Abstract
The proapoptotic protein Siva-1 plays an important role in some of the extrinsic and intrinsic apoptosis signaling pathways in cancer cells. Previously, we showed that Siva-1 inhibited the activity of the prosurvival transcription factor NF-κB. In the present study, upon TCR cross-linking of Jurkat T leukemia cells, we demonstrated that the inhibitory target of Siva-1 is upstream of the IKK complex in the NF-κB signaling pathway. Additionally, Siva-1 also suppressed the activity of another crucial transcription factor AP-1, and a common mediator of both these pathways is the adaptor protein TRAF2. Further, we observed that Siva-1 indeed interacted with TRAF2 and negatively regulated its activity by promoting K48-linked polyubiquitination. Siva-1 specifically interacted with the ring finger domain of TRAF2, which is essential for its E3 ligase activity and its ability to subsequently activate NF-κB. TCR cross-linking of Jurkat T cells that lacked Siva-1 revealed significantly lowered K48- but elevated K63-ubiquitinated TRAF2 levels upon TCR cross-linking, suggesting that the differential pattern of ubiquitination in these cells essentially contributed to a robust and sustained activation of NF-κB. The above results demonstrated an important role for endogenous Siva-1 in negatively regulating NF-κB activation by targeting TRAF2.
Keywords: Siva-1, NF-kappaB, AP-1, TRAF, K48, K63, NIK, TCR
Introduction
NF-κ B signaling is crucial for regulating inflame-mation, innate and adaptive immunity, and cell survival. Dysregulated NF-κB activity is known to promote tumorigenesis.1 In T-cell receptor (TCR) signaling, the adaptor proteins TRAF2 and TRAF6 play crucial roles by relaying signals from the CARMA1-BCL10-MALT1 (CBM) complex to the IKK complex, through the TGF-β-associated kinase 1 (TAK1), which activates both NF-κB and AP-1.2
Ubiquitination plays a central role in the activation of both canonical and noncanonical limbs of the NF-κB pathway. Ubiquitin has 7 lysine residues, and polyubiquitination occurs through different lysine linkages of the ubiquitin moiety generating functionally distinct signals. Typically, proteins bearing K48-linked polyubiquitin (K48-Ub) chains get proteasomally degraded,3,4 whereas those having K63-linked (K63-Ub) chains provide signals for kinase activation.5 Although predominantly K48 and K63 ubiquitin linkages have been demonstrated to have a role in the NF-κB pathway, linkages through the other lysine residues of ubiquitin, such as K11, K33, K27, and K6, have also been found in cells.6 TRAF2 and TRAF6 are E3 ligases that undergo K63-Ub to activate NF-κB in TNF- and TCR-mediated signaling events.2,7 On the other hand, K48-linked ubiquitination of TRAF2 and RIP results in their degradation and subsequent termination of NF-κB signaling upon TNF-α stimulation.8,9
This article focuses on the mechanism by which the p53- and E2F1-induced proapoptotic protein Siva-110 inhibits NF-κB activity.11 Siva-1 was originally discovered as an interacting partner of the co-stimulatory TNF family receptor CD2712 and appears to promote both extrinsic and intrinsic apoptotic pathways.13,14 Recently, we demonstrated that Siva-1 plays a nonredun-dant role in TCR-mediated activation-induced cell death (AICD) by inhibiting the activity of prosurvival transcription factor NF-κB but not NFAT. Knockdown of Siva-1 expression in Jurkat T cells results in hyperactivation of both canonical and noncanonical NF-κB pathways through TCR stimulation.11 Here, we report that Siva-1 also inhibits the transcriptional activity of AP-1 and specifically interacts with a common upstream mediator, TRAF2. Siva-1 promotes K48-linked ubiquitination of TRAF2, which suppresses the TCR-mediated NF-κB activation by an as yet unknown mechanism. T cells devoid of Siva-1 lack the negative regulatory effect-mediated by K48-ubiquitinated TRAF2, therefore resulting in a sustained activation of NF-κB, as seen by the persisting levels of K63-ubiquitinated TRAF2.
Materials and Methods
Transfection and treatment
Electroporation of Jurkat T cells and cross-linking of their TCRs using anti-CD3 antibodies have been described.11 The proteasome inhibitor MG-132 was added to the cell cultures at a final concentration of 25 µM for 1 hour before TCR cross-linking was performed. The calcium phosphate method was used for transfection of 293T cells.
Plasmids
Flag-TRAF2 and Flag-TRAF6 plas-mids were obtained from Dr. Zhijian Chen (UT Southwestern, TX, USA), myc-NIK was from Dr. Xin Lin (M. D. Anderson Center, TX, USA), and Flag-IKKβ was from Dr. Richard Ye (University of Illinois at Chicago, Chicago, IL, USA). HA-tagged mutants of ubiquitin retaining only K48 or K63 were a kind gift from Dr. Vishva Dixit (Genen-tech, CA, USA). Siva-1-expressing plasmids, such as GST-Siva-1 and pEF-Siva-1, have been described earlier.15 Generation of the lentiviral constructs expressing control and Siva-1-specific siRNA, as well as NF-κB- and AP1-luciferase reporter vec- tors and the β-galactosidase plasmid, have been described earlier.11
Antibodies
Anti-IKKβ, anti-TRAF2, anti-NIK, and anti-myc antibodies were obtained from Santa Cruz Biotech, CA. Anti-Flag and anti-HA epitope monoclonal antibodies were purchased from Sigma-Aldrich (St. Louis, MO, USA). The generation of Siva-1 polyclonal antibody has been described earlier.12 The anti-CD3 antibody (Clone OKT3) was obtained from eBiosciences (CA, USA). For immunoprecipitation studies, the True blot secondary anti-mouse and anti-rabbit-HRP antibodies were also from eBiosciences.
Immunoprecipitation
Cell lysates were prepared as described earlier.16 For immunoprecipi-tations, cell lysates were prepared using RIPA lysis buffer containing 0.01% SDS, and 1 µg final concentration of various antibodies and their respective isotype controls were used. A 50% slurry of proteinA/G beads equilibrated in the lysis buffer was used to pull down the protein-antibody complexes. For separation of ubiquit-inated proteins, gradient gels (4%–15%) were used (Biorad, CA, USA). Blots were developed using ECL (Pierce, IL, USA). In case of nickel beads (Qiagen, CA, USA) pull down or GST-pull down (Amersham Pharmacia Biotech, Sweden), respective beads were added to the lysates for 4 hours at 4°C. The rest of the protocol was similar to the above procedure.
Luciferase and β-galactosidase assays
Both assays were performed as described previously.11 The luci ferase assay was performed using substrate from Promega (WI, USA) and a single-tube luminometer (Turner Biosystems, CA, USA). β-galactosidase assay was performed using substrate containing ortho-nitro-phenyl-galactoside (ONPG) (Sigma-Aldrich).
Statistical analysis
All the values for the lu-ciferase assays were presented as mean +/− SD. The Student’s t test was used to calculate the statistical significance of various groups. A p value of < 0.01 was found to be significant. The luminescence in the luciferase assays was normalized to β-galactosidase expression to avoid variation arising due to transfection efficiencies. Typically, in all the experiments, the normalized luminescence of control siRNA unstimulated cells was taken as 1. The fold luminescence of the other groups was calculated and plotted relative to this group.
Results
Siva-1 Targets Proteins Upstream of the IKK Complex
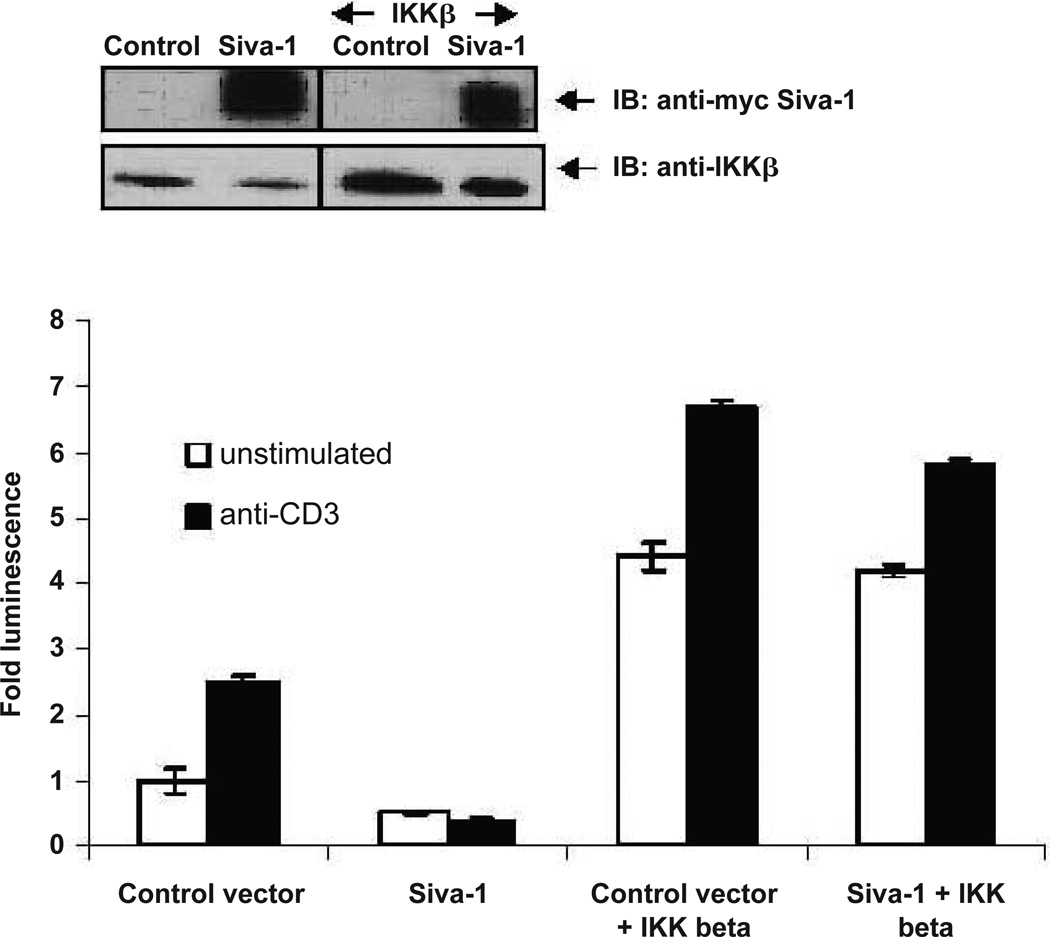
Our earlier study has shown that Siva-1 promotes TCR-mediated AICD by inhibiting the NF-κB activity.11 Therefore, to understand the underlying mechanism and identify the target of Siva-1 in this pathway, Jurkat T cells were co-transfected with Siva-1 and/or IKKβ-expressing vectors along with the NF-κB luciferase reporter and β-galactosidase plasmid. After 24 hours, cells were either left un-stimulated or subjected to TCR-cross-linking for 6 hours. Relative levels of NF-κB activity were then determined and normalized to the β-galactosidase activity in these cells. As expected, expression of IKKβ alone resulted in significantly high NF-κB activity, which was further enhanced upon TCR cross-linking. Although Siva-1 expression alone inhibited both the basal and TCR-induced NF-κB activity, co-expression of Siva-1 with IKKβ did not show a significant inhibition of the basal and induced levels of NF-κB activity. This suggests that IKKβ acts downstream of Siva-1 and hence, exogenous IKKβ expression can relieve the inhibitory effect of Siva-1 on the NF-κB signaling pathway (Fig. 1).
FIGURE 1.
Siva-1 acts upstream of the IKK complex in the TCR-mediated NF-κB signaling pathway. Jurkat cells were co-transfected with either Siva-1-myc and/or IKKβ vectors along with the NF-κB-luciferase reporter and β-galactosidase vectors. The inset shows immunoblots of whole-cell lysates with anti-myc antibody and anti-IKKβ antibodies. Results shown are drawn from three independent experiments. Abrogation of endogenous Siva-1 resulted in a significant increase in TCR-induced AP-1 reporter activity (p < 0.01), and, conversely, expression of Siva-1 led to a dramatic inhibition of both basal and TCR-induced AP-1 reporter activity (p < 0.001).
Siva-1 Inhibits TCR-Induced AP-1 Activity
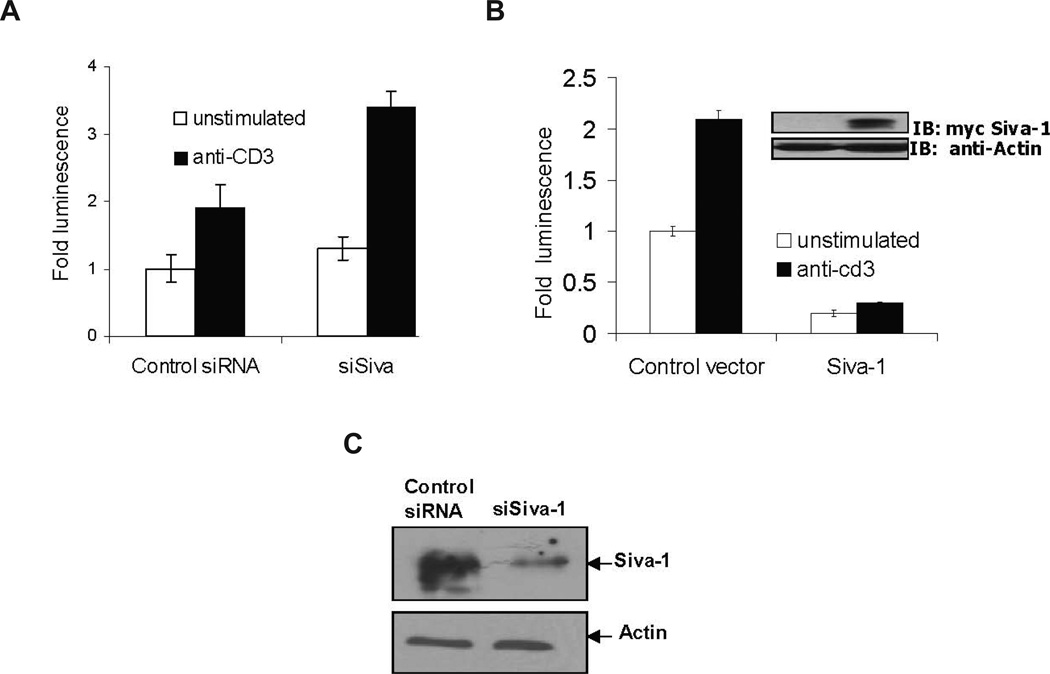
NF-κB, NFAT, and AP-1 are the three major prosurvival transcription factors that are induced upon T-cell activation. We have previously shown that Siva-1 appears to have no effect on TCR-induced NFAT activity,11 whereas the effect of Siva-1 on AP-1 was not known. Because both NF-κB and AP-1 activation involves some common mediators, in order to further understand the mechanism of action of Siva-1 in inhibiting TCR-induced NF-κB activity, we examined whether Siva-1 expression has any effect on TCR-mediated activation of AP-1. Jurkat cells were co-transfected with AP-1 luciferase reporter and β-galactosidase plasmids in conjunction with either the control siRNA or Siva-1-specific siRNA (siSiva)-expressing vectors. The effectiveness of siSiva in abrogating Siva-1 expression has been previously demonstrated11 and is depicted in Figure 2C. These cells were then tested for luciferase activity, with and without anti-CD3 cross-linking, after 6 hours. As observed in Figure 2A, the abrogation of Siva-1 expression in T cells using siSiva resulted in enhanced CD3 cross-linking-induced AP1 activity. On the other hand, co-expression of Siva-1 resulted in a profound inhibition of the basal as well as the TCR-induced AP1 activity (Fig. 2B). The inhibition of basal AP-1 activity suggests that the effects of Siva-1 are not limited to TCR stimulation. This is in line with our observation that Siva-1 also inhibits TNF-α-induced NF-κB and AP-1 activities (unpublished observation). The above results suggest that the target of Siva-1 is upstream of the IKK complex and is required for the TCR-mediated activation of both NF-κB and AP1.
FIGURE 2.
Siva-1 inhibits TCR-induced AP1 activity. Jurkat cells were co-transfected with either control siRNA or siSiva (A) or human Siva-1-expressing or control vector (B) along with the AP-1 luciferase reporter vector, and the Siva-1 expression was confirmed (inset). Luciferase assay was performed after 6 hours, and the normalized fold activity was plotted in comparison with unstimulated control cells. Results shown are representative of three independent experiments and are statistically significant (p < 0.01). Lysates of lentivirus-infected Jurkat cells expressing either control siRNA or siSiva-1 siRNA were immunoblotted with polyclonal anti-Siva-1 antibody to demonstrate the knockdown of endogenous Siva-1 expression in them. Actin levels serve as loading controls (C).
Siva-1 Inhibits TRAF2- and NIK- but Not TRAF6-Induced NF-κB Activation
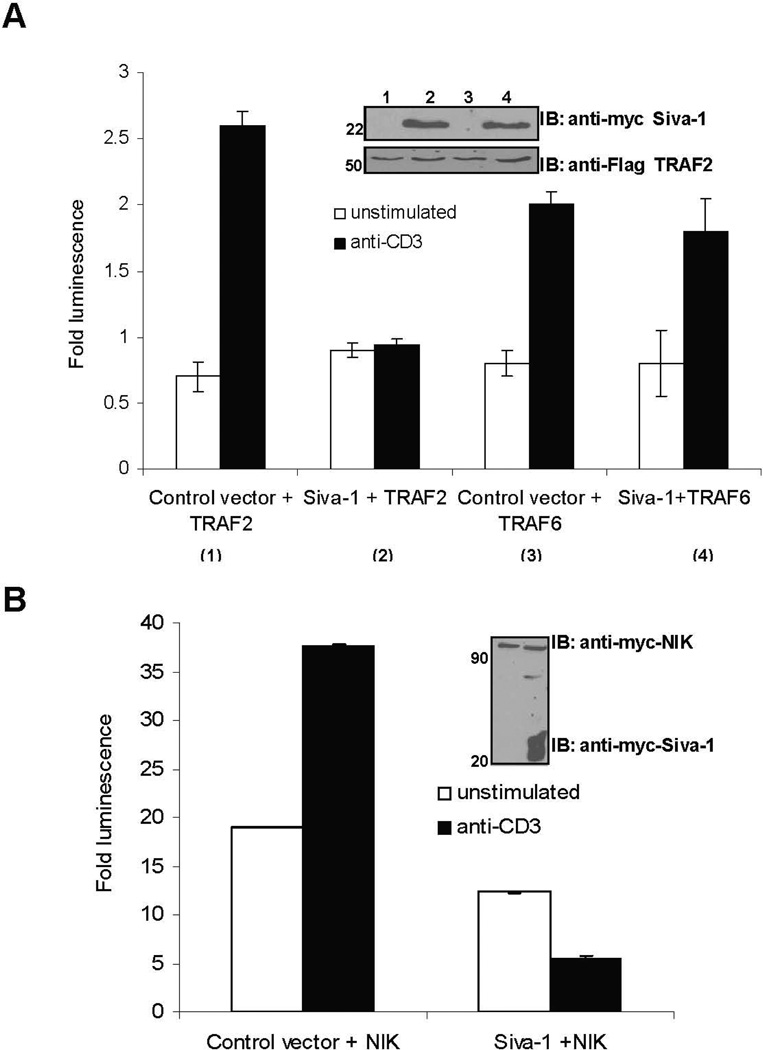
Some of the known common mediators that activate NF-κB and AP-1 are the adaptor proteins TRAFs and the kinases such as NF-κB inducing kinase (NIK) and TAK1.17 The adaptor proteins TRAF2 and TRAF6 activate TAK1, which is known to act upstream of the IKK complex. TAK1, in turn, activates the mitogen-activated protein kinase (MAPKKK), which initiates the c-Jun kinases (Jnk) and p38 MAP kinase pathways responsible for translocation of the AP-1 complexes to the nucleus.18 A kinase inactive mutant of NIK was able to inhibit NF-κB and AP-1 activation induced by TRAF2, suggesting that NIK acts downstream of TRAFs.18 Hence, we next examined whether Siva-1 targets the TRAFs or NIK by measuring the NF-κB activation-induced luciferase activity in cells co-transfected with Siva-1 and TRAF2, TRAF6, or NIK expression vectors. Jurkat cells were transfected with control or myc-Siva-1-expressing vectors and Flag-TRAF2-, Flag-TRAF6-, or myc-NIK-expressing plasmids along with the NF-κB luciferase construct. As shown in Figure 3A, the TCR cross-linking-induced NF-κB activation was significantly higher in cells overexpressing TRAF2, TRAF6, and NIK by themselves. Although Siva-1 expression by itself significantly inhibited the TCR-induced NF-κB activity (data not shown), co-expression of Siva-1 specifically inhibited the TCR-mediated NF-κB activity in TRAF2 and NIK, but not TRAF6 overexpressing cells (Figs. 3A and 3B). Luciferase activity in NIK- and Siva-1-expressing cells was measured at the same time as TRAF2 and TRAF6 with all the controls but has been represented as a separate panel because NIK, being a kinase, is a potent activator of NF-κB. Interestingly, exogenous expression of Siva-1 could still inhibit the activation of NF-κB induced by a potent activator such as NIK.
FIGURE 3.
Siva-1 inhibits TRAF2- and NIK- but not TRAF6-induced NF-κB activity. Jurkat cells were co-transfected with control and Siva-1-myc vectors with either Flag-TRAF2 or Flag-TRAF6 (A) or myc-NIK constructs (B), along with the NF-κB-luciferase reporter vector. Cells were left unstimulated or stimulated by TCR cross-linking, and the normalized fold activity was plotted in comparison with unstimulated cells transfected with control vector. Expression levels were confirmed by immunoblotting (inset). Results shown were compiled from a minimum of three independent experiments. Co-expression of Siva-1 with TRAF2 or NIK resulted in a significant inhibition of TCR-induced activation of NF-κB (p < 0.01) and are statistically significant (p < 0.01), but not when TRAF6 was co-expressed.
Siva-1 Interacts Specifically with TRAF2, and RING Finger Motif of TRAF2 Mediates This Association
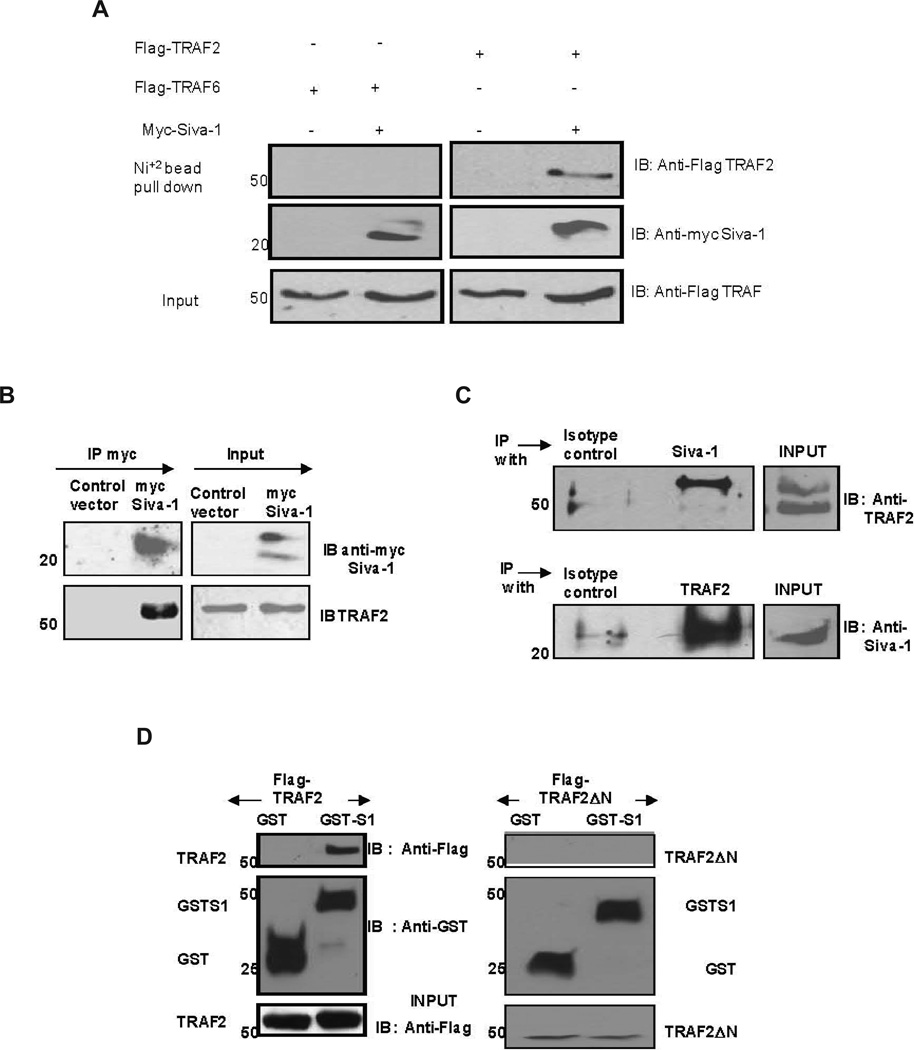
Because Siva-1 overexpression resulted in the inhibition of TRAF2- and NIK-mediated activation of NF-κB, we hypothesized that Siva-1 regulates NF-κB activation by interacting with TRAF2 and/ or NIK. Therefore, we examined these interactions in 293T cells exogenously overexpressing Siva-1 and TRAF2, TRAF6, or NIK. To test for interaction with the TRAFs, the His-myc tagged Siva-1 and Flag-tagged TRAF2 and TRAF6 proteins were co-expressed in 293T cells. Siva-1 complexes were isolated using nickel beads and analyzed by immunoblotting. Probing with the anti-Flag antibody revealed significant co-precipitation of TRAF2 but not TRAF6 with Siva-1, although comparable amounts of Siva-1 were pulled down with an anti-myc antibody (Fig. 4A). In a similar experiment, we did not observe any interaction between Siva-1 and NIK (data not shown). This data suggests that Siva-1 appears to be interacting only with TRAF2, but not TRAF6 and NIK.
FIGURE 4.
Siva-1 interacts with TRAF2. 293T cells were co-transfected with either control or Siva-1-myc/His vectors along with either Flag-TRAF2 or Flag-TRAF6 constructs. Anti-flag and anti-myc antibodies were used for immunoblotting (A). 293T cells were transfected with control or myc-tagged Siva-1 vectors. Siva-1 was immunoprecipitated using anti-myc antibody, and immunoblotting was performed using anti-TRAF2 and anti-myc antibodies (B). Endogenous complexes of Siva-1 and TRAF2 were immunoprecipitated from Jurkat cells using anti-Siva-1 and anti-TRAF2 antibodies (C). Ring finger motif of TRAF2 is required for association with Siva-1. 293T cells were co-transfected with either GST or GST-Siva-1 and Flag-TRAF2 (left panel) or GST or GST-Siva-1 and Flag-TRAF2ΔN (right panel) expressing plasmids. Glutathione beads were used to precipitate GST-tagged proteins, and the complexes were separated and immunoblotted with anti-FLAG and anti-GST antibodies (D).
The TRAF2-Siva-1 interaction was confirmed further by immunoprecipitating myc-tagged Siva-1 using the anti-myc antibody in 293T cells and probing for endogenous TRAF2 binding (Fig. 4B) as well as by immunoprecipitating endo genous Siva-1 and TRAF2 complexes from lysates of Jurkat cells using anti-Siva-1 and anti-TRAF2 antibodies (Fig. 4C). As seen in Figures 4B and 4C, signifi cant amounts of TRAF2 and Siva-1 co-precipitated along with Siva-1 and TRAF2 when anti-myc or anti-Siva-1 and anti-TRAF2 antibodies, respectively, were used. These observations unequivocally demonstrate that Siva-1 regulates NF-κB activation through its interaction with TRAF2.
Ubiquitination of TRAF2 plays a prominent role in regulating NF-κB activation. The RING fi nger domain of TRAF2, which lies between the amino acid residues 1–83, is critical for this process.19 A dominant negative mutant of TRAF2 that lacks the ring fi nger domain (TRAF2ΔN) was overex-pressed along with GST-tagged Siva-1 exogenously and tested for their interaction using the glutathione beads. As expected, pull down of exogenously expressed GST-Siva-1 resulted in co-precipitation of full-length TRAF2 but not TRAF2ΔN (Fig. 4D, compare left and right panels). This suggests that the ring finger domain in TRAF2 is necessary for its interaction with Siva-1.
Siva-1 Inhibits K63-Polyubiquitination-Mediated Activation of NF-κB by TRAF2
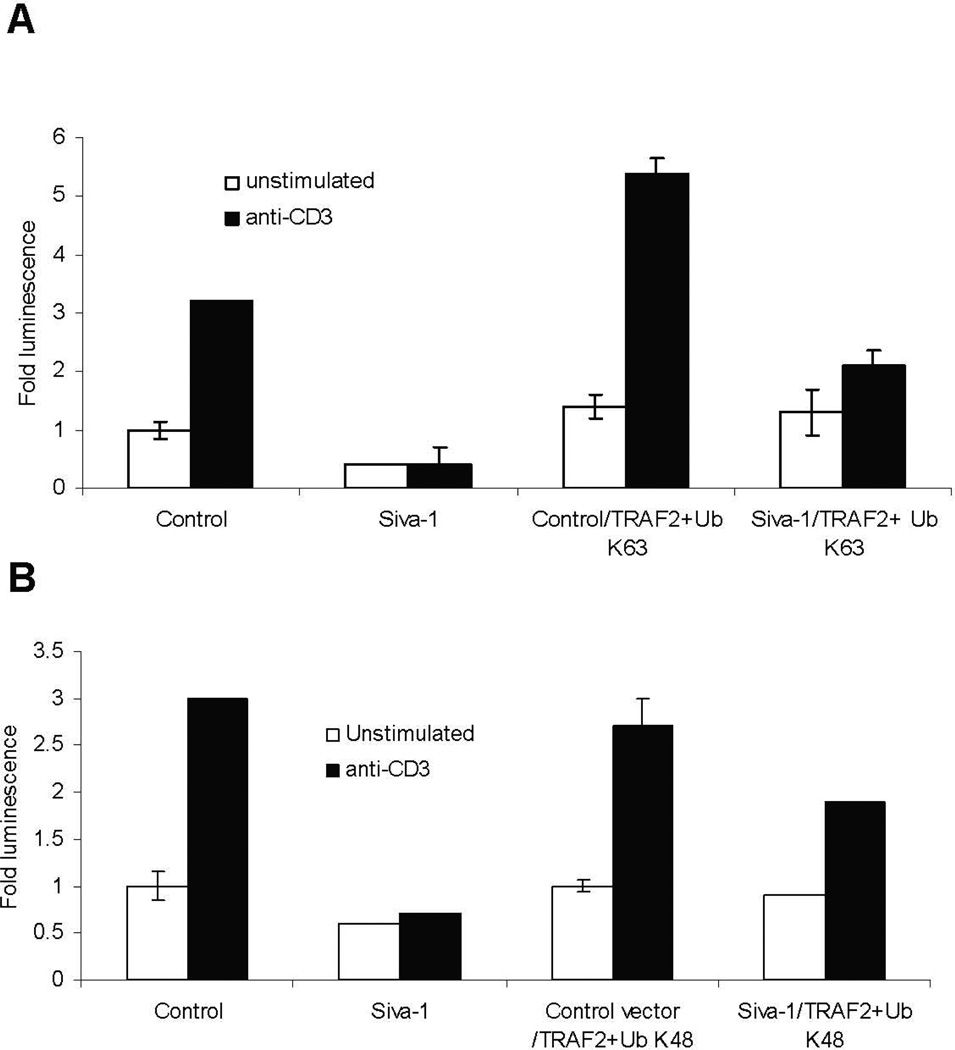
The dependence of Siva-1 interaction on the ring finger motif of TRAF2 led us to investigate the role of Siva-1 on the ubiquitination of TRAF2. Intracellular levels of TRAF2, as well as its function, are regulated through ubiquitination. Although it has been shown that K48-Ub of TRAF2 results in its proteasomal degradation and, therefore, termination of NF-κB activity upon TNF-α treatment,7 K48-ubiquitination-mediated degradation of TRAF2 upon TCR-cross-linking per se has not yet been shown. K63-Ub-mediated ubiquitination of TRAF2 is essential for the activation of its downstream signaling component TAK1.2 Hence, using vector constructs encoding for Siva-1, TRAF2, and mutant forms of ubiquitin that retain only the lysine at positions 63 or 48,9 we studied the effect of Siva-1 on the ubiquitination of TRAF2 in a luciferase reporter assay system. As observed in Figures 5A and 5B, exogenous overexpression of TRAF2 in the presence of both ubiquitin mutants resulted in a significant activation of NF-κB after TCR-cross-linking. However, TRAF2-mediated NF-κB activation was signifi-cantly lower in the presence of K48 compared to K63 transfected cells. Importantly, Although co-expression of Siva-1 inhibited the K63-Ub- TRAF2-mediated NF-κB activation profoundly (Fig. 5A), it had only a marginal inhibitory effect on K48-Ub-mediated NF-κB activation (Fig. 5B) (compare 40% decrease in A to 10% decrease in B). These observations suggest that Siva-1 differentially affects K48-Ub- and K63-Ub-mediated ubiquitination of TRAF2.
FIGURE 5.
Exogenous expression of Siva-1 dramatically inhibits the K63- but not K48-ubiquitylated TRAF2-mediated NF-κB activation. Jurkat cells were co-transfected with either the control or Siva-1 vectors along with the TRAF2 construct and either K63-Ub- (A) or K48-Ub-expressing plasmids (B) and the NF-κB luciferase reporter vector. Luciferase assay was performed after 6 hours, and the fold activity was plotted in comparison with unstimulated control siRNA cells. Results were compiled from three independent experiments.
Siva-1 Promotes K48-Linked Polyubiquitination of TRAF2 in 293T Cells
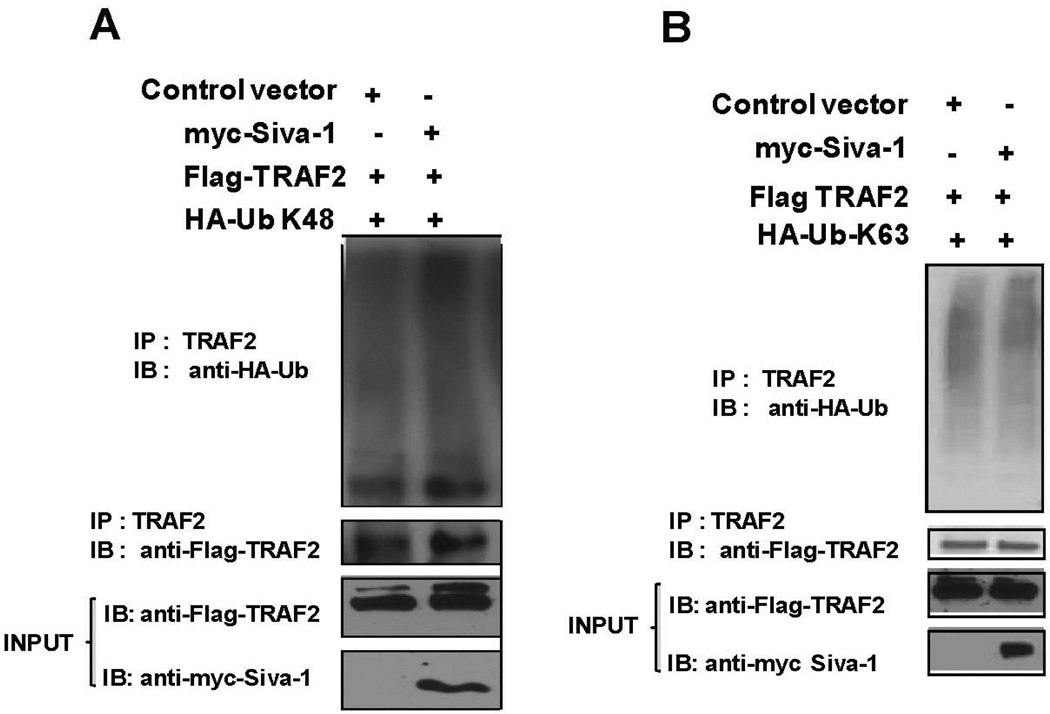
To further understand the influence of Siva-1 in K48-Ub- and K63-Ub-mediated ubiquitination of TRAF2, we examined the levels of K63- and K48-ubiquitinated TRAF2 in the presence or absence of exogenous Siva-1 in 293T cells. Control or his-myc-tagged Siva-1 vector was co-transfected with Flag-tagged TRAF2 along with either HA-tagged K48-Ub or K63-Ub expressing vectors, as described in Figures 6A and 6B, respectively. TRAF2 protein complexes were immunopre-cipitated using anti-TRAF2 antibody and protein A/G beads. The proteins were then separated on a gradient gel (4%–15%) and immunoblotted. As indicated in Figure 6A, the top panel reveals the K48 ubiquitination pattern of TRAF2 as discerned from anti-HA antibody immunoblotting. Overexpression of Siva-1 resulted in increased K48 ubiquitination of TRAF2, although the amount of Flag-tagged TRAF2 pulled down in both cases was equivalent. In a similar experiment, wherein HA-K63 Ub was co-expressed, no significant difference in the polyubiquitination of TRAF2 between control and Siva-1 expressing cells was observed (Fig. 6B). These results indicate that Siva-1 promotes K48- but not K63-mediated polyubiquitination of TRAF2.
FIGURE 6.
Siva-1-expression results in increased K48 but not K63 polyubiquitination of TRAF2. 293T cells were co-transfected with control or Siva-1 and TRAF2 constructs along with either HA-K63-Ub- (A) or HA-K48-Ub-expressing plasmids (B). Immunoprecipitation of the lysates was performed using anti-TRAF2 antibody. The complexes were separated and immunoblotted with the HA antibody to visualize the ubiquitination of TRAF2. Siva-1- and TRAF2-expression levels in the lysates were determined using anti-myc and anti-Flag antibodies.
Siva-1 Knockdown T Cells Demonstrate Decreased K48 and Increased K63 Ubiquitination of TRAF2
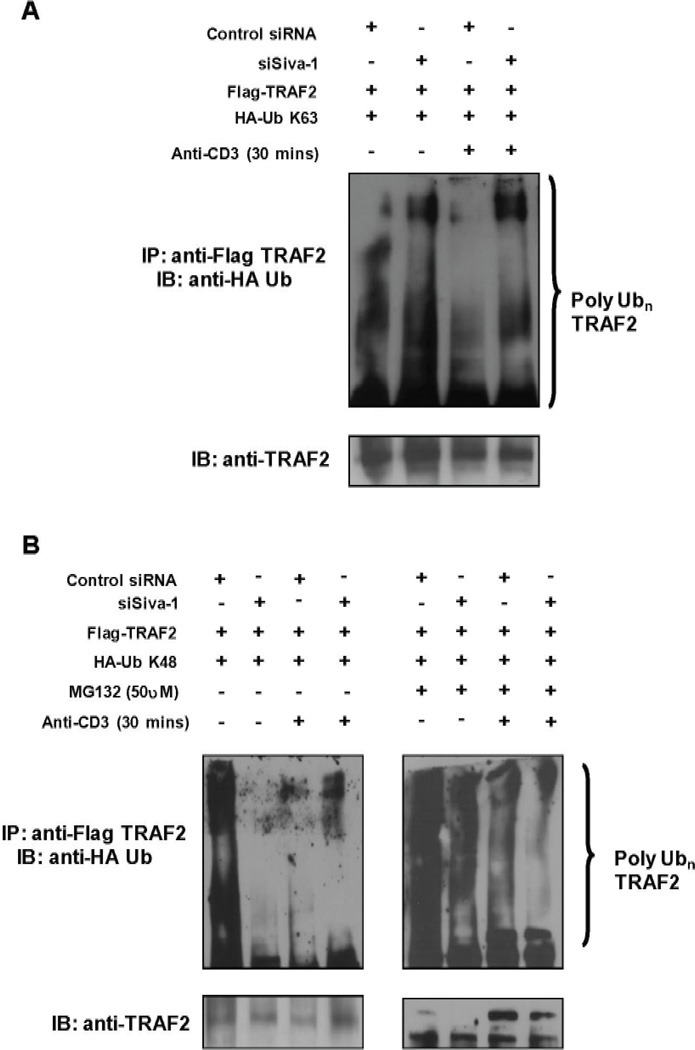
To further elucidate the role of Siva-1 in poly-ubiquitination of TRAF2, we determined the TCR cross-linking-induced ubiquitination status of TRAF2 in Jurkat cells that express endogenous and knockdown levels of Siva-1. Control siRNA or siSiva-expressing Jurkat cells were co-transfected with Flag-TRAF2 in combination with HA-K63-Ub- or HA-K48-Ub-expressing plasmids. Cells were either left unstimulated or subjected to TCR cross-linking for 30 minutes. A parallel experiment was carried out using cells that were pretreated with the inhibitor MG-132 (25 µM) to prevent proteasomal degradation of TRAF2. The exogenously expressed TRAF2 was immunoprecipitated from cell lysates using an anti-Flag antibody. The basal as well as TCR-cross-linking-induced K63 polyubiquitination of TRAF2 was significantly higher (Fig. 7A, lanes 2 and 4) in the Siva-1 knockdown cells as compared to the control cells (Fig. 7A, lanes 1 and 3). This is in line with our earlier observation that Siva-1 knockdown T cells have higher basal and TCR-induced NF-κB activities.
FIGURE 7.
Siva-1 knockdown T cells demonstrate decreased K48 and increased K63 ubiquitination of TRAF2. Control siRNA or siSiva Jurkat T cells were co-transfected with Flag-TRAF2 and K63-Ub (A) or K48-Ub constructs (B). After 36 hours, T cells were either left unstimulated or subjected to TCR cross-linking for 30 minutes. TRAF2 complexes from detergent cell lysates containing 0.01% SDS were collected by immunoprecipitation with anti-Flag antibody. Complexes were separated on gradient gels, and immunoblotting was performed with anti-HA and anti-TRAF2 antibodies to check ubiquitina-tion and TRAF2 levels. Data from K48-Ub-transfected cells pretreated with MG-132 for 1 hour and then subjected to TCR cross-linking are shown in the right panel of B.
On the contrary, the pattern of K48-ubiq-uitinated TRAF2 was significantly different in control and Siva-1 knockdown cells. A dramatic reduction in the basal K48-ubiquitinated TRAF2 levels in the Siva-1 knockdown cells was observed compared to the control cells (Fig. 7B, left panel, lane 2 versus lane 1). Both control and Siva-1 knockdown cells, however, had comparable levels of K48-polyubiquitinated TRAF2 (Fig. 7B, left panel, lane 3 and lane 4) upon CD3 stimulation. Interestingly, in the presence of proteasome inhibitor MG-132, the accumulation of K48-Ub complexes of TRAF2 was significantly less in Siva-1 knockdown cells compared to the control cells under resting condition (Fig. 7B, right panel, lane 2 vs. 1). In addition, the levels of K48-Ub-polyubiquitinated TRAF2 were also lower in cells stimulated with anti-CD3 Ab (Fig. 7B, right panel, lane 4 vs. 3) as compared to unstimulated cells (Fig. 7B, right panel, lanes 1 and 2 versus lanes 3 and 4) upon MG132 treatment. This observation, that the relative amounts of K48-polyubiquitinated TRAF2 being profoundly less under both un-stimulated as well as TCR-stimulatory conditions in Siva-1 knockdown cells, suggests that Siva-1 is required for mediating the K-48 ubiquitination of TRAF2.
Collectively, these results demonstrate that Siva-1 promotes K48-mediated ubiquitination and, thereby, inhibits NF-κB activation. Our data also indirectly suggest that during the TCR-cross-linking-induced NF-κB activation process, there is a continuous degradation of K48-ubiquitinated TRAF2 by the proteasome (Fig. 7B, left panel, lanes 3 and 4). This can be concluded from the accumulation of these K48-complexes upon treatment of the proteasomal inhibitor, MG-132 (Fig. 7B, right panel, lanes 2, 3, and 4). However, as mentioned earlier, this accumulation is significantly reduced in the absence of Siva-1, suggesting a lack of K48-ubiquitinated TRAF2 being generated.
Hence, Siva-1 primarily promotes the K48-Ub-mediated ubiquitination of TRAF2, and the increased K63-Ub-mediated ubiquitination observed in the Siva-1 knockdown T cells is very likely to be an effect secondary to the absence of the negative regulatory effect of Siva-1 on TRAF2 through K48-Ub (Figs. 7A and 7B). Therefore, the lower K48-Ub but the higher persisting levels of K63-Ub levels of TRAF2 might translate into enhanced NF-κB and AP-1 activities that could have a detrimental effect and lead to T-cell tumorigenesis, suggesting Siva-1 to be an important regulatory protein for the basal NF-κB signaling machinery.
Discussion
In our previous work, we have shown that Siva-1 is expressed in double positive thymocytes15 and in highly activated T cells that are targeted for TCR-mediated AICD, which led us to investigate the role of Siva-1 in AICD. Using specific siRNA against Siva-1 (siSiva), we clearly demonstrated a significant role for Siva-1 in AICD using two transformed T-cell lines—Jurkat and DO11.10. Abrogation of endogenous Siva-1 resulted in a profound increase in TCR-mediated NF-κB activation in the AICD model, leading to elevation of the key NF-κB-induced antiapoptotic gene products—Bcl-xL and c-FLIP.11
In this study, we have investigated the mechanism underlying Siva-1-mediated inhibition of NF-κB. Using constitutively active IKKβ, we found that Siva-1 acts upstream of the IKK signalo-some (Fig. 1A) in the NF-κB signaling pathway. TNF and LIGHT are known activators of the NF-κB pathway,20 and our unpublished results show that Siva-1 negatively regulates NF-κB activity also induced by these stimuli. On the basis of this additional information and the ability of Siva-1 to also inhibit AP-1 activation (Figs. 2A and 2B), we surmised that Siva-1 could be targeting the upstream elements, TRAFs. In fact, our results clearly demonstrate that Siva-1 interacts with TRAF2, but not TRAF6. Although other studies have shown that TRAF6 but not TRAF2 associates directly with the CARMA1-BCL10-MALT1 complex, significant suppression of TCR-induced NF-κB activity was seen only when expression of both the TRAFs was abrogated.2 However, in our studies, we have observed that the ectopic expression of Siva-1 which targets TRAF2, is sufficient to inhibit TCR-mediated increase in NF-κB activity.
The interaction between Siva-1 and TRAF2 is intriguing because both molecules can independently associate with the cytoplasmic tails of some of the TNFR family members, such as CD27, GITR, 4-1BB, and OX-40.13,19,21 Therefore, it is likely that elevated levels of Siva-1 can prevent the binding of TRAF2 to the above receptors and vice versa, thus either inhibiting NF-κB activation and promoting apoptosis or facilitating NF-κB activation, respectively. Interestingly, CD27, GITR, OX40, and 4-1BB are expressed on memory T cells.19 At the end of an immune response, most of the activated T cells undergo cell death (AICD).22 Very few T cells survive in circulation as memory T cells. Therefore, Siva-1 may be important for the regulation of immunological memory through its interaction with the co-stimulatory receptors GITR, CD27, and 4-1BB.
Lack of endogenous Siva-1-expression results in significant activation of both the canonical and noncanonical NF-κB pathways.11 In this study, we have demonstrated that Siva-1 can inhibit the NF-κB activation induced by the exogenous expression of TRAF2 and NIK (Figs. 3A and 3B); however, it interacts specifically with TRAF2, but not with NIK and TRAF6 (Fig. 4A). In this context, it is important to note that in TRAF2−/− B cells, the noncanonical pathway is highly active, revealing a regulatory role for TRAF2 in NF-κB activation.23 NIK is one of the key molecules required in this pathway. In addition, intracellular NIK-expression levels are tightly regulated by the adaptor protein TRAF3.24 Because TRAF2 is known to target TRAF3 for proteasomal degradation,25 it is possible that the inhibitory effect rendered by Siva-1 on NIK-induced NF-κB activity could be secondary to its suppression of TRAF2-mediated activation of NF-κB. Therefore, it is not surprising that Siva-1 knockdown cells have hyperactive TRAF2 (elevated K63-Ub, Fig. 7A, lanes 2 and 4), which downmodulates TRAF3, thereby facilitating NIK-mediated activation of the noncanonical pathway.11
Cellular deubiquitinases play a vital role in the regulation of NF-κB activity. For instance, CYLD deubiquitinates the K63-Ub chains of TRAF2 and, to some extent, TRAF6, and inhibits NF-κB activity.26 A20, another deubiquitinase that has the DUB domain in addition to the Ring finger, mediates sequential deubiquitination of K63 followed by K48-Ub of RIP, resulting in its proteasomal degradation and arrest of NF-κB signaling.8 In comparison, Siva-1 has only a B-box and cysteine-rich C-terminal region, and yet, it promotes K48-Ub of TRAF2. Because Siva-1 lacks a true Ring finger domain, it is unlikely to harbor any ligase activity by itself. Interestingly, a yeast two-hybrid study using Siva-1 as bait has revealed its binding ability with an E1-like enzyme (unpublished observation). Therefore, we can speculate that Siva-1 promotes ubiquitination of TRAF2 by interacting with the E1-like enzyme and is part of the basal ubiquitination machinery. Further studies are needed to understand the mechanism by which Siva-1 mediates the ubiquitination of TRAF2 at the K48 residue. The increased K63-Ub of TRAF2 observed in siSiva T cells is possibly a consequence of the lowered K48-Ub TRAF2 levels, suggesting lowered negative regulation by TRAF2 on the NF-κB pathway. Our data, for the first time, demonstrate the possible degradation of TRAF2 through K48-polyubiquitination upon TCR-cross-linking, and Siva-1 has an important role in this process (Fig. 7B, compare left panel, lane 3 versus right panel lane 3).
In summary, we have demonstrated a specific interaction between Siva-1 and TRAF2 that offers a mechanistic explanation for the observed inhibitory effect of endogenous Siva-1 on NF-κB activation. The primary consequence of the above interaction appears to be an increase in K48-Ub of TRAF2 that, in turn, decreases the K63-Ub of TRAF2 and inhibits the NF-κB activity. Our findings suggest that Siva-1, through its interaction with TRAF2, is likely to have a significant impact on T-cell homeostasis and autoimmune disorders as well as T-cell memory.
Acknowledgments
We thank Drs. Richard Ye (University of Illinois at Chicago [UIC]), Zhijian Chen (University of Texas [UT] Southwestern), Gail Bishop (University of Iowa), Vishva Dixit (Genentech), and Xin Lin (M. D. Anderson Cancer Center) for kindly providing various plasmids upon request. We also thank UIC for funding this project. B.S.P. is supported by National Institutes of Health (NIH) grants R01 CA107506-02 and R01 AI058190-02.
Abbreviations
- TCR
T-cell receptor
- NF-κB
nuclear factor-kappaB
- AP-1
activator protein-1
- TRAF2
tumor necrosis factor receptor-associated factor 2
- TRAF6
tumor necrosis factor receptor-associated factor 6
- IKK
Ikappa B kinase
- K48-Ub
ubiquitination at lysine 48 position
- K63-Ub
ubiquitination at lysine 63 position
- AICD
activation-induced cell death
References
- 1.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 2.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell. 2004;7:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 3.Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243:1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- 4.Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Spence J, Gali RR, Dittmar G, Sherman F, Karin M, Finley D. Cell cycle-regulated modification of the ribosome by a variant multiubiquitin chain. Cell. 2000;102:67–76. doi: 10.1016/s0092-8674(00)00011-8. [DOI] [PubMed] [Google Scholar]
- 6.Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 7.Shi CS, Kehrl JH. Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2) J Biol Chem. 2003;278:15429–15434. doi: 10.1074/jbc.M211796200. [DOI] [PubMed] [Google Scholar]
- 8.Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Monack DM, Kayagaki N, Wertz I, Yin J, Wolf B, Dixit VM. Yersinia virulence factor YopJ acts as a deubiquitinase to inhibit NF-kappa B activation. J Exp Med. 2005;202:1327–1332. doi: 10.1084/jem.20051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortin A, MacLaurin JG, Arbour N, Cregan SP, Kushwaha N, Callaghan SM, Park DS, Albert PR, Slack RS. The proapoptotic gene SIVA is a direct transcriptional target for the tumor suppressors p53 and E2F1. J Biol Chem. 2004;279:28706–28714. doi: 10.1074/jbc.M400376200. [DOI] [PubMed] [Google Scholar]
- 11.Gudi R, Barkinge J, Hawkins S, Chu F, Manicassamy S, Sun Z, Duke-Cohan JS, Prasad KV. Siva-1 negatively regulates NF-kappaB activity: effect on T-cell receptor-mediated activation-induced cell death (AICD) Oncogene. 2006;25:3458–3462. doi: 10.1038/sj.onc.1209381. [DOI] [PubMed] [Google Scholar]
- 12.Prasad KV, Ao Z, Yoon Y, Wu MX, Rizk M, Jacquot S, Schlossman SF. CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapopto tic pro tein. Proc Natl Acad Sci U S A. 1997;94:6346–6351. doi: 10.1073/pnas.94.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spinicelli S, Nocentini G, Ronchetti S, Krausz LT, Bianchini R, Riccardi CS, Krausz LT, Bianchini R, Riccardi C. GITR interacts with the pro-apoptotic protein Siva and induces apoptosis. Cell Death Differ. 2002;12:1382–1384. doi: 10.1038/sj.cdd.4401140. [DOI] [PubMed] [Google Scholar]
- 14.Chu F, Barkinge J, Hawkins S, Gudi R, Salgia R, Kanteti PV. Expression of Siva-1 protein or its putative amphipathic helical region enhances cisplatin-induced apoptosis in breast cancer cells: effect of elevated levels of BCL-2. Cancer Res. 2005;65:5301–5309. doi: 10.1158/0008-5472.CAN-04-3270. [DOI] [PubMed] [Google Scholar]
- 15.Xue L, Chu F, Cheng Y, Sun X, Borthakur A, Ramarao M, Pandey P, Wu M, Schlossman SF, Prasad KV. Siva-1 binds to and inhibits BCL-X(L)-mediated protection against UV radiation- induced apoptosis. Proc Natl Acad Sci U S A. 2002;99:6925–6930. doi: 10.1073/pnas.102182299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu F, Borthakur A, Sun X, Barkinge J, Gudi R, Hawkins S, Prasad KV. The Siva-1 putative am-phipathic helical region (SAH) is sufficient to bind to BCL-XL and sensitize cells to UV radiation induced apoptosis. Apoptosis. 2004;9:83–95. doi: 10.1023/B:APPT.0000012125.01799.4c. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 18.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 19.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 20.Weih F, Caamaño J. Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway. Immunol Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto H, Kishimoto T, Minamoto S. NF-kappaB activation in CD27 signaling: involve- ment of TNF receptor-associated factors in its signaling and identification of functional region of CD27. J Immunol. 1998;161:4753–4759. [PubMed] [Google Scholar]
- 22.Nossal GJ. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 23.Grech AP, Amesbury M, Chan T, Gardam S, Basten A, Brink R. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity. 2004;21:629–642. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 25.Moore CR, Bishop GA. Differential regulation of CD40-mediated TNF receptor-associated factor degradation in B lymphocytes. J Immunol. 2005;175:3780–3789. doi: 10.4049/jimmunol.175.6.3780. [DOI] [PubMed] [Google Scholar]
- 26.Kovalenko A, Chable-Bessia C, Cantarella G, Israël A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]