Abstract
Important roles played by invariant natural killer T (iNKT) cells in asthma pathogenesis have been demonstrated. We identified functional iNKT cells and CD1d molecules in pig lungs. Pig iNKT cells cultured in the presence of α-GalCer proliferated and secreted Th1 and Th2 cytokines. Like in other animal models, direct activation of pig lung iNKT cells using α-GalCer resulted in acute airway hyperreactivity (AHR). Clinically, acute AHR-induced pigs had increased respiratory rate, enhanced mucus secretion in the airways, fever, etc. In addition, we observed petechial hemorrhages, infiltration of CD4+ cells, and increased Th2 cytokines in AHR-induced pig lungs. Ex vivo proliferated iNKT cells of asthma induced pigs in the presence of C-glycoside analogs of α-GalCer had predominant Th2 phenotype and secreted more of Th2 cytokine, IL-4. Thus, baby pigs may serve as a useful animal model to study iNKT cell-mediated AHR caused by various environmental and microbial CD1d-specific glycolipid antigens.
Keywords: iNKT cells, airway hyperreactivity, pigs, cytokines
Introduction
Asthma is a complex human disease. There are fundamental differences between the spontaneous asthma symptoms in man compared to in experimentally induced counterparts [1]. To reconcile these differences, use of non-human primates and/or other large animal model may be beneficial. Like humans, pigs are outbred in nature and are more similar to humans in terms of anatomy, immunology, biochemistry, physiology, size, and genetics [2, 3]. Indeed, the pig has been used as an animal model for cystic fibrosis [4, 5]. Porcine lungs have marked similarities to humans in terms of its tracheobronchial tree structure, lung physiology, airway morphology, abundance of airway submucosal glands, and the patterns of glycoprotein synthesis [6–8].
Natural killer T (NKT) cells are CD1d-restricted T lymphocyte subpopulation [9]. Majority of NKT cells possess invariant TCR that are called “invariant NKT” (iNKT) [9]. iNKT cells respond to CD1d-bound lipid antigens with a unique α-anomerically linked sugar, one such well-known antigen is α-GalCer [10]. Recently, invariant TCRα of iNKT cells and full-length CD1d transcript in pigs have been identified [11, 12]. Molecular modeling predicts that porcine invariant TCRα chain/poCD1d/α-GalCer form complexes that are highly homologous to the human complex [11]. CD1d molecules are structurally similar to MHC class I and are expressed on the surface of dendritic cells (DCs), macrophages (Mφs), B cells, subset of activated T cells, γδ T cells, and non-hematopoietic cells (keratinocytes, hepatocytes, intestinal, and lung cells) [11–15]. iNKT cells have been grouped into CD4+ (high IL-4 and IL-13 producer) and double-negative (high IL-17 and IL-22 producer) subsets [16, 17]. iNKT cells constitutively express cytokine mRNAs and within minutes of activation rapidly secrete a large amount of cytokines, which in turn activate DCs, Mφs, and T and B cells [18, 19]. iNKT cells in a CD1d-dependent manner can skew adaptive immunity toward Th2 responses, and they have been confirmed to play pivotal role in regulating the development of asthma and allergy [18, 20, 21].
Human and murine CD1d tetramers stain human and murine NKT cells, also in a species cross-reactive manner [11], so it is not surprising that those tetramers can also recognize NKT cells present in other species of animals. C-glycoside analogs of α-GalCer (GCK127 and GCK152) differentially activate murine and human iNKT cells [22, 23]. The GCK152 exhibited a stronger stimulatory activity against human iNKT cells and a much weaker activity against murine iNKT cells, but the opposite trend was detected with GCK127. Invariant TCR of iNKT cells (but not CD1d) is responsible for species-specific preferential activity of C-glycosides [22]. Even though T cells with NK cell phenotype (perforin+CD3+CD11b+CD16+DAP10+−DAP12+) and transcripts of invariant TCRα of pig NKT cells were identified in pigs [11, 24], their physiological relevance has not been explored. In the present study, we identified the functional iNKT cell population and CD1d molecules in the pig lungs. Furthermore, we validated a pig model of iNKT cell-mediated airway hyperreactivity (AHR). A preferential activation and cytokine production by pig iNKT cells to α-GalCer and its C-glycoside analogs was also revealed. These useful insights on pig iNKT cells may provide an opportunity to consider the pig model to explore the iNKT cell-mediated AHR for therapeutic benefits.
Materials and Methods
Glycolipids, Antibodies, and Reagents
α-Galactosylceramide was purchased (Toronto Research Chemicals Inc., Canada). C-glycoside analogs of α-GalCer (GCK127 and GCK152) were synthesized by Drs. Richard W. Franck and Guangwu Chen [25]. Mouse anti-CD1d mAb (clone 1H6) and other eight CD1d mAb [26, 27] and their respective isotype control mAb hybridomas were provided by Dr. Randy Brutkiewicz (Indiana University School of Medicine, Indianapolis, IN, USA). Both the CD1d specific and its isotype mAb were grown and purified. Empty and α-GalCer analog PBS-57-loaded CD1d tetramers conjugated with APC were provided by the NIH Tetramer Core Facility. Anti-pig CD4α mAb-conjugated with FITC (BD Biosciences), anti-pig CD3ε mAb-conjugated with R-PE, CD172-conjugated with R-PE (SouthernBiotech, Birmingham, AL, USA), and purified pan anti-cytokeratin mAb (clone PCK-26; Sigma-Aldrich) were purchased. The clone PCK-26 binds to type II cytokeratins 1, 5, 6, and 8 present only on epithelial cells [28].
Pigs
Weaned-specific pathogen-free Large White–Duroc crossbred piglets raised in farms belonging to The Ohio State University were transported to our BSL-2 animal facility at the FAHRP, OARDC. Pigs were allowed to acclimate for an additional week before initiation of experiments. Pigs were maintained and in vivo experiments were performed in strict accordance with the recommendations by Public Health Service Policy, United States Department of Agriculture Regulations, the National Research Council’s Guide for the Care and Use of Laboratory Animals, the Federation of Animal Science Societies’ Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, and all relevant institutional, state, and federal regulations and policies regarding animal care and use at The Ohio State University. The protocol was approved by the Committee on the Ethics of Animal Experiments, the Institutional Laboratory Animal Care and Use Committee at The Ohio State University.
Identification of Pig CD1d Reactive mAb and iNKT Cell Reagents
A pig CD1d cross-reactive mouse anti-CD1d mAb was identified from a panel of 9 mAb [26, 27] by flow cytometric analysis. Briefly, pig bronchoalveolar lavage fluid (BAL) cells and PBMCs were immunostained using a panel of Alexa488-conjugated mouse anti-CD1d mAb (clone 1H6) [26] along with a respective isotype control mAb. We identified the cross reactivity of mouse and human CD1d tetramers with pig iNKT cells and further standardized the staining conditions to study them.
Isolation of Bronchial and Lung Epithelial Cells
Pig bronchial airway and lung tissues were collected in DMEM (Hyclone) supplemented with FBS and in the presence of antibiotics. Tissues were minced and treated with a mixture of DNase I (Sigma-Aldrich; 40 µg/ml) and collagenase type II (1.5 mg/ml; Invitrogen) in DMEM for 48 h at 4°C. The enzyme activity was stopped by addition of DMEM containing 10% FBS and then passed through a 70-µm cell strainer to harvest single cell suspension. Cells were then subjected to 43% and 70% Percoll density gradient centrifugation, and the pellet was washed and resuspended in enriched RPMI (Hyclone; E-RPMI; 10% FBS, gentamicin (100 µg/ml), ampicillin (20 µg/ml), 20 mM HEPES, 2 mM l-glutamine, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, and 50 nM 2-ME).
Isolation of Pig PBMCs, Splenocytes, Lung Mononuclear Cells, and BAL Cells
From euthanized pigs, blood (in acid citrate dextrose solution), spleen, and lung tissues were collected in DMEM. Isolation of BAL cells and PBMCs were performed as described previously [29]. Isolation of splenocytes was performed using standard procedures [30], using 43% and 70% discontinuous Percoll density gradient centrifugation. Isolation of lung mononuclear cells (MNCs) was performed as described previously [31], by treatment of minced lung tissue with type II collagenase and DNase I.
Induction of AHR in Pigs
Pigs were anesthetized using Telazol® and intubated with the help of laryngoscope and then inoculated with α-GalCer 500 µg/m2 in saline or mock-inoculated (vehicle control) intratracheally. Body surface area of pigs was calculated as described previously [32]. The dose of α-GalCer used was optimized in pigs to induce acute AHR based on a previous study conducted in monkeys [21]. Pigs were monitored every 3 h for breathing pattern and respiratory rate. The body temperature of pigs was recorded at 9 and 24 h post-inoculation.
Evaluation of Gross Lesions, Immunohistochemistry, and Hematoxylin-and-Eosin Staining
At necropsy, the trachea, airways, and lungs were examined for gross pathology. A small portion of the lung tissue was frozen using optimal cutting temperature compound (embedding medium for frozen tissue specimens, Tissue Tek). Frozen lung sections (3 µm) were immunostained as described previously [33]. Briefly, sections were dewaxed, dehydrated, and quenched. The sections were washed with PBS and blocked using 2% goat serum and then treated with biotinylated anti-pig-specific CD4 (Southern Biotech) or isotype control mAb. The sections were then treated with ABC peroxidase staining kit (Vectastain Elite, Vector Labs), and then labeling was “visualized” by application of the DAB (3, 3′-diaminobenzidine) substrate (Vector Laboratories) and counterstained with hematoxylin. Frozen lung sections (3 µm) were also stained using hematoxylin-and-eosin (H&E) as described previously [34].
In Vitro Proliferation of Pig iNKT Cells Using α-GalCer and Its C-Glycoside Analogs
PBMCs and lung MNCs isolated from control, vehicle, or α-GalCer-inoculated pigs were cultured in 96- or 48-well tissue culture plates in E-RPMI in the presence α-GalCer, GCK127, GCK152 (10, 100, or 1,000 ng/ml) or vehicle. Lyophilized α-GalCer, GCK127, and GCK152 were dissolved in DMSO (1 mg/ml), aliquoted, and stored at −80°C. Further, glycolipids were dissolved in E-RPMI, sonicated, and then used to induce iNKT cell proliferation. On every third day, 75% of the supernatant was replaced with fresh medium containing glycolipid or vehicle, and the supernatant harvested was stored at −20°C for cytokine analysis.
Determination of Cytokine Concentrations in Serum and Lung Homogenates
The lung tissue from all the euthanized pigs was collected in DMEM without serum, and the lung homogenate was prepared as described previously [35] and stored at −20°C. Serum, cell culture supernatants harvested on day 4 cultures of PBMCs and lung MNCs as described above, and lung homogenates were analyzed for cytokines IL-4, IL-6, IL-10, IL-13, and IFNγ by ELISA as described previously [35, 36]. Amount of cytokines detected in lung homogenates of each pig was recalculated and expressed as picograms per gram of lung tissue based on the quantity of lung tissue used to prepare the homogenate. Amounts of cytokines detected in medium control wells were subtracted from the test wells.
Flow Cytometry
BAL cells, splenocytes, lung cells, both fresh and 8 days cultured PBMCs, and lung MNCs were washed in HBSS (Sigma) containing BSA (0.1%) and sodium azide (0.02%; FACS buffer) and then treated with 2% pig serum to block the Fc receptors. Cells were treated with purified mouse anti-CD1d mAb (1H6; IgG2a), pan-cytokeratin mAb (IgG1), CD172-R-PE, or their respective isotype control mAb and then incubated for 30 min on ice. Cells were washed and treated with respective anti-species isotype-specific secondary antibody conjugated with R-PE or APC and then fixed using 1% paraformaldehyde. The immunostained cells were acquired using FACS AriaII (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR, USA). To immunostain pig iNKT cells, after blocking Fc receptors, cells were treated with CD3ε-PE, pre-titrated dilution of mouse PBS-57/CD1d tetramers-APC (1:500) and CD4α-FITC for 1 h at 4°C. Respective isotype control mAb and empty CD1d tetramers-APC were included in the assay. Cells were fixed and analyzed as described above. The frequencies of CD1d expression on pig cells and iNKT cell populations were analyzed from a total of 50,000 to 100,000 events.
Data Analyses
Statistical analyses were performed using nonparametric unpaired Mann–Whitney U test using the software GraphPad Instat Biostatistics version 3.0 for windows. Combined data from two experiments (n=6) were used for statistical purpose. Statistical significance was assessed as P<0.05. Error bars represent the standard error from the mean.
Results
Pigs Express Functional CD1d Molecules in Their Lungs
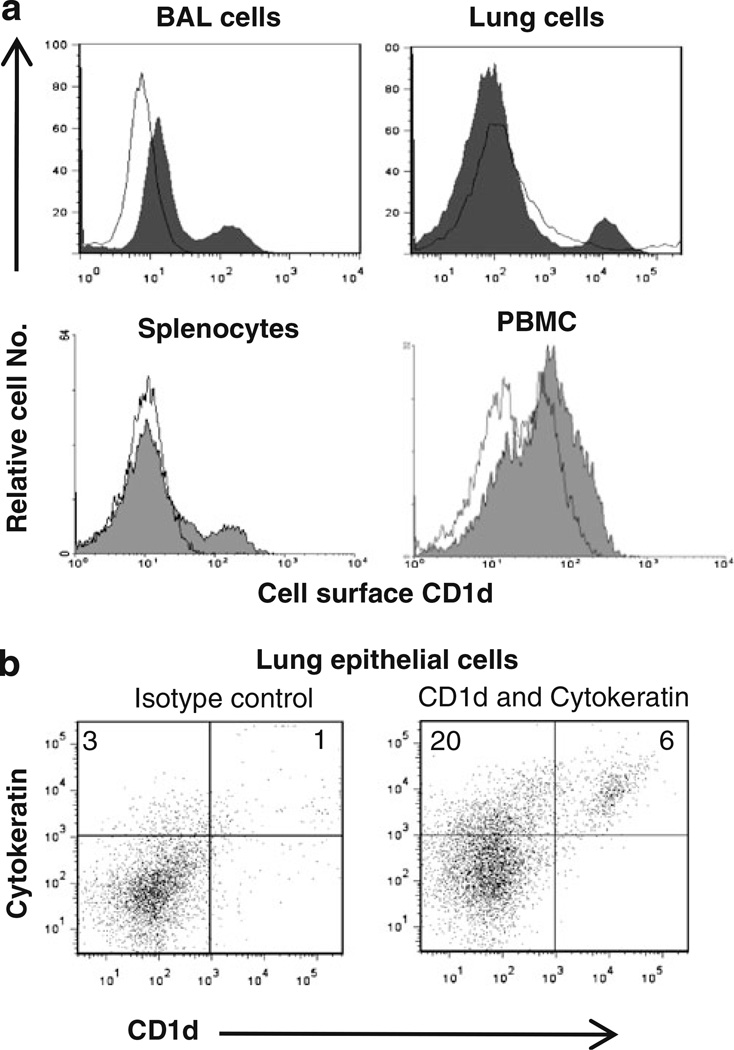
In our study to analyze the expression of pig CD1d, a cross-reactive mouse anti-CD1d mAb was used. Expression of CD1d was observed on the pig BAL cells, lung cells, splenocytes, and PBMCs (Fig. 1a). Further, the CD1d expression on lung epithelial cells was also observed (Fig. 1b). Approximately 25% of the pig lung cells were epithelial cells, and a fraction of them (~25%) did express CD1d (cytokeratin+CD1d+; Fig. 1b).
Fig. 1.
Cell surface CD1d expression on pig cells. a Flow cytometric analysis of pig BAL cells, lung cells, splenocytes, and PBMCs was performed using a pig CD1d cross-reactive mouse anti-CD1d mAb (filled histogram) and an isotype control mAb (open histogram). b Two-color flow cytometric analysis of lung cells for the cell surface CD1d and cytokeratin expression. Numbers in the quadrant indicate percentage of cells, and the data shown are representative of four to ten pigs from two to three independent experiments
Pigs Possess Functional iNKT Cells in Their Lungs
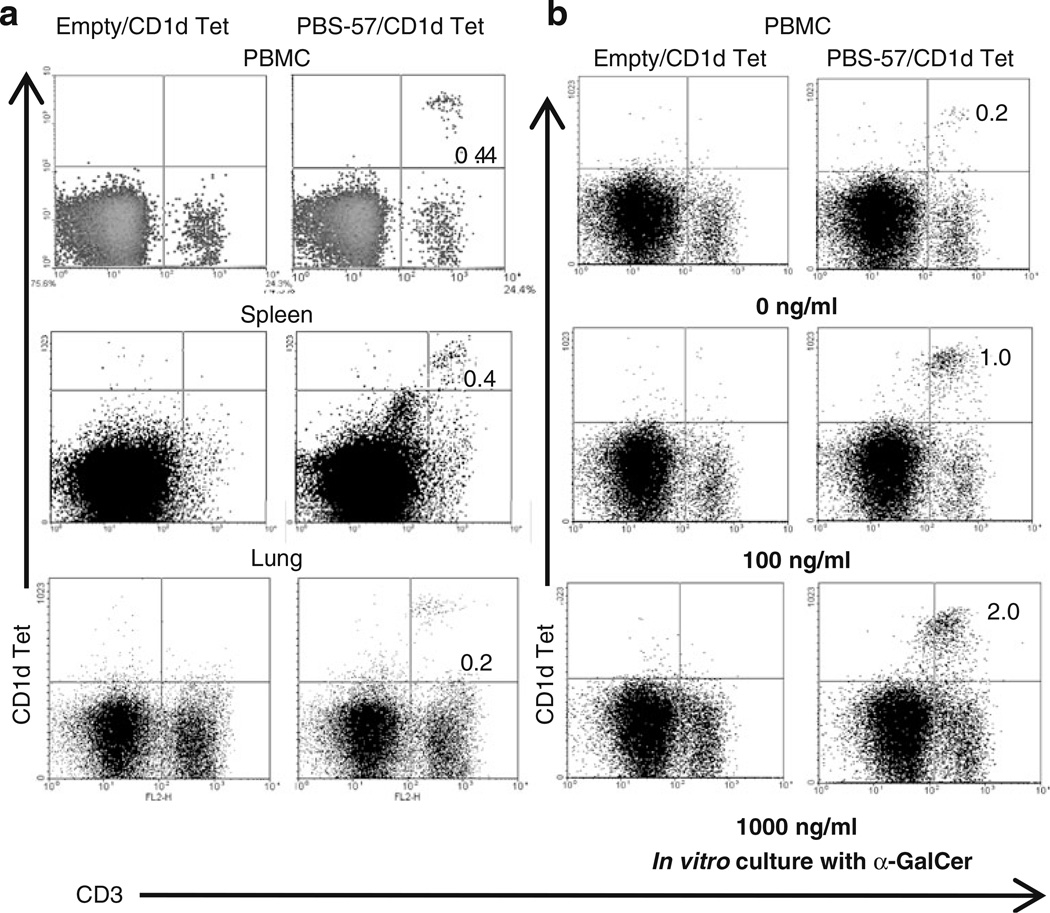
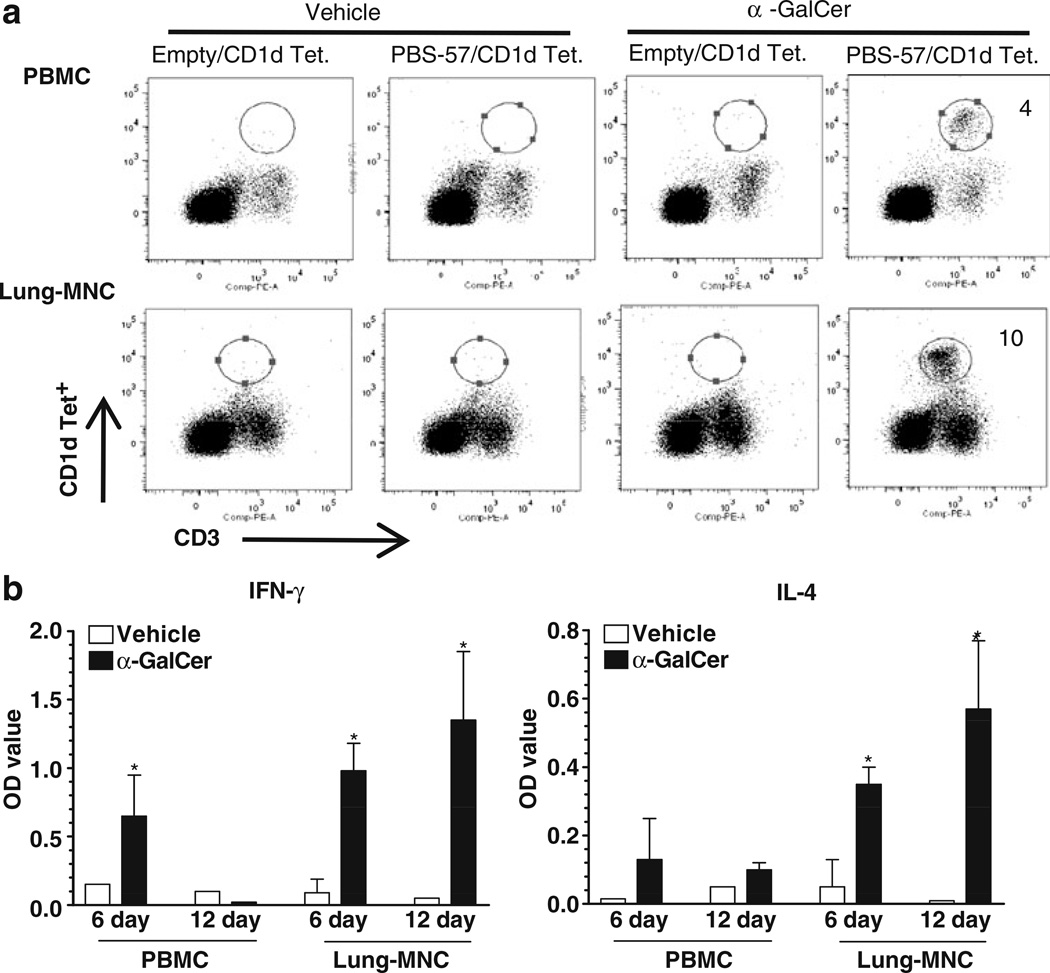
Until now, we have analyzed pig iNKT cells in more than 100 conventional pigs and consistently detected up to 1% (0.01% to 1%) of iNKT cells in blood, spleen, lung, tracheobronchial lymph nodes, and BAL fluid. A representative data of iNKT cells present in the PBMCs, splenocytes, and lung MNCs are shown (Fig. 2a). To identify the antigen-specific activation of pig iNKT cells, PBMCs were cultured in the presence of different concentrations of α-GalCer and detected a dose-dependent proliferation of iNKT cells (Fig. 2b). In addition, lung MNCs and PBMCs when cultured in the presence of α-GalCer, proliferation of iNKT cells, and also secretion of both Th1 (IFNγ) and Th2 (IL-4) cytokines were observed (Fig. 3a, b). As expected, lung MNCs and PBMCs cultured in the presence of vehicle did not proliferate and secret any cytokines (Fig. 3a, b). The cytokines were detected in the culture supernatants harvested at post-3, 6, 9, and 12 day old iNKT cultures in the presence of α-GalCer (Fig. 3b and data not shown), suggesting that pig iNKT cells were actively proliferating in the presence of α-GalCer. In addition, we found CD8α expression on all the CD1d tetramer positive cells (data not shown).
Fig. 2.
iNKT cells are present in pigs. a Two-color flow cytometric analysis of fresh pig PBMCs, splenocytes, and lung MNCs using pig anti-CD3ε mAb conjugated with R-PE and APC-conjugated mouse empty or PBS-57-loaded CD1d tetramers. b Pig PBMCs was cultured in vitro in the presence of various concentrations of α-GalCer for 4 days, and iNKT cells were analyzed as described above. Numbers in the quadrant indicate percentage of cells and the data shown are the representative of five to ten pigs from five independent experiments
Fig. 3.
Functional iNKT cells are present in pig lungs. Pig lung MNCs and PBMCs (control) were cultured in the presence of α-GalCer (1,000 ng/ml) or vehicle for 12 days. a Immunostained using fluorochrome conjugated anti-pig CD3ε and mouse empty or PBS-57 loaded CD1d tetramers and analyzed by flow cytometry. b Culture supernatants harvested on post-6 and 12 day cultures were analyzed for IFNγ and IL-4 using pig cytokine-specific ELISA. Each bar represents the average cytokines OD from three pigs±SEM and * denote statistically significant difference (P<0.05) in the amount of cytokines secreted by cells cultured in the presence of vehicle vs α-GalCer. The data shown are representative of six pigs in three independent experiments
Pig iNKT Cells Mediated Lung Inflammation and Airway Hyperreactivity
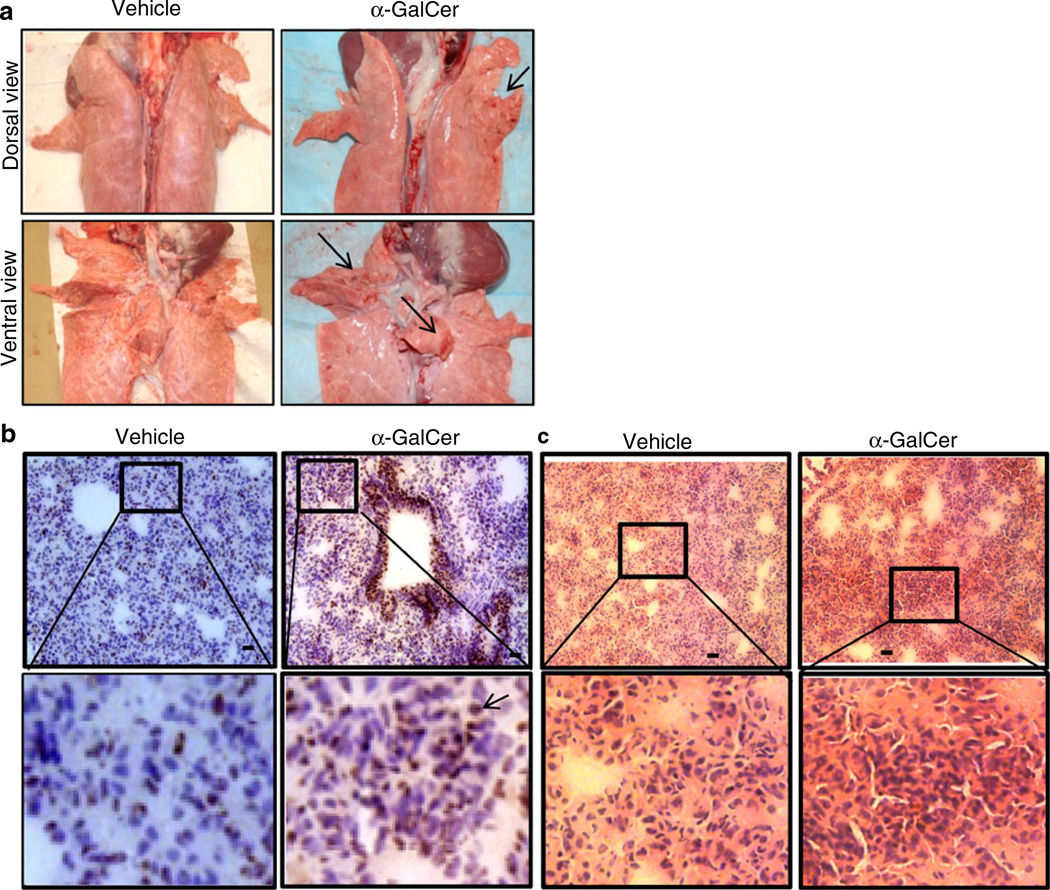
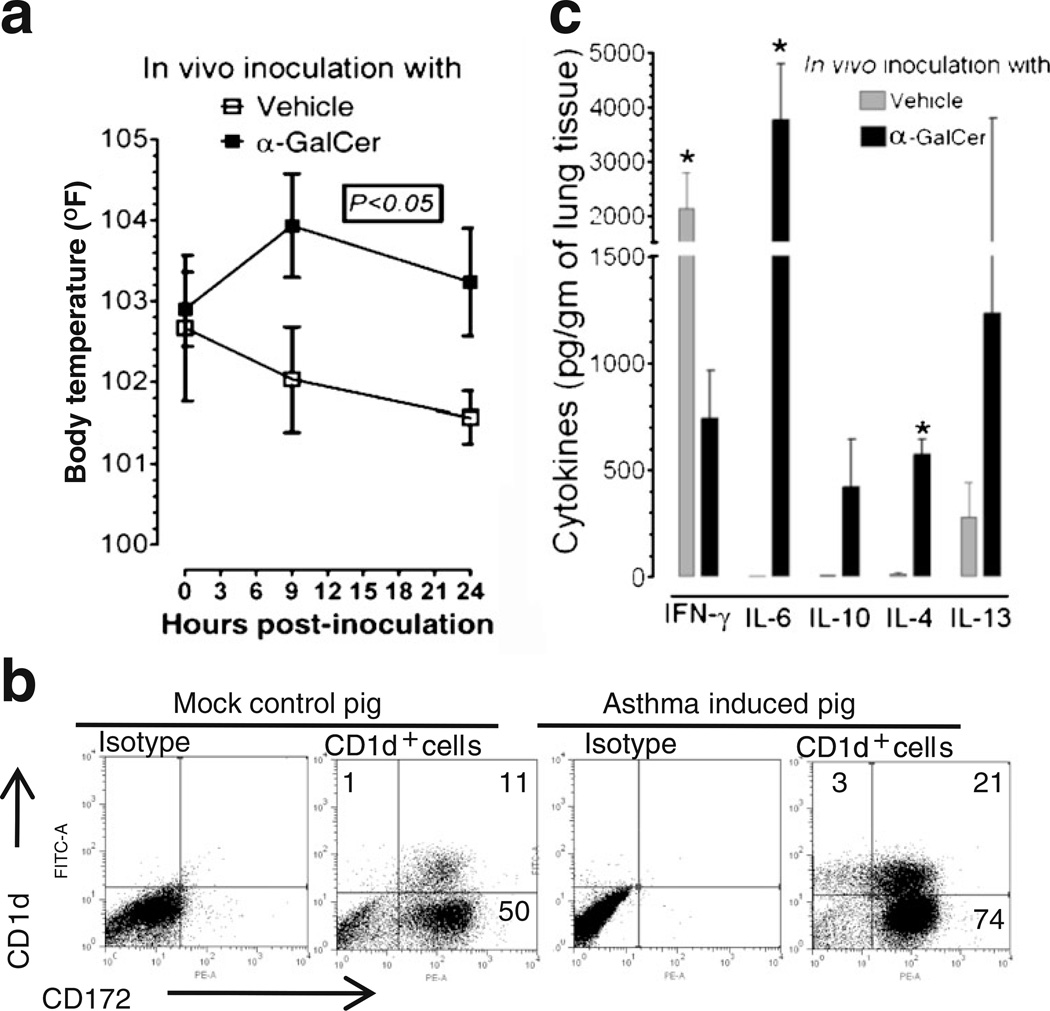
After confirming functional iNKT cells in the pig lungs, we wanted to determine whether pigs can be used to study iNKT cell-mediated AHR. Previously, AHR was successfully induced by direct activation of lung iNKT cells using α-GalCer in mice [37] and non-human primates [21]. During necropsy, in acute AHR-induced pig lungs, petechial hemorrhages (tiny pinpoint red marks, an important sign of asphyxia) on both dorsal and ventral surfaces of cranial, middle, and accessory lobes of lungs was observed (Fig. 4a). In the airways, hypersecretion of mucus was observed, and microscopically in the lung parenchyma excessive infiltration of CD4+ cells (Fig. 4b) and accumulation of severely infiltrated inflammatory leukocytes (Fig. 4c) was also detected in AHR-induced pig lungs compared to in the lungs of vehicle-treated pigs. Clinically, α-GalCer-inoculated pigs were restless with increased respiratory rate (50–60 breaths per minute) compared to control pigs (20–40 breaths per minute) from 6 h post-α-GalCer inoculation. In addition, the body temperature of AHR-induced pigs was approximately 2°F higher compared to vehicle control pigs at 9 and 24 h post-inoculation that we recorded (Fig. 5a).
Fig. 4.
Acute AHR-induced pig lungs had petechial hemorrhages with excessive CD4+ and myeloid cells infiltration. a A representative pig lung macroscopic picture of vehicle or α-GalCer-inoculated pig (n=3 per group) with petechial hemorrhages on both the dorsal and ventral surfaces (arrows indicate areas of hemorrhages) is shown. b A representative pig lung section was subjected to immunohistochemistry, showing more of infiltrated CD4+ cells in α-GalCer received pig lungs compared to vehicle controls. Bar, 10 µm. c A representative pig lung section was subjected to H&E staining, showing massive infiltration of inflammatory leukocytes in α-GalCer received pig lungs. Bar, 5 µm. Frozen sections of vehicle or α-GalCer-inoculated pigs (n=3 per group) were immunostained for pig-specific CD4+ cells (arrowheads) and then counterstained with hematoxylin. Similar results were obtained in another independent experiment
Fig. 5.
Elevated body temperature and increased pro-inflammatory and Th2-cytokine responses were mediated by lung iNKT cells in the acute AHR-induced pigs. Pigs were intratracheally inoculated with α-GalCer or vehicle. a Body temperature was recorded at 9 and 24 h post-inoculation. Mean temperature from three pigs in each treatment group±SEM is shown, and P value was calculated by nonparametric Kruskal–Wallis test. b BAL cells harvested from pigs were immunostained to analyze CD1d on myeloid cells (CD172+) by flow cytometry. The data shown were from a representative pig from vehicle or α-GalCer received pigs (n=3 pigs/group). Numbers in the quadrant indicate percentage of cells. c Lung homogenates prepared from pig lungs 24 h post-inoculation were analyzed for indicated cytokines by ELISA. Each bar represents the average cytokine concentration from three pigs±SEM and * denote statistically significant difference (P<0.05) between vehicle vs α-GalCer-inoculated pigs
To elucidate immune responses in the respiratory tract of acute AHR-induced pigs, pigs were euthanized at 24 h post-inoculation and analyzed for lung cytokines and the frequency of myeloid cells (CD172+). Pig myeloid cells with CD172+ expression are immature myelomonocytic precursors, tissue macrophages, and granulocytes [38]. In the acute AHR-induced pig lungs, a two-fold increase in the total BAL cells and a 1.5-fold increase in the frequency of myeloid cells, in addition, a two-fold increase in the frequency of CD1d expressing myeloid cells (CD172+CD1d+) was detected compared to vehicle-treated mock pigs (Fig. 5b). Distinct CD1d expression on non-myeloid cells, albeit less in frequency (3%), was also noticed (Fig. 5b), suggesting that those cells could be of epithelial origin. We did not detect any increase in the frequency of iNKT cells in both lungs and blood of α-GalCer-inoculated pigs at post-24 h of inoculation (data not shown). To evaluate the cytokines response in acute AHR-induced pigs, the lung homogenates were analyzed and detected an increased secretion of the proinflammatory cytokine IL-6 and Th2 cytokines IL-4, IL-10, and IL-13 compared to mock pigs (Fig. 5c). In contrast, significantly less of IFNγ in lungs of AHR-induced pigs was detected compared to vehicle-inoculated mock pigs (Fig. 5c).
C-Glycoside Analogs of α-GalCer Differentially Activated the Pig iNKT Cells
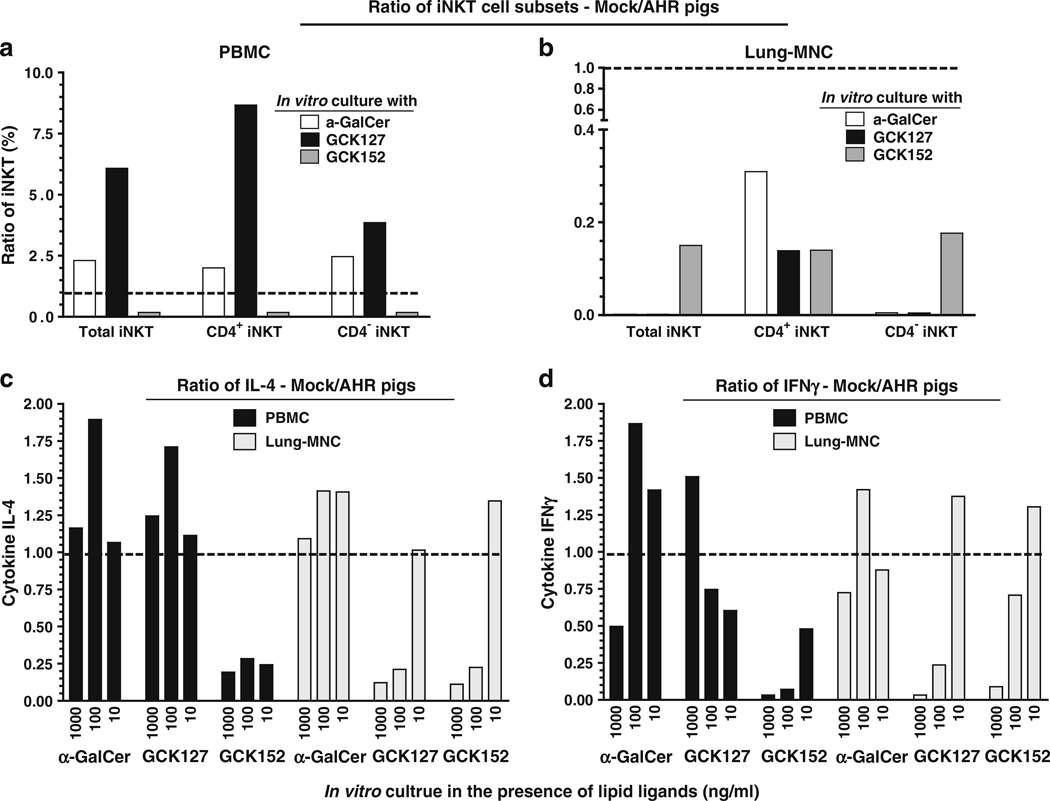
The knowledge pertaining to the phenotype and function of iNKT cells present in the lungs of acute AHR patients is limited. In experimental studies, a species-specific differential activation of iNKT cells to C-glycoside analogs of α-GalCer (GCK127 and GCK152) is reported [22, 23]. To determine the response of iNKT cells to restimulation ex vivo, the α-GalCer received pigs PBMCs and lung MNCs were cultured in the presence of vehicle, α-GalCer, or C-glycoside analogs α-GalCer and then analyzed both the phenotype of iNKT cells and their cytokine response. Our results indicated that GCK127 strongly stimulated the proliferation of iNKT cells (CD3+CD1d Tet+) present in the PBMCs of vehicle-treated pigs compared to GCK152, as indicated by the ratio of mock/AHR-induced pig iNKT cells frequency (Fig. 6a, S1a, and S2a). Concomitantly, iNKT cells of vehicle-treated pig PBMCs secreted higher amounts of both IL-4 and IFNγ upon stimulation with GCK127 compared to GCK152, as indicated by their respective cytokine ratios (Figure 6c, d and S1c, e). In contrast, higher amounts of IL-4 was secreted by iNKT cells present in the PBMCs of acute AHR-induced pigs upon GCK152 stimulation compared to GCK127, as indicated by substantially reduced ratio of IL-4 of mock/AHR pigs (Fig. 6c and S1e). However, comparable levels of IFNγ were secreted upon stimulation with α-GalCer and GCK127 by iNKT cells present in the PBMCs of acute AHR-induced pigs (Fig. 6d). Approximately 30% of the normal healthy (vehicle received) pig iNKT cells express CD4α (Fig. S2). GCK127 and GCK152 favored the preferential proliferation of CD4+ iNKT cells (and IL-4 production) present in the PBMCs of vehicle received and α-GalCer-inoculated pigs, respectively (Fig. 6a, c and S1a). These data indicated the preferential Th2-biased response by GCK152 in AHR-induced pigs.
Fig. 6.
Acute AHR-induced pig iNKT cells present in the PBMCs and lung MNCs were preferentially proliferated into CD4+ phenotype and secreted more of IL-4 than IFNγ ex vivo. Pigs were intratracheally inoculated with α-GalCer or vehicle and euthanized at post-24 h of inoculation. a PBMCs and b lung MNCs isolated from pigs were cultured for 8 days in the presence of vehicle, α-GalCer, GCK127, or GCK152 at indicated concentrations. Cells were immunostained using fluorochrome conjugated anti-pig CD3ε, CD4α, and mouse empty or PBS-57-loaded CD1d tetramers and subjected to flow cytometry. CD3+ gated cells were analyzed for mouse CD1d tetramer+ and CD4α± iNKT cells. The supernatants harvested from cultures were analyzed for c IL-4 and d IFNγ by ELISA. Each bar represents the ratio (vehicle/α-GalCer received pigs) of average percentage of iNKT cells or amounts of cytokine from three pigs in one independent experiment. A trend line was drawn on the y-axis at “1”. Ratio of < or > one means that iNKT cells frequency or secreted cytokines were that many fold < or > in AHR-induced pigs compared to respective mock control, respectively. Similar results were observed in another independent experiment
The iNKT cells present in the lung MNCs of AHR-induced pigs compared to mock pigs proliferated to higher frequency when cultured in the presence of both α-GalCer and its C-glycoside analogs, as indicated by substantially reduced ratio of iNKT cells of mock/AHR pigs (Fig. 6b and S1b). Interestingly, the frequency of proliferated CD4± iNKT cells in the lung MNCs of AHR-induced pigs was comparable when cultured in the presence of all three glycolipids (Fig. 6b and S1b). But relatively more IL-4 and less IFNγ secretion by iNKT cells present in the lung MNCs of AHR-induced pigs cultured in the presence of both GCK127 and GCK152 compared to vehicle-treated pigs was detected (Fig. 6c, d and S1d, f). In contrast, comparable amounts of both Th1 and Th2 cytokines were secreted by iNKT cells present in the lung MNCs of both AHR-induced and vehicle control pigs cultured in the presence of α-GalCer (Fig. 6c, d). Overall, our data suggested that there is a Th2 bias in the acute AHR-induced pig lung iNKT cells upon restimulation by CD1d-specific glycolipids having minor structural differences.
Discussion
Invariant NKT cells express several features of innate immunity, including the ability to rapidly secrete cytokines on activation and the expression of a germline-encoded “the invariant TCR” that is remarkably conserved in sequence and function across species [9]. In an iNKT cell-dependent manner, AHR was induced by allergens [20, 39], virus [40], and ozone [41] in mice. Direct activation of lung iNKT cells resulted in AHR in man [42, 43], monkeys [21], and mice [37] and also demonstrated now in pigs by us. All these studies confirmed that absolute frequency of iNKT cells present in the lungs is not important, but their functional capacity when activated to contribute to the development of AHR is critical. Even we did not detect any increase in the frequency of iNKT cells in acute AHR-induced pigs.
For the first time, both functional iNKT cells and CD1d molecules in pig lungs were identified. The frequency of iNKT cells detected in different tissues of pigs (0.01–1%) is comparable to similar frequencies normally present in humans [44, 45]. Further, we noticed a differential proliferation and cytokine production by AHR-induced pig lung iNKT cells to C-glycoside analogs of α-GalCer. Consistent with the previous reports [15, 44], expression of CD1d on myeloid cells and lung epithelial cells was also detected in pigs. It has been shown that CD1d bound α-GalCer activates iNKT cells exclusively, both in vivo and in vitro [46, 47]. Our results in pigs also suggested that α-GalCer stimulates iNKT cells. Further studies using a stable pig CD1d expressing cell line and NKT cell clones are essential to include the valuable pig system in NKT cell research.
A critical role played by infiltrated CD4+ cells in acute asthma pathogenesis in the lungs of patients and animal models has been demonstrated [48, 49]. As per many recent reports, children suffering from severe asthma (<5 years) and acute bronchiolitis (<1 year) also suffered from high fever [50–52]. The pro-inflammatory cytokine IL-6 is one of the important players responsible for induction of fever in both children and pigs [53–55]. Consistent with the symptoms and immune reactions detected in children with severe asthma, pigs suffering from acute AHR also suffered from fever, many fold increase in the concentration of IL-6, and their lungs were excessively infiltrated with CD4+ cells. Thus, the baby pig model of acute AHR mediated by iNKT cells may serve as a useful addition to the asthma research.
Recent in vitro studies using both murine and human CD1d-expressing cell lines and NKT cell hydridomas or clones detected marked differences in the activation of NKT cells to C-glycoside analogs of α-GalCer (GCK127 and GCK152) [22, 23]. The only difference between these C-glycoside analogs is in their lipid portion; GCK152 has an aromatic ring in the acyl chain, and therefore, lipid chain binding to CD1d molecules results in a minor conformation shift of the sugar head of glycolipids which interacts with the TCR of iNKT cells [22]. Thus, the role of invariant TCR of iNKT cells (but not CD1d molecules) is responsible for differential activation of iNKT cells to lipid antigens with minor structural differences [22]. Many bacterial lipids [56] and plant pollen lipids [57] have been identified to be CD1d ligands capable of activating iNKT cells. But how iNKT cells of AHR-induced animals/patients respond when exposed to different environmental CD1d-specific lipid antigens is not known and that basic information is important to advance in the field of iNKT cell-mediated asthma research. In our study, the response of AHR-induced pig iNKT cells to GCK127 and GCK152 has provided interesting results. An increase in the frequency of CD4+ iNKT cells with concomitant increased production IL-4 upon stimulation of AHR-induced pig iNKT cells to GCK152 compared to GCK127 was detected. In contrast, near opposite results were detected by mock pig iNKT cells in response to GCK127 compared to GCK152. Thus, our data suggest that there are fundamental differences in the iNKT cell proliferation and cytokine secretion in healthy and AHR conditions mediated through iNKT cells upon exposure/re-exposure to environmental CD1d-specific antigens.
iNKT cells present in the PBMCs and lung MNCs of AHR-induced pigs tend to secrete more of IL-4 than IFNγ ex vivo, and in vivo also more of IL-4, IL-10, and IL-13 were detected in pig lungs. Th2-biased immune response in patients suffering from allergic asthma conditions is common [58]. Unlike Th2 cells, iNKT cells are resistant to the effects of corticosteroids and continue to produce cytokines at doses that inactivate Th2 cells [59]. In severe persistent corticosteroid-resistant asthma, iNKT cells could be directly triggered by glycolipids from infectious microbes, plant pollen, or airways insult mediated by viruses and air pollutants [41, 56, 57, 60]. Therapies that disrupt the activation or effector function of pulmonary iNKT cells may be beneficial in the treatment of multiple forms of asthma, and such research could be easily taken up in pig models. In addition, ability of C-glycosides analogs of α-GalCer to differentially activate iNKT cells of AHR-induced pigs has provided useful impetus for future investigations.
Conclusion
Functional pig CD1d molecules and iNKT cells were identified in pigs, in particular in their lungs. Importantly, iNKT cell-mediated acute AHR in pigs was demonstrated. In addition, ability of C-glycoside analogs of α-GalCer (GCK127 and GCK152) to differentially stimulate pig iNKT cells from healthy and AHR-induced pigs has provided useful impetus for future investigations.
Supplementary Material
Acknowledgments
We acknowledge the technical help of Ms. Ruthi Patterson, Ms. Kathryn Dodson, Dr. Juliette Hanson, Mr. Todd Root, and Ms. Kingsly Berlin. We would like to thank NIH Tetramer Core Facility for providing CD1d Tetramers, Dr. Randy Brutkiewicz for providing mouse anti-CD1d and its isotype control mAb, and Drs. Richard W. Franck and Guangwu Chen for providing GCK127 and GCK152 analogs. We also thank Drs. Prosper Boyoka and Dechamma Joyappa for critically reviewing the manuscript. The research in Dr. Gourapura’s laboratory is funded by the US National Pork Checkoff (NPB #08-187) and USDA (PRRS CAP NIFA award 2008-55620-19132). Salaries and research support were provided by state and federal funds appropriated to OARDC, The Ohio State University. Funds were provided from Cytheris, Otsuka Pharmaceutical Company, the Irene Diamond Foundation to MT.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10875-010-9476-4) contains supplementary material, which is available to authorized users.
Contributor Information
Gourapura J. Renukaradhya, Email: gourapura.1@osu.edu, Food Animal Health Research Program, Ohio Agricultural Research and Development Center, Department of Veterinary Preventive Medicine, The Ohio State University, 1680 Madison Ave., Wooster, OH 44691, USA.
Cordelia Manickam, Food Animal Health Research Program, Ohio Agricultural Research and Development Center, Department of Veterinary Preventive Medicine, The Ohio State University, 1680 Madison Ave., Wooster, OH 44691, USA.
Mahesh Khatri, Food Animal Health Research Program, Ohio Agricultural Research and Development Center, Department of Veterinary Preventive Medicine, The Ohio State University, 1680 Madison Ave., Wooster, OH 44691, USA.
Abdul Rauf, Food Animal Health Research Program, Ohio Agricultural Research and Development Center, Department of Veterinary Preventive Medicine, The Ohio State University, 1680 Madison Ave., Wooster, OH 44691, USA.
Xiangming Li, HIV and Malaria Vaccine Program, Aaron Diamond AIDS Research Center, The Rockefeller University, New York, NY 10016, USA.
Moriya Tsuji, HIV and Malaria Vaccine Program, Aaron Diamond AIDS Research Center, The Rockefeller University, New York, NY 10016, USA.
Gireesh Rajashekara, Food Animal Health Research Program, Ohio Agricultural Research and Development Center, Department of Veterinary Preventive Medicine, The Ohio State University, 1680 Madison Ave., Wooster, OH 44691, USA.
Varun Dwivedi, Food Animal Health Research Program, Ohio Agricultural Research and Development Center, Department of Veterinary Preventive Medicine, The Ohio State University, 1680 Madison Ave., Wooster, OH 44691, USA.
References
- 1.Coffman RL, Hessel EM. Nonhuman primate models of asthma. J Exp Med. 2005;201(12):1875–1879. doi: 10.1084/jem.20050901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006;13(6):488–499. doi: 10.1111/j.1399-3089.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 3.Rogers CS, Abraham WM, Brogden KA, et al. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295(2):L240–L263. doi: 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baskerville A. Development of the early lesions in experimental enzootic pneumonia of pigs: an ultrastructural and histological study. Res Vet Sci. 1972;13(6):570–578. [PubMed] [Google Scholar]
- 5.Rogers CS, Stoltz DA, Meyerholz DK, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321(5897):1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi HK, Finkbeiner WE, Widdicombe JH. A comparative study of mammalian tracheal mucous glands. J Anat. 2000;197(Pt 3):361–372. doi: 10.1046/j.1469-7580.2000.19730361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham S, Meng QH, Klein N, McAnulty RJ, Hart SL. Evaluation of a porcine model for pulmonary gene transfer using a novel synthetic vector. J Gene Med. 2002;4(4):438–446. doi: 10.1002/jgm.270. [DOI] [PubMed] [Google Scholar]
- 8.Ballard ST, Trout L, Garrison J, Inglis SK. Ionic mechanism of forskolin-induced liquid secretion by porcine bronchi. Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L97–L104. doi: 10.1152/ajplung.00159.2005. [DOI] [PubMed] [Google Scholar]
- 9.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 10.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 11.Looringh van Beeck FA, Reinink P, Hermsen R, et al. Functional CD1d and/or NKT cell invariant chain transcript in horse pig African elephant and guinea pig but not in ruminants. Mol Immunol. 2009;46:1424–1431. doi: 10.1016/j.molimm.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eguchi-Ogawa T, Morozumi T, Tanaka M, Shinkai H, Okumura N, Suzuki K, et al. Analysis of the genomic structure of the porcine CD1 gene cluster. Genomics. 2007;89(2):248–261. doi: 10.1016/j.ygeno.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Lalazar G, Preston S, Zigmond E, Ben Yaacov A, Ilan Y. Glycolipids as immune modulatory tools. Mini Rev Med Chem. 2006;6(11):1249–1253. doi: 10.2174/138955706778742722. [DOI] [PubMed] [Google Scholar]
- 14.Exley M, Garcia J, Wilson SB, et al. CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology. 2000;100(1):37–47. doi: 10.1046/j.1365-2567.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieuwenhuis EE, Matsumoto T, Exley M, et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002;8(6):588–593. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 16.Taniguchi M, Seino K, Nakayama T. The NKT cell system: bridging innate and acquired immunity. Nat Immunol. 2003;4(12):1164–1165. doi: 10.1038/ni1203-1164. [DOI] [PubMed] [Google Scholar]
- 17.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21(11):573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 18.Matangkasombut P, Pichavant M, Dekruyff RH, Umetsu DT. Natural killer T cells and the regulation of asthma. Mucosal Immunol. 2009;2(5):383–392. doi: 10.1038/mi.2009.96. [DOI] [PubMed] [Google Scholar]
- 19.Strowig T, Brilot F, Munz C. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol. 2008;180(12):7785–7791. doi: 10.4049/jimmunol.180.12.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lisbonne M, Diem S, de Castro Keller A, et al. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003;171(4):1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 21.Matangkasombut P, Pichavant M, Yasumi T, Hendricks C, Savage PB, Dekruyff RH, et al. Direct activation of natural killer T cells induces airway hyperreactivity in nonhuman primates. J Allergy Clin Immunol. 2008;121(5):1287–1289. doi: 10.1016/j.jaci.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Shiratsuchi T, Chen G, Dellabona P, Casorati G, Franck RW, et al. Invariant TCR rather than CD1d shapes the preferential activities of C-glycoside analogues against human versus murine invariant NKT cells. J Immunol. 2009;183(7):4415–4421. doi: 10.4049/jimmunol.0901021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Chen G, Garcia-Navarro R, Franck RW, Tsuji M. Identification of C-glycoside analogues that display a potent biological activity against murine and human invariant natural killer T cells. Immunology. 2009;127(2):216–225. doi: 10.1111/j.1365-2567.2008.02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denyer MS, Wileman TE, Stirling CM, Zuber B, Takamatsu HH. Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of natural killer, cytotoxic T, natural killer T and MHC un-restricted cytotoxic T-cells. Vet Immunol Immunopathol. 2006;110(3–4):279–292. doi: 10.1016/j.vetimm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Chen G, Chien M, Tsuji M, Franck RW. E and Z alpha-C-galactosylceramides by Julia–Lythgoe–Kocienski chemistry: a test of the receptor-binding model for glycolipid immunostimulants. Chembiochem. 2006;7(7):1017–1022. doi: 10.1002/cbic.200500386. [DOI] [PubMed] [Google Scholar]
- 26.Roberts TJ, Sriram V, Spence PM, et al. Recycling CD1d1 molecules present endogenous antigens processed in an endocytic compartment to NKT cells. J Immunol. 2002;168(11):5409–5414. doi: 10.4049/jimmunol.168.11.5409. [DOI] [PubMed] [Google Scholar]
- 27.Renukaradhya JG, Sriram V, Polakova K, Russ G, Brutkiewicz RR. Development of a quantitative cell-based intracellular ELISA for the screening of B cell hybridoma supernatants: a novel rapid assay to detect positive clones. Hybrid Hybridomics. 2004;23(6):373–379. doi: 10.1089/hyb.2004.23.373. [DOI] [PubMed] [Google Scholar]
- 28.Kofler K, Ainoedhofer H, Hollwarth ME, Saxena AK. Fluorescence-activated cell sorting of PCK-26 antigen-positive cells enables selection of ovine esophageal epithelial cells with improved viability on scaffolds for esophagus tissue engineering. Pediatr Surg Int. 2010;26(1):97–104. doi: 10.1007/s00383-009-2512-x. [DOI] [PubMed] [Google Scholar]
- 29.Davis GS, Pfeiffer LM, Hemenway DR. Expansion of interferon-gamma-producing lung lymphocytes in mouse silicosis. Am J Respir Cell Mol Biol. 1999;20(4):813–824. doi: 10.1165/ajrcmb.20.4.3407. [DOI] [PubMed] [Google Scholar]
- 30.Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host’s innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111(12):5637–5645. doi: 10.1182/blood-2007-05-092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loving CL, Brockmeier SL, Sacco RE. Differential type I interferon activation and susceptibility of dendritic cell populations to porcine arterivirus. Immunology. 2007;120(2):217–229. doi: 10.1111/j.1365-2567.2006.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley KW, Curtis SE, Marzan GT, Karara HM, Anderson CR. Body surface area of female swine. J Anim Sci. 1973;36(5):927–930. doi: 10.2527/jas1973.365927x. [DOI] [PubMed] [Google Scholar]
- 33.Grumelli S, Corry DB, Song LZ, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1(1):e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung K, Alekseev KP, Zhang X, Cheon DS, Vlasova AN, Saif LJ. Altered pathogenesis of porcine respiratory coronavirus in pigs due to immunosuppressive effects of dexamethasone: implications for corticosteroid use in treatment of severe acute respiratory syndrome coronavirus. J Virol. 2007;81(24):13681–13693. doi: 10.1128/JVI.01702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renukaradhya GJ, Alekseev KP, Jung K, Fang F, Saif LJ. Porcine reproductive and respiratory syndrome virus induced immunosuppression exacerbate the inflammatory response to porcine respiratory coronavirus in pigs. Viral Immunol. 2010;23(5):457–466. doi: 10.1089/vim.2010.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung K, Renukaradhya GJ, Alekseev KP, Fang Y, Tang Y, Saif LJ. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections. J Gen Virol. 2009;90(Pt 11):2713–2723. doi: 10.1099/vir.0.014001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer EH, Goya S, Akbari O, et al. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci USA. 2006;103(8):2782–2787. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ezquerra A, Revilla C, Alvarez B, Perez C, Alonso F, Dominguez J. Porcine myelomonocytic markers and cell populations. Dev Comp Immunol. 2009;33(3):284–298. doi: 10.1016/j.dci.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Akbari O, Stock P, Meyer E, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9(5):582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 40.Kim EY, Battaile JT, Patel AC, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14(6):633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pichavant M, Goya S, Meyer EH, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205(2):385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matangkasombut P, Marigowda G, Ervine A, et al. Natural killer T cells in the lungs of patients with asthma. J Allergy Clin Immunol. 2009;123(5):1181–1185. doi: 10.1016/j.jaci.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, et al. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006;354(11):1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 44.Eger KA, Sundrud MS, Motsinger AA, Tseng M, Van Kaer L, Unutmaz D. Human natural killer T cells are heterogeneous in their capacity to reprogram their effector functions. PLoS ONE. 2006;1:e50. doi: 10.1371/journal.pone.0000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers PR, Matsumoto A, Naidenko O, Kronenberg M, Mikayama T, Kato S. Expansion of human Valpha24+ NKT cells by repeated stimulation with KRN7000. J Immunol Methods. 2004;285(2):197–214. doi: 10.1016/j.jim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Brutkiewicz RR. CD1d ligands: the good the bad the ugly. J Immunol. 2006;177(2):769–775. doi: 10.4049/jimmunol.177.2.769. [DOI] [PubMed] [Google Scholar]
- 47.Kamijuku H, Nagata Y, Jiang X, et al. Mechanism of NKT cell activation by intranasal coadministration of alpha-galactosylceramide, which can induce cross-protection against influenza viruses. Mucosal Immunol. 2008;1(3):208–218. doi: 10.1038/mi.2008.2. [DOI] [PubMed] [Google Scholar]
- 48.Hasegawa A, Hayashi K, Kishimoto H, et al. Color-coded real-time cellular imaging of lung T-lymphocyte accumulation and focus formation in a mouse asthma model. J Allergy Clin Immunol. 2010;125(2):461–468. e6. doi: 10.1016/j.jaci.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 49.Robinson DS, Bentley AM, Hartnell A, Kay AB, Durham SR. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48(1):26–32. doi: 10.1136/thx.48.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahib El-Radhi A, Patel S. The clinical course of childhood asthma in association with fever. Clin Pediatr (Phila) 2009;48(6):627–631. doi: 10.1177/0009922809335320. [DOI] [PubMed] [Google Scholar]
- 51.El-Radhi AS, Barry W, Patel S. Association of fever and severe clinical course in bronchiolitis. Arch Dis Child. 1999;81(3):231–234. doi: 10.1136/adc.81.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butnariu A, Chindris AM, Giurgiu D, Leucuta A. Correlation between fever and the clinical severity of acute bronchiolitis. Pneumologia. 2005;54(3):154–157. [PubMed] [Google Scholar]
- 53.Steinmetz HT, Herbertz A, Bertram M, Diehl V. Increase in interleukin-6 serum level preceding fever in granulocytopenia and correlation with death from sepsis. J Infect Dis. 1995;171(1):225–228. doi: 10.1093/infdis/171.1.225. [DOI] [PubMed] [Google Scholar]
- 54.Rummel C, Sachot C, Poole S, Luheshi GN. Circulating interleukin-6 induces fever through a STAT3-linked activation of COX-2 in the brain. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1316–R1326. doi: 10.1152/ajpregu.00301.2006. [DOI] [PubMed] [Google Scholar]
- 55.Van Reeth K, Van Gucht S, Pensaert M. In vivo studies on cytokine involvement during acute viral respiratory disease of swine: troublesome but rewarding. Vet Immunol Immunopathol. 2002;87(3–4):161–168. doi: 10.1016/S0165-2427(02)00047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434(7032):520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 57.Agea E, Russano A, Bistoni O, et al. Human CD1-restricted T cell recognition of lipids from pollens. J Exp Med. 2005;202(2):295–308. doi: 10.1084/jem.20050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003;111(3):291–297. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milner JD, Kent SC, Ashley TA, Wilson SB, Strominger JL, Hafler DA. Differential responses of invariant Valpha 24J alpha Q T cells and MHC class II-restricted CD4+ T cells to dexamethasone. J Immunol. 1999;163(5):2522–2529. [PubMed] [Google Scholar]
- 60.Meyer EH, DeKruyff RH, Umetsu DT. T cells and NKT cells in the pathogenesis of asthma. Annu Rev Med. 2008;59:281–292. doi: 10.1146/annurev.med.59.061506.154139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.