0.13 (1/788). Breeding sites corresponded to stream (n = 7), flooded excavations (n = 4), flooded forest (n = 1), wetlands (n = 2), and an abandoned water reservoir (n = 1). An. darlingi predominated in these sites in both seasons. Based on these data, An. darlingi is the main local malaria vector, and we recommend that local prevention and control efforts focus on strengthening entomological surveillance to determine potential changes of species biting behavior and time to reduce human–vector interactions.
Keywords: Anopheles darlingi, malaria, Amazonia, Colombia
Colombia consistently reports the second highest annual malaria incidence in Latin America after Brazil (World Malaria Report 2011). Overall, in Colombia, Plasmodium vivax (Grassi and Feletti) is responsible for ≈74% of malaria incidence, and Plasmodium falciparum (Welch) for the remaining 26%. More than 85% of Colombia's land area has the potential for endemic malaria transmission (Mendoza et al. 2000). The highest risk zones have been identified as the Pacific Coast, the Cauca River Valley, and the Urabá, Orinoquía, and Amazonia regions (Padilla and Peña 2002). The major malaria vectors in Colombia, all in subgenus Nyssorhynchus, are Anopheles darlingi Root, 1926, Anopheles albimanus Wiedemann, 1920, and Anopheles nuneztovari Gabaldón, 1940 (Montoya-Lerma et al. 2011).
The Department of Guaviare is situated in the Amazonia region of eastern Colombia and has an area of 42,327 km2. It extends from 0° 32′ to 3° 09′ N, and from 69° 47′ to 73° 47′ W (Fig. 1) and includes several municipalities, e.g., San José del Guaviare, El Retorno, Calamar and Miraflores (Instituto Amazónico de Investigaciones Científicas [SINCHI] 2000). According to the World Malaria Report (2011), this is a region of high transmission, with an API (annual parasite index; number of malaria cases per 1,000 inhabitants) of 10–50. Malaria is endemic in Guaviare, and during 2010 it had the highest API in Colombia (58.8; Instituto Nacional de Salud [INS] 2010). In 2010, the municipality of Miraflores had the highest API (56.4) in Guaviare, reporting 66.1% P. vivax, 19.5% P. falciparum, and 4.27% mixed P. falciparum and P.vivax cases (INS 2010). This level of malaria contributes to unstable endemic transmission throughout Colombia (Padilla et al. 2011). Furthermore, for the year 2010, the number of reported cases by state suggested a situation of epidemic malaria (Chaparro et al. 2013). The current study was performed in periurban Miraflores, and is the first entomological report for this municipality.
Fig. 1.
Map of Colombia depicting location of four municipalities, including Miraflores, Guaviare Department where anopheline study was carried out in 2010.
Materials and Methods
Study Site
Miraflores is situated in southeastern Amazonian Colombia (Fig. 1) at 213 m, and has an average annual temperature of 27°C, average relative humidity of 86%, and average annual rainfall of 2692.3 mm/yr. Tropical wet rainforest is the dominant local Holdridge life zone of Amazonian Colombia (SINCHI 2000). Miraflores has an area of 12,756 km2 and is designated as an Amazonian forest reserve within the National Wildlife Reserve Nukak (Ministerio del Medio Ambiente 1989). It is ≈150 km from San José del Guaviare, the capital of Guaviare, and is accessible only by river (≈10 d along the Vaupés River from Calamar municipality) or by air (1.5 h from San José del Guaviare). The economy is based on agriculture and logging. Miraflores is located on the northeastern margin of the Vaupés River. The rural area is occupied by more than 10 ethnic groups such as Nukak Makú, Tucanos, and Carijonas. At least five of these groups are also found in the periurban area, as a result of displacement from armed conflict (Miraflores, Guaviare, 2013).
The urban area is occupied by settlers who have arrived as migrants attracted by economic booms including the exotic skin trade and coca cultivation. It is the major regional commercial center for food, clothing, and medicine. Local institutional capacity is weak, influenced by groups involved in armed conflict. In general the population self-medicates and has poor nutritional status. The risk factors inherent in this situation favor malaria endemicity (CORPES 2004).
Mosquito Collections
In March (dry season) and September (rainy season) 2010, mosquito collections were conducted during three consecutive nights in the periurban neighborhood of La Paz (01° 19′0.01″ N, 71° 57.2′39″ W; Fig. 2), where malaria cases were previously reported. The house where mosquitoes were collected has wooden walls and few bricks with only one entrance for both entry and exit. The roof is made of wood, with a dirt floor, and the only bedroom is completely made of wood without windows. The kitchen is open without walls or windows; it was connecting directly to the courtyard where vegetation is found. Sampling was carried out using human landing catch (HLC) indoors and outdoors from 1800 to 0600 hours, by a team of four collectors. Collectors worked in pairs, one collector indoors and one outdoors, and rotated every two hours to avoid collector bias (World Health Organization [WHO] 1975). Live females were placed in plastic bottles labeled with collection information and provided with a 10% sugar solution. They were transported to the Laboratory of Entomology, Faculty of Agronomy, Universidad Nacional de Colombia (UNC)—Bogotá, blood fed on Mus musculus L. (protocol identification below) and identified using the keys of Faran and Linthicum (1981), González and Carrejo (2007), and Rubio-Palis (2000). After the wild-caught females oviposited (or died), they were maintained over desiccant for subsequent identification of Plasmodium species circumsporozoite (CS) protein using enzyme-linked immunosorbent assay (ELISA). Isofamilies were obtained by forced mating (one leg and wing removed) after which each female was lightly anesthetized with ethyl acetate and placed in water for oviposition (Estrada et al. 2003). Wild females and their isofamilies were used for taxonomic identification, and in both cases one leg and wing of each was mounted on a slide with Canada balsam and retained as a voucher. The number of mosquitoes caught per day per person (human-biting rate—HBR; WHO 1975) was estimated for the most abundant species, An. darlingi.
Fig. 2.
A detailed map of periurban Miraflores, Guaviare, Colombia, 2010. Five immature anopheline breeding site types and the wild adult female collection locality are depicted. Carrera 7 is the main road through the town of Miraflores. Nearby Vaupes River and two tributary streams, Picho and Umari, are labeled.
Plasmodium Detection
Only heads and thoraces were tested for the CS protein of P. falciparum and P. vivax 210 and 247 by ELISA, following standard protocols (Wirtz et al. 1987a,b, 1991, 1992). Mosquitoes were tested in pools of five specimens by collection method and date, to ensure that the probability of detecting more than one infected mosquito per pool is <1% (Rubio-Palis et al. 1992, Galardo et al. 2007, Magris et al. 2007). Negative controls were from the An. albimanus colony, Buenaventura strain, maintained at the Laboratory of Entomology, Faculty of Agronomy, UNC. The number of CS-positive Plasmodium pools was equivalent to the number of CS-positive mosquitoes; therefore, the sporozoite rate was estimated as the percentage of CS-positive mosquitoes divided by the total number of mosquitoes assayed.
Anopheline Larval Collections
Breeding sites in periurban Miraflores were surveyed to detect and collect Anopheles larvae, using 500-ml ladles every 5 m, for a total of 50 dips per breeding site. The number of individual larvae collected per dip was recorded. Each collection was georeferenced, and hydrological parameters (temperature, depth, pH, apparent color, current, shade) and associated vegetation cover and type were recorded for subsequent analysis (not included in this manuscript). At least 10 breeding sites could not be sampled due to the probable presence of landmines.
Ethics
HLCs were conducted using an informed consent agreement under a protocol and collection procedures that were reviewed and approved by the Ethics Committee of the Faculty of Medicine of UNC and by the Institutional Review Board of the Wadsworth Center, NY State Department of Health (NYSDOH), Albany, NY protocol no. 02-028. Blood feeding of the mosquito females followed the vertebrate management protocols of the Faculty of Veterinary Medicine of UNC, the Ethics Committee on Animal Care of the National Institutes of Health—Office of Laboratory Animal Welfare (assurance no. A5791-01), and Institutional Animal Care and Use Committee (IACUC) committee protocol no. 11-421, NYSDOH.
Results
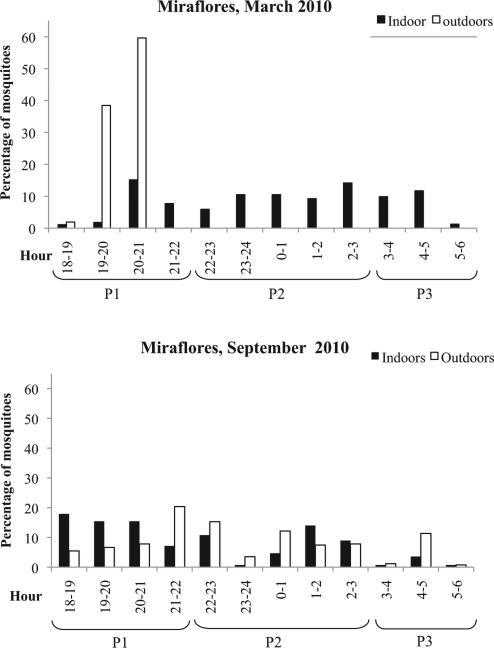
In total, 828 female anopheline mosquitoes representing four species were collected using HLC in 36 h indoors and outdoors, during March and September. The most abundant species was An. darlingi (n = 789), followed by Anopheles braziliensis (Chagas) (n = 28), and Anopheles oswaldoi s.l. (Peryassu) (n = 6). The mean biting rate for An. darlingi was 8.6, 1.3 for An. braziliensis, and 0.7 for An. oswaldoi s.l. The biting activity of An. darlingi was analyzed to consider exposure during three periods determined by habits of the human population (Fig. 3). The only time Miraflores has electricity is during the first period (P1), 1800–2200 hours; during the second period (P2), 2200–0300 hours, people are in their homes; and during the last period (P3), 0300–0600 hours, people begin daily activities (Fig. 3). During the dry season, exophagic activity was confined to P1; endophagy was the only biting behavior during P2–P3. In contrast, during the rainy season, both feeding behaviors occurred throughout the night (P1–P3). All 828 mosquitoes, representing 166 pools, were assayed by ELISA. Only An. darlingi was positive for P. vivax 210, with a sporozoite rate of 0.13% (1 of 788). The infected mosquito was captured in March between 2400–0100 hours indoors.
Fig. 3.
Percentage of adult female anopheline mosquitoes (y-axis) collected per hour, between 1800 and 0600 hours (x-axis) by collection location (indoor, black vertical bar; outdoor, white vertical bar) and season in Miraflores, Colombia, in 2010. Upper graph shows results from March—dry season; lower graph shows results from September—rainy season.
The main breeding sites in Miraflores were grouped into stream (n = 7), flooded excavations (n = 4), flooded forest (n = 1), wetlands (n = 2), and an abandoned water reservoir (n = 1; Fig. 2). During the rainy season, water levels of the Vaupés River rise and flood part of the municipality, forming two major anopheline breeding sites (flooded forest and flooded excavation; Table 1). Three species were collected in these breeding sites: An. darlingi (64.5%) was the most abundant, followed by An. oswaldoi s.l. (25.8%). An. braziliensis was present, but uncommon. A significant number of larvae (n = 38) could not be identified owing to high mortality of early instars during transportation to Bogotá.
Table 1.
Anopheles immature forms collected from each breeding site type in Miraflores, Guaviare, Colombia, March and September, 2010
| Month | Species | Breeding site types |
||||
|---|---|---|---|---|---|---|
| Stream (n = 7) | Flooded excavation (n = 4) | Flooded forest (n = 1) | Wetlands (n = 2) | Abandoned water reservoir (n = 1) | ||
| Mar. | An. darlingi | 5 | 2 | 1 | 1 | 0 |
| An. oswaldoi s.l. | 1 | 3 | 1 | 1 | 0 | |
| An. braziliensis | 0 | 1 | 0 | 0 | 1 | |
| Sept. | An. darlingi | 5 | 1 | 3 | 1 | 1 |
| An. oswaldoi s.l. | 1 | 0 | 1 | 0 | 0 | |
| An. braziliensis | 0 | 0 | 0 | 0 | 1 | |
Discussion
An. darlingi has long been associated with a rain-forest environment (Faran and Linthicum 1981, Consoli and Lourenço-de-Oliveira 1994), and it is considered the main Amazonian malaria vector (Zimmerman 1992, Lounibos and Conn 2000, Rubio-Palis 2000, Montoya-Lerma et al. 2011). In Miraflores, An. darlingi was caught throughout the night showing mainly exophagy (Fig. 3). Exophagic behavior is common in An. darlingi, but it also feeds endophagically, depending on environment and host availability (Hiwat and Bretas 2011, Moutinho et al. 2011). Low densities were due to limited sampling effort; therefore, we could not obtain adequate data to define biting patterns for this species in the study area.
An. darlingi was found naturally infected with P. vivax, and the peak seasonal, indoor–outdoor biting times (Table 2) were related to human activities (Fig. 3). However, because of the small sample size (n = 1 infected specimen), An. darlingi is a presumed vector in this locality, but additional studies should be undertaken for confirmation. The behavior of the vector species influences the local epidemiological pattern, and the same populations of An. darlingi may show behavioral variation in response to environmental change in addition to seasonal fluctuation, thus increasing the complexity of malaria transmission dynamics (Charlwood 1996, Voorham 2002, Santos et al. 2009). Even with low An. darlingi capture rates, transmission by this species has been recorded in the Amazon region (Lanelli et al. 1998, Tadei et al. 1998, Conn et al. 2002).
Table 2.
HBR and numbers of adult female An. darlingi collected in Miraflores, Guaviare, Colombia, 2010, during three periods of nightly activity in both dry (March) and rainy (September) seasons
| HBR | ||||||||
|---|---|---|---|---|---|---|---|---|
| Period of night (P) and hour | Indoor |
Outdoor |
||||||
| Mar. | Total | Sept. | Total | Mar. | Total | Sept. | Total | |
| P1 | ||||||||
| 18-19 | 0.3 | 2 | 8.3 | 50 | 0.1 | 1 | 2.3 | 14 |
| 19-20 | 0.5 | 3 | 7.1 | 43 | 3 | 20 | 2.8 | 17 |
| 20-21 | 4.1 | 25 | 7.1 | 43 | 5.1 | 31 | 3.3 | 20 |
| 21-22 | 2.1 | 13 | 3.3 | 20 | 0 | 0 | 8.6 | 52 |
| P2 | ||||||||
| 22-23 | 1.6 | 10 | 5 | 30 | 0 | 0 | 6.5 | 39 |
| 23-24 | 2.8 | 17 | 0.3 | 2 | 0 | 0 | 1.5 | 9 |
| 0-1 | 2.8 | 17 | 2.1 | 13 | 0 | 0 | 5.16 | 31 |
| 1-2 | 2.5 | 15 | 6.5 | 39 | 0 | 0 | 3.16 | 19 |
| 2-3 | 3.8 | 23 | 4.1 | 25 | 0 | 0 | 3.3 | 20 |
| P3 | ||||||||
| 3-4 | 2.6 | 16 | 0.3 | 2 | 0 | 0 | 0.5 | 3 |
| 4-5 | 3.1 | 19 | 1.6 | 10 | 0 | 0 | 4.8 | 29 |
The Miraflores municipality may be in a permanent state of malaria outbreak. Possible reasons for this include migration of members of indigenous communities from high malaria transmission areas, uncontrolled movement of gold miners, the presence of a local army force where members also rotate among high and low transmission areas, and movement of regional traders. Furthermore, drastic changes in the ecosystem, such as the cut and burn method of the forest clearing for crops and livestock, have likely contributed to risk factors for local Plasmodium transmission.
Knowledge of fauna composition and vector behavior in wild areas is essential for the adoption of potential alternative vector control strategies aimed at preventing transmission. During the rainy season An. darlingi exhibited both exo- and endophagic biting behaviors, with exophagy more pronounced during P2–P3 compared with dry season behavior, as was previously observed in Roraima state, Amazonian Brazil (Barros et al. 2007), and in other parts of the Amazon forest (Lanelli et al. 1998, Sá et al. 2005, Hiwat and Bretas 2011).
The main breeding sites were natural water collections as expected for the ecological conditions of Miraflores, where people live on the fringes of rainforest, only occupying a small area associated with the aircraft landing strip, but are continually expanding into the forest. Breeding was associated with human-made excavations because people use gravel for multiple purposes such as mining or house construction. All of the breeding sites are well defined in the dry season, but during the rainy season, the Vaupés River floods the periurban area, forming two huge breeding sites. An. darlingi was found in all types of breeding sites, so in theory it can emerge from a breeding site, obtain a bloodmeal and then rest in vegetation, likely in the forest, where vector control is impractical. In this way, populations of An. darlingi can be maintained all year, contributing to both abundance and transmission.
Vector control is an important component of malaria control programs because it is one of the most efficient strategies to prevent transmission (Brochero and Quiñones 2008); however, forest malaria may be more difficult to control than nonforest malaria (Tadei and Dutary Thatcher 2000, Galardo et al. 2009, Santos et al. 2009). Forest vectors are often partially or wholly exophilic and exophagic and do not normally enter houses protected by indoor residual spraying (Enayati and Hemingway 2010). In Miraflores, it may be impractical to try to control An. darlingi immatures in breeding sites because this species appears to be locally ubiquitous. Furthermore, such a strategy in periurban and urban areas of Miraflores is impossible due to the presence of landmines and armed conflict. Health Authorities could try to implement long-lasting insecticidal nets in all houses after considering local community attitudes, beliefs, and practices, and use the results to evaluate changes in abundance or behavior of An. darlingi populations.
Furthermore, biological characteristics of wild female An. darlingi, which are behaviorally plastic (Voorham 2002), often biting outdoors, combined with their nocturnal activity, suggest that multiple control strategies might be more successful. Prevention and control strategies could focus on improved entomological surveillance to detect potential changes in behavioral patterns and relative species incrimination in local transmission. The reduced diversity of Anopheles species detected in this preliminary study in Miraflores may be the result of the limited number and frequency of collections, suggesting that further studies should be undertaken for a more comprehensive understanding of local malaria transmission patterns.
Acknowledgments
The authors thank Humberto Mosquera for assistance with field collections. We thank Laureano Mosquera, Entomological Unit coordinator, and technical personnel of the Guaviare Health Department, Colombia, for logistic support in Miraflores. We thank Maria Camila Ramirez for assistance with the maps (Figs. 1 and 2). We are grateful to Robert A. Wirtz, Centers for Disease Control (CDC), Entomology Branch, Atlanta, GA, for ELISA reagents. This work was supported by U.S. National Institutes of Health grant (AI) R01 54139 “Malaria Vector Biology in Brazil: Genetics and Ecology” (to J.E.C.), and Universidad Nacional de Colombia—Bogotá, Faculty of Agronomy Quipu 201010012197 (to H.B.).
References Cited
- Barros FS, Arruda ME, Vasconcelos SD, Luitgards-Moura JF, Confalonieri U, Rosa-Freitas MG, Tsouris P, Lima-Camara TN, Honório NA. Parity and age composition for Anopheles darlingi Root (Diptera: Culicidae) and Anopheles albitarsis Lynch-Arribálzaga (Diptera: Culicidae) of the northern Amazon Basin, Brazil. J. Vector Ecol. 2007;32:54–68. doi: 10.3376/1081-1710(2007)32[54:paacfa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Brochero H, Quiñones ML. Retos de la entomología médica para la vigilancia en salud pública en Colombia: reflexión para el caso de malaria. Biomedica. 2008;28:18–24. [PubMed] [Google Scholar]
- Chaparro P, Padilla J, Vallejo A, Herrera S. Characterization of a malaria outbreak in Colombia in 2010. Malar. J. 2013;12:330. doi: 10.1186/1475-2875-12-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlwood JD. Biological variation in Anopheles darlingi Root. Memórias do Instituto Oswaldo Cruz. 1996;91:391–398. doi: 10.1590/s0074-02761996000400001. [DOI] [PubMed] [Google Scholar]
- Conn JE, Wilkerson RC, Segura MN, de Souza RT, Schlichting CD, Wirtz RA, Póvoa MM. Emergence of a new neotropical malaria vector facilitated by human migration and changes in land use. Am. J. Trop. Med. Hyg. 2002;66:18–22. doi: 10.4269/ajtmh.2002.66.18. [DOI] [PubMed] [Google Scholar]
- Consoli RAGB, Lourenço-de-Oliveira R. Principais mosquitos de importância sanitária no Brasil. Fiocruz, Rio de Janeiro. 1994:225. [Google Scholar]
- (CORPES) Consejo Regional de Planificación—Gobernación del Guaviare Miraflores–Guaviare. Esquema de ordenamiento territorial Municipio de Miraflores. Documento técnico. Componente Rural. 2004:216. [Google Scholar]
- Enayati A, Hemingway J. Malaria management: past, present, and future. Annu. Rev. Entomol. 2010;55:569–591. doi: 10.1146/annurev-ento-112408-085423. [DOI] [PubMed] [Google Scholar]
- Estrada DA, Quinones ML, Sierra DM, Ruiz F, Erazo HF, Linton YM. Egg morphology as an indirect method to identify Anopheles benarrochi, Anopheles oswaldoi and Anopheles rangeli (Diptera: Culicidae). Biomedica. 2003;23:388–395. [PubMed] [Google Scholar]
- Faran M, Linthicum K. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae). Mosq. Syst. 1981;13:1–81. [Google Scholar]
- Galardo AKR, Arruda M, D'Almeida Couto AAR, Wirtz RA, Lounibos LP, Zimmerman RH. Malaria vector incrimination in three rural riverine villages in the Brazilian Amazon. Am. J. Trop. Med. Hyg. 2007;76:461–469. [PubMed] [Google Scholar]
- Galardo AK, Zimmerman RH, Lounibos LP, Young LJ, Galardo CD, Arruda M, D'Almeida Couto AA. Seasonal abundance of anopheline mosquitoes and their association with rainfall and malaria along the Matapí River, Amapá, Brazil. Med. Vet. Entomol. 2009;23:335–349. doi: 10.1111/j.1365-2915.2009.00839.x. [DOI] [PubMed] [Google Scholar]
- González R, Carrejo N. Introducción al estudio taxonómico de Anopheles de Colombia, claves taxonómicas y notas de distribución. Universidad del Valle; Cali: 2007. p. 237. [Google Scholar]
- Hiwat H, Bretas G. Ecology of Anopheles darlingi Root with respect to vector importance: a review. Parasit. Vectors. 2011;4:177. doi: 10.1186/1756-3305-4-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (INS) Instituto Nacional de Salud Sistema nacional de vigilancia en salud pública (SIVIGLIA). Inf. Quinc. Epidemiol. Nac. 2010;11:33–48. [Google Scholar]
- Lanelli RV, Honório NA, Lima DC, Lourenço-de- Oliveira R, Santos RV, Coimbra CEA., Jr. Faunal composition and behavior of anopheline mosquitoes in the Xavante Indian reservation of Pimentel Barbosa, Central Brazil. Parasite. 1998;5:197–202. doi: 10.1051/parasite/1998052197. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Conn JE. Malaria vector heterogeneity in South America. Am. Entomol. 2000;46:237–248. [Google Scholar]
- Magris M, Rubio-Palis Y, Menares C, Villegas L. Vector bionomics and malaria transmission in the Upper Orinoco River, Southern Venezuela. Mem. Inst. Oswaldo Cruz. 2007;102:303–311. doi: 10.1590/s0074-02762007005000049. [DOI] [PubMed] [Google Scholar]
- Mendoza M, Nicholls R, Olano VA, Cortés L. Manual de manejo integral de la malaria. Instituto Nacional de Salud; Bogota: 2000. Situación de la Malaria en Colombia. [Google Scholar]
- Ministerio del Medio Ambiente . Unidad Administrativa Especial del Sistema de Parques Naturales Nacionales de Colombia-UAESPNN. Unidad de parques; Bogotá.: 1989. [Google Scholar]
- Miraflores Guaviare. Sitio oficial de Miraflores en Guaviare. Colombia: 2013. ( http://miraflores-guaviare.gov.co/index.shtml#2) [Google Scholar]
- Montoya-Lerma J, Solarte YA, Giraldo-Calderon GI, Quinoñes ML, Ruiz-Lopez F, Wilkerson RC, González R. Malaria vector species in Colombia -a review. Mem. Inst. Oswaldo Cruz. 2011;106(Suppl. I):223–238. doi: 10.1590/s0074-02762011000900028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutinho PR, Gil LH, Cruz RB, Ribolla PE. Population dynamics, structure and behavior of Anopheles darlingi in a rural settlement in the Amazon rainforest of Acre, Brazil. Malar. J. 2011;10:174. doi: 10.1186/1475-2875-10-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Peña S. Situación epidemiológica de la malaria en Colombia. Inf. Quinc. Epidemiol. Nac. 2002;7:333–345. [Google Scholar]
- Padilla J, Alvarez G, Montoya R, Chaparro P, Herrera S. Epidemiology and control of malaria in Colombia. Mem. Inst. Oswaldo Cruz. 2011;106(Suppl. 1):114–122. doi: 10.1590/s0074-02762011000900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Palis Y. Escuela de Malariología y Saneamiento Ambiental Dr. Arnoldo Gabaldon, Proyecto Control de Enfermedades Endémicas. Venezuela. Maracay: 2000. Anopheles (Nyssorhynchus) de Venezuela: taxonomía, bionomía, ecología e importancia médica. p. 121. [Google Scholar]
- Rubio-Palis Y, Wirtz RA, Curtis CF. Malaria entomological rates in Western Venezuela. Acta Trop. 1992;52:167–174. doi: 10.1016/0001-706x(92)90033-t. [DOI] [PubMed] [Google Scholar]
- Sá DR, Souza-Santos R, Escobar AL, Coimbra CE., Jr. Malaria epidemiology in the Pakaanóva (Wari') Indians, Brazilian Amazon. Bull. Soc. Pathol. Exot. 2005;98:28–32. [PubMed] [Google Scholar]
- Santos RL, Padilha A, Costa MD, Costa EM, Dantas-Filho Hde C, Póvoa MM. Malaria vectors in two indigenous reserves of the Brazilian Amazon. Rev. Saúde Pública. 2009;43:859–868. doi: 10.1590/s0034-89102009000500016. [DOI] [PubMed] [Google Scholar]
- (SINCHI) Instituto Amazónico de Investigaciones Científicas . Plan de ordenamiento territorial. Gobernación del Guaviare; Colombia: 2000. [Google Scholar]
- Tadei WP, Dutary Thatcher B. Malaria vectors in the Brazilian amazon: Anopheles of the subgenus Nyssorhynchus. Rev. Inst. Med. Trop. Sao Paulo. 2000;42:87–94. doi: 10.1590/s0036-46652000000200005. [DOI] [PubMed] [Google Scholar]
- Tadei WP, Thatcher BD, Santos JMM, Scarpassa VM, Rodrigues IB, Rafael MS. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am. J. Trop. Med. Hyg. 1998;59:325–335. doi: 10.4269/ajtmh.1998.59.325. [DOI] [PubMed] [Google Scholar]
- Voorham J. Intra-population plasticity of Anopheles darlingi's (Diptera, Culicidae) biting activity patterns in the state of Amapá, Brazil. Rev. Saude Publica. 2002;36:75–80. doi: 10.1590/s0034-89102002000100012. [DOI] [PubMed] [Google Scholar]
- (WHO) World Health Organization . Methods and techniques. II. WHO; Geneva, Switzerland: 1975. Manual of practical entomology in malaria. [Google Scholar]
- Wirtz RA, Burkot TR, Graves PM, Andre RG. Field evaluation of enzyme-linked immunosor-bent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. J. Med. Entomol. 1987a;24:433–437. doi: 10.1093/jmedent/24.4.433. [DOI] [PubMed] [Google Scholar]
- Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, Esser KM, Beaudoin RL, Andre RG. Bull. Vol. 65. World Health Organ; 1987b. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. pp. 39–45. [PMC free article] [PubMed] [Google Scholar]
- Wirtz RA, Charoenvit Y, Burkot TR, Esser KM, Beaudoin RL, Collins WE, Andre RG. Eval uation of monoclonal antibodies against Plasmodium vivax sporozoites for ELISA development. Med. Vet. Entomol. 1991;5:17–22. doi: 10.1111/j.1365-2915.1991.tb00515.x. [DOI] [PubMed] [Google Scholar]
- Wirtz RA, Sattabongkot J, Hall T, Burkot TR, Rosenberg R. Development and evaluation of an enzyme-linked immunosorbent assay for Plasmodium vivax-VK247 sporozoites. J. Med. Entomol. 1992;29:854–857. doi: 10.1093/jmedent/29.5.854. [DOI] [PubMed] [Google Scholar]
- World Malaria Report World Malaria Report 2011. 2011 ( http://www.who.int/malaria/world_malaria_report_2011/en/)
- Zimmerman RH. Ecology of malaria vectors in the Americas and future directions. Mem. Inst. Oswaldo Cruz. 1992;87(Suppl. III):371–383. doi: 10.1590/s0074-02761992000700064. [DOI] [PubMed] [Google Scholar]