Abstract
Pancreatic neoplasms are morphologically and genetically heterogeneous and include wide variety of neoplasms ranging from benign to malignant with an extremely poor clinical outcome. Our understanding of these pancreatic neoplasms has improved significantly with recent advances in cancer sequencing. Awareness of molecular pathogenesis brings in new opportunities for early detection, improved prognostication, and personalized gene-specific therapies. Here we review the pathological classification of pancreatic neoplasms from their molecular and genetic perspective.
All of the major tumor types that arise in the pancreas have been sequenced, and a new classification that incorporates molecular findings together with pathological findings is now possible (Table 1). This classification has significant implications for our understanding of why tumors aggregate in some families, for the development of early detection tests, and for the development of personalized therapies for patients with established cancers. Here we describe this new classification using the framework of the standard histological classification.
Ductal Adenocarcinoma
Pancreatic ductal adenocarcinoma (PDAC) is the most common malignant neoplasm of pancreas. Unfortunately it is one of the most lethal of all of the solid malignancies. The American Cancer Society (Cancer Facts & Figures 2014; www.cancer.org) has estimated that 46,420 Americans will be diagnosed with pancreatic cancer in 2014 and that 39,590 will die of the disease.1
Grossly, most ductal adenocarcinomas form poorly demarcated and firm white-yellow masses. The adjacent non-neoplastic pancreas is typically atrophic and fibrotic, and the pancreatic ducts may be dilated from the obstructive effects of the carcinoma. Microscopically, these neoplasms vary from well-differentiated duct forming carcinomas, which may be so well-differentiated as to mimic non-neoplastic glands, to poorly-differentiated carcinomas with glandular differentiation demonstrable only onimmunolabeling.2 Ductal adenocarcinomas typically elicit an intense stromal reaction, and this reaction has been postulated to serve as a barrier to chemotherapy.3, 4
The pancreatic parenchyma adjacent to invasive ductal adenocarcinomas often contains epithelial proliferations in smaller pancreatic ducts, called pancreatic intraepithelial neoplasia (PanIN) lesions. PanINs are one of the precursors to invasive ductal adenocarcinoma.5 PanINs contain many of the genetic alterations that are present in invasive ductal adenocarcinomas.6–9 PanINs are graded histologically from PanIN-1 to PanIN-3 based on architectural complexity and cytological atypia, with PanIN-3 lesions having the most atypia.10
Molecular Genetics of pancreatic ductal adenocarcinoma
The genomes or exomes of a large number of ductal adenocarcinomas have been sequenced, significantly increasing our understanding of the molecular drivers of pancreatic cancer.11, 12 Although the genetic changes identified are complex, the key to understanding of pancreatic tumorigenesis lies in recognition and appreciation that these mutations target a core set of pathways and processes. The core genes and pathways targeted in ductal adenocarcinomas include KRAS, p16/CDKN2A, TP53, the TGF-β signaling pathway as well as other less commonly targeted pathways. The KRAS, p16/CDKN2A, TP53 and SMAD4 genes are each mutated in more than 50% of ductal adenocarcinomas. (Table 1)
Table 1.
Frequently targeted genes in pancreatic neoplasms
| Pancreatic neoplasm | Targeted gene | Alteration prevalence | Altered gene function |
|---|---|---|---|
| PDCA | KRAS | 90% | Cell cycle activation (MAPK and PIK3CA pathway) |
| P16/CDKN2A | 95% | CDK4 and CDK6 inhibition | |
| TP53 | 75% | Cellular stress response | |
| SMAD4 | 55% | Loss of TGF-β induced tumor suppression | |
| IPMN | KRAS | 80% | Cell cycle activation (MAPK and PIK3CA pathway) |
| P16/CDKN2A | Present only in high grade dysplasia and carcinoma | CDK4 and CDK6 inhibition | |
| TP53 | Cellular stress response | ||
| SMAD4 | Loss of TGF-β induced tumor suppression | ||
| GNAS | 60–65% IPMN variants Intestinal – 100% Pancreatobiliary – 71% Gastric type – 51% |
Uncontrolled growth signaling | |
| RNF43 | 75% | Wnt signaling regulation | |
| MCN | KRAS | 30% – 80% Progressive increase with dysplasia grade |
Cell cycle activation |
| RNF43 | 40% | Wnt signaling regulation | |
| P16/CDK2NA, TP53, SMAD4 | Only in high grade tumors | ||
| SPN | CTNNB1 | 95% | Wnt/β-catenin signaling pathway activation |
| PanNET | MEN1 | 45% | |
| DAXX and ATRX | 45% | Chromatin remodeling | |
| PIK3CA, PTEN and TSC2 | 14% | mTOR pathway | |
| VHL | 25% | HIF-1 α pathway | |
| ACC | APC–β-catenin | 25% | Cell signaling and adhesion |
| KRAS | Rare | Cell cycle activation | |
| TP53 | Rare | Cellular stress response | |
| Fanconi anemia pathway genes | 45% | DNA repair mechanism | |
| CTNNB1 | 5% | Cell signaling and adhesion | |
| PB | APC–β-catenin | 86% | Cell signaling and adhesion |
| CTNNB1 | 55% | Cell signaling and adhesion |
PDCA: pancreatic ductal adenocarcinoma, IPMN: intraductal papillary and mucinous neoplasm, MCN: mucinous cystic neoplasm, SPN: solid and pseudopapillary neoplasm, PanNET: pancreatic neuroendocrine tumor, ACC: acinar cell carcinoma, PB: pancreatoblastoma
KRAS
The oncogene KRAS (short arm of chromosome 12p) is the most commonly mutated gene in ductal adenocarcinomas, with alterations present in >90% of the cancers. These mutations occur early in tumorigenesis, as KRAS gene mutations are present in greater than 90% of PanIN-1 lesions.7
The KRAS oncogene encodes Kirsten rat sarcoma viral oncogene homolog (KRAS) protein which belongs to the GTPase family of proteins. Somatic activating mutations in KRAS most frequently target codon 12 of the gene, resulting in substitution of glycine with aspartic acid, valine, arginine or serine in the protein-product of the gene.11 These amino acid changes constitutively activate the KRAS protein by decreasing its intrinsic GTPase activity and rendering the mutant protein insensitive to GTPase-activating proteins. Constitutive KRAS activation leads to activation of a number of complex downstream pathways such as the RAF-MEK-ERK pathway that governs proliferation, cell survival, differentiation and gene expression. As will be discussed in greater detail later in this review, pancreatic ductal adenocarcinomas that do not harbor a KRAS gene mutation, are often microsatellite unstable and have a unique medullary histologic phenotype with poor differentiation and pushing borders.13
It has recently been suggested that oncogenic KRAS alters the regulation of metabolism in neoplastic cells.14–16 Regulated metabolic pathways include glutamine metabolism supporting the maintenance of redox state in the tumor cells,17 and activation of detoxification of reactive oxygen species18 by inducing the expression of NRF2. Other key cellular functions regulated by KRAS include adaptation to higher energy needs of tumor cells. Activated oncogenic KRAS may also promote the direct acquisition of albumin from extracellular space by macropinocytosis allowing the neoplastic cells to utilize albumin for Krebs cycle intermediates.19 KRAS has also been implicated in inducing autophagy, which is a regulated catabolic cell organelle and macromolecule utilization pathway, required for maintainence and progression of PDAC.20
Several downstream effector pathways are affected by mutant KRAS. Of these, the mitogen activated protein kinase (MAPK) and PI3K pathways have been the most extensively studied. The MAPK pathway is a kinase cascade involving activation of RAF kinase by KRAS and eventual activation of MEK1/2. MEK kinases further activate ERK1/2 via phosphorylation. This signaling is active in PanIN lesions as well as invasive ductal adenocarcinomas.21,22 These downstream effector pathways are clinically important because they may be therapeutically targetable. The hope is to exploit the vulnerability of downstream effector pathways, in particular RAF – MEK – ERK, to develop specifically targeted therapies using kinase inhibitors.
It has also been suggested that oncogenic KRAS, in addition to transforming growth factor β (TGF-β) signaling, affects the fibroinflammatory stromal response characteristic of PDAC. The reversibility of inducible KRAS in iKras mouse has been exploited to demonstrate the regression of cellular stroma to an inactive scar like histology, loss of proliferation and loss of smooth muscle actin expression in the tumor associated stroma following inactivation of KRASG12D.21–23 Mutant KRAS mediated upregulation of GLI1/IL6 axis via Sonic Hedgehog augments the symbiotic paracrine signaling with the active fibroblast rich stroma.24–26 Suppression of host immunosurveillance had also been postulated as one of the mechanism driven by mutant KRAS pathway.27 The precursor PanIN lesions are shown to adapt to the host immunologic defenses at an early stage by establishing an immune suppressive environment consisting predominantly of regulatory T cells and myeloid derived suppressor cells and lack of tumor infiltrating effector or cytotoxic T cells.28, 29
Activating point mutations in KRAS therefore play an early, important and multifaceted role in the development of ductal adenocarcinoma of the pancreas. The biology of exactly how mutant KRAS promotes tumorigenesis is complex, as the protein product of the KRAS gene impacts a large number of different pathways.
p16/CDKN2A
A number of tumor suppressor genes are targeted in ductal adenocarcinomas. These include p16/CDKN2A, TP53 and SMAD4/DPC4.11,30–35
The p16/CDKN2A gene (chromosome 9p) is the most commonly inactivated tumor suppressor gene, targeted in 95% of ductal adenocarcinomas. The protein product of the p16/CDKN2A gene, p16INK4A, normally functions to inhibit the G1 phase of the cell cycle by inhibiting the cyclin D dependent kinases (CDK4 and CDK6) and therefore the phosphorylation of retinoblastoma protein.33 Loss of p16INK4A occurs early in pancreatic tumorigenesis and plays a significant role in disease progression.7,34 The CDKN2A locus also encodes p14ARF in humans (p19ARF in mouse). The protein p14ARF stabilizes the p53tumor suppressor protein through neutralization of MDM2. Loss of p14ARF can be seen in up to 40% of ductal adenocarcinomas due to homozygous deletion of CDKN2A.35 The resulting MDM2 activation promotes degradation of p53 through ubiquitinization in an early stage of pancreatic carcinogenesis causing faster progression of Pan-IN lesions to an invasive tumor. Thus, the almost universal inactivation of p16/CDKN2A results in the loss of an important cell cycle control mechanism in pancreatic cancer.
SMAD4
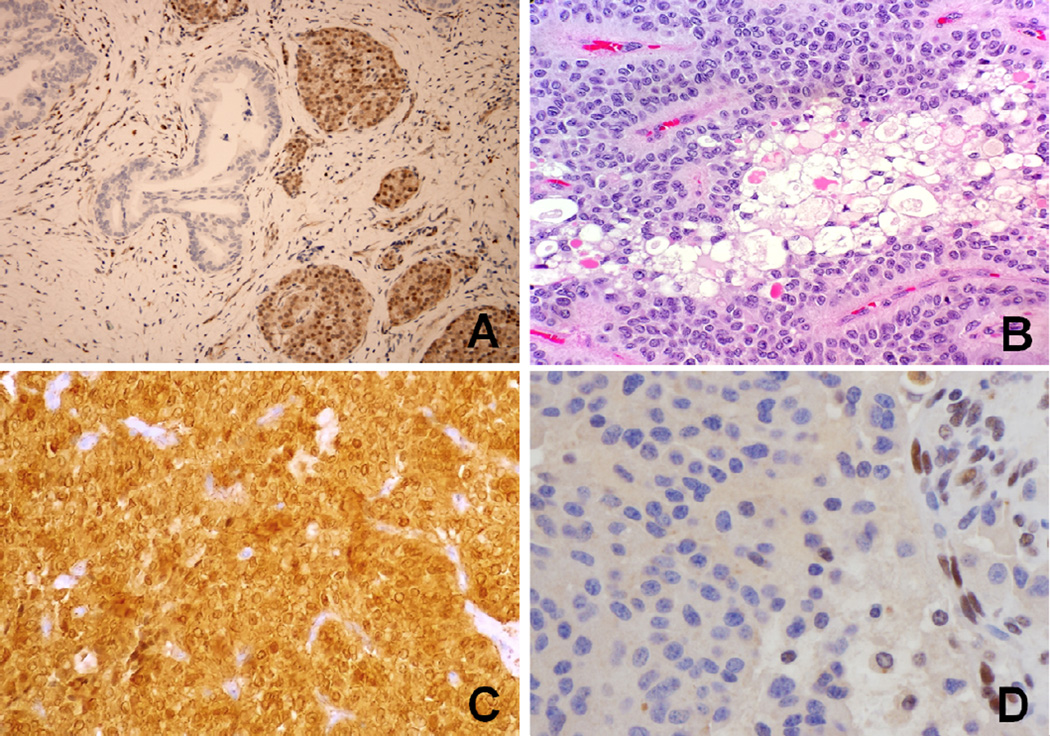
Tumor suppressor gene SMAD4 (DPC4, MADH4) is inactivated in ~55% of ductal adenocarcinomas.11,36 SMAD4 (chromosome 18q21.2) codes for the protein Smad4, which is involved in transforming growth factor β (TGF-β) signaling. In ductal adenocarcinomas, the gene can be inactivated through homozygous deletions or deletion of one allele coupled with an intragenic mutation in the second allele.36,37 The result is loss of TGF-β induced tumor suppression. Immunolabeling for the SMAD4 gene product, Smad4, has been shown to be sensitive and specific marker for SMAD4 gene status and may be used to support the diagnosis of a metastasis as from a pancreatic primary.38 This diagnostic utility is valid only when there is complete absence of protein on immunohistochemical labeling (Fig 1A), which is seen in 55% of invasive pancreatic cancers. In addition, SMAD4 gene status of primary tumor has been shown to correlate with a poorer outcome39 and with widespread metastases in autopsy studies.40 SMAD4 loss has also been associated with worse survival in surgically resected patients.41
Figure 1.
A: DPC4 immunohistochemistry with complete loss of immunolabeling in the pancreatic ductal adenocarcinoma. Note the intact staining in non-neoplastic islet cells. (×100)
B: Solid-pseudopapillary neoplasm of the pancreas showing hyaline globules and bland monomorphic neoplastic cells with nuclear grooves, pale chromatin and inconspicuous nucleoli. (Hematoxylin &Eosin × 200)
C: β-Catenin immunolabeling of solid-pseudopapillary neoplasm with strong nuclear and cytoplasmic staining. (×200)
D: Pancreatic neuroendocrine tumor with loss of nuclear immunolabeling for the protein product of ATRX (α thalassemia/mental retardation syndrome X-linked) chromatin remodeling gene. Note the intact nuclear staining in non-neoplastic endothelial cells. (×400)
TP53
TP53 (chromosome 17p) is inactivated in ~75% of ductal adenocarcinomas, almost always by missense mutations coupled with loss of the remaining allele.11,30 The protein encoded by TP53 (p53) plays an important role in DNA repair mechanisms, cell growth arrest and activation of apoptosis following the cellular injury. TP53 is targeted late in PanIN progression, usually not until PanIN-3.8 Of interest, Kanda et al have recently reported the detection of mutant TP53 alleles in pancreatic juice samples collected endoscopically from patients with lesions with high-grade dysplasia in their pancreata, highlighting the potential of these mutations as a strategy for early detection.8
Telomeres
In comparison to normal chromosomes, telomeres are shortened during pancreatic tumorigenesis. Telomeres are located at the ends of chromosomes and are composed of repetitive nucleotide sequences and associated proteins. Telomeres prevent the free ends of chromosomes from sticking to each other. Telomere shortening is an early event in tumorigenesis in the pancreas as telomere shortening has been observed in PanIN lesions.42 Similarly telomere shortening is present in acinar to ductal metaplasia lesions associated with PanIN but not in isolated acinar-to-ductal metaplasia.43 When telomeres are shortened, the ends of chromosomes may inappropriately stick together, causing the formation of anaphase bridges and chromosome instability.
Variants of pancreatic ductal adenocarcinoma
A number of morphological variants of ductal adenocarcinoma have been characterized at the molecular level, and these histologically and molecularly defined variants have distinct clinical features. For example, colloid carcinoma is characterized by the production of copious amounts of extracellular mucin, and these distinctive neoplasms almost always arise in association with an intestinal-type intraductal papillary mucinous cystic neoplasm (IPMN).44 The majority of intestinal-type intraductal papillary mucinous neoplasms (as well as their associated carcinomas) harbors GNAS gene mutations, and, of interest, colloid carcinomas are often lower-stage and therefore associated with a better prognosis than ductal adenocarcinomas.45–47
The medullary variant of pancreatic cancer is a poorly differentiated variant of pancreatic cancer. The medullary variant has a higher prevalence of microsatellite instability related to defects in mismatch repair and BRAF mutations, but these neoplasms lack somatic KRAS mutations.13,48 Although the medullary variant is associated with pancreatic cancers that have defects in mismatch repair, not allmedullary pancreatic cancers have microsatellite instability.13,49 The intense host immune response associated with microsatellite unstable cancers suggests that they are more recognizable by the host immune system, and the instability may damage genes coding for proteins needed for the neoplastic cells to function. Thus, the medullary variant of pancreatic cancer is a great example in which an understanding of the genetics (microsatellite instability) helps us understand why a poorly differentiated neoplasm is associated with a better prognosis.
Undifferentiated carcinomas of the pancreas (pancreatic cancers with non-cohesive phenotype) are characterized by loss of expression of e-cadherin protein expression, sometimes through promoter hypermethylation.50 Some of these pancreatic cancers with loss of e-cadherin expression have signet ring features. These carcinomas are important to recognize because they are associated with a worse prognosis. Interestingly, focal loss of e-cadherin expression is seen in many pancreatic ductal adenocarcinomas and is associated with a poorer outcome.51
Other variants of pancreatic cancer, such as the adenosquamous carcinoma, undifferentiated carcinomas, and undifferentiated carcinomas with osteoclast-like giant cells are all important to recognize because they are associated with poor prognosis.2
Cystic Neoplasms of the Pancreas
Pancreatic cysts are not uncommon, and more pancreatic cysts are being detected as imaging techniques improve. As many as 20% of autopsied patients have a cystic lesion in their pancreas.52 Similarly, the prevalence of pancreatic cysts among patients undergoing pancreatic MRI for suspected disease is high. (~ 19.6%)53 MRI/MRCP is a more sensitive test for detecting pancreatic cysts than CT54 but pancreatic cysts are still commonly detected among patients having CT scans performed for non-pancreatic indications (detection rate of 2.6% in asymptomatic patients).55 Among healthy individuals who have undergone a screening MRI, the prevalence of pancreatic cysts increases with age, with a prevalence of >10% among those aged 70 or more.56 These cysts are important because some, such as the intraductalpapillary mucinous neoplasm, are curable precursors to invasive ductal adenocarcinoma, while others, such as serous cystadenomas, are virtually always benign. Recent genetic sequencing has shown that each of the major types of cystic neoplasms of the pancreas has its own mutational profile.
Intraductal Papillary Mucinous Neoplasm
Intraductal papillary mucinous neoplasms (IPMNs) account for at least 25–30% of all neoplastic cysts. By definition, intraductal papillary mucinous neoplasms are mucin-producing epithelial neoplasms that involve the duct system and are equal to or larger than 1cm in size. These neoplasms are noninvasive and can harbor varying degrees of dysplasia. IPMNs may involve the main or smaller branch pancreatic ducts. Most arise in the head of the pancreas; however, they may also arise in the tail of the gland, and some even involve the entire pancreas. Intraductal papillary mucinous neoplasms may be multicenteric, and therefore the presence of one lesion should heighten the clinical suspicion for additional lesions and mandate careful follow-up.
Macroscopically, IPMNs are characterized by dilatation of the main or branch pancreatic ducts.2 Papillary fronds of neoplastic epithelium and tenacious luminal mucin are often present. Microscopically, by definition, the neoplastic epithelium involves the larger pancreatic ducts. The neoplastic epithelium can be papillary or flat, and can show one of four directions of differentiation: intestinal, gastric, pancreatobiliary or oncocytic differentiation.5 The intestinal type includes goblet cells and intestinal-type mucin, the gastric type is composed of foveloar and pyloric epithelium resembling gastric glands, the oncocytic has abundant eosinophilic granular cytoplasm, and the pancreatobiliary type is more cuboidal cells with minimal mucin. Although IPMNs are typically classified into a histological subtype, more than one epithelial subtypes can be present within the same IPMN.5
The degree of dysplasia in intraductal papillary mucinous neoplasms is graded from low to intermediate to high based on cytological as well as architectural microscopic features.2 Among patients with IPMNs who go to pancreatic resection, ~ 30% will have IPMNs that have an associated invasiveadenocarcinoma.46,57 This invasive adenocarcinoma can be either colloid carcinoma (mucinous non-cystic adenocarcinoma) or a conventional tubular-type adenocarcinoma.58 As noted earlier, the colloid carcinomas almost always arise in association with an intestinal-type intraductal papillary mucinous neoplasm.59,60
Patients with an invasive carcinoma arising in association with an intraductal papillary mucinous neoplasm have a better prognosis than do patients with a conventional ductal adenocarcinoma not arising in association with an IPMN, but some of this improved prognosis is lost when one controls for stage.46,61
Molecular Genetics of IPMNs
The genetic alterations in intraductal papillary mucinous neoplasms have been characterized using whole exome sequencing. IPMNs harbor fewer genetic alterations than do invasive ductal adenocarcinomas. Some of the genes targeted in intraductal papillary mucinous neoplasms are also targeted in ductal adenocarcinomas (such as KRAS, p16/CDKN2A, TP53 and SMAD4), while others are more specific for the IPMN pathway (such as GNAS and RNF43).47,62 Somatic mutations in KRAS are common and early events in the development of intraductal papillary mucinous neoplasms (up to 80% cases)63,64 These mutations can be detected in fluid aspirated from the cysts.47 TP53 gene mutations have also been reported in intraductal papillary mucinous neoplasms, and the prevalence of these mutations increases with the grade of dysplasia, whereas SMAD4 mutations are uncommon in IPMNs unless there is an associated invasive adenocarcinoma.65–70
Whole-exome sequencing of intraductal papillary mucinous neoplasms also revealed mutations in two genes that are not commonly targeted in usual ductal adenocarcinomas. The GNAS oncogene located on chromosome 20q13.3 encodes for G protein stimulating α subunit and is mutated at single hotspot codon201 in 65% of IPMNs.45,47,64 Mutations in the GNAS gene result in the constitutive activation of Gsα and its effector adenylate cyclase, leading to autonomous synthesis of cyclic AMP (cAMP) and uncontrolled growth signaling.71 GNAS mutations are most common in intestinal type IPMNs (100%) followed by pancreatobiliary (70%) and gastric types (50 %). Interestingly, patients with McCune Albright syndrome which arises from post-zygotic oncogenic mutations in GNAS have been reported to have pancreatic cysts.72 Of note, the GNAS mutations present in IPMNs can be detected in cyst fluid aspirates and even in duodenal fluids collected at the time of endoscopy. For example, Kanda et al. reported that GNAS mutations are detectable in two-thirds of pancreatic secretions collected from duodenal contents of patients with an IPMN. Remarkably, GNAS mutations were detected in some patients who did not have an IPMN detectable by imaging at the time they were studied, but who later developed an IPMN.45,73 The combination of testing for KRAS and GNAS mutations may prove a very sensitive approach to analyzing cyst fluids and to the early detection of pancreatic neoplasms, as 95% of IPMNs have mutations in one of these two oncogenes.
The RNF43 gene located on chromosome 17q23.2 encodes for a protein with intrinsic E3 ubiquitin ligase activity, and RNF43 is commonly (up to 75% of studied cases) inactivated in IPMNs.62 The protein product of the RNF43 gene is involved in regulating Wnt signaling, and may be therapeutically targetable.74–76 Jiang et al have shown that inactivating mutations in RNF43 confer Wnt pathway dependency, and that cells with an RNF43 gene mutation are particularly sensitive to the porcupine inhibitor LGK974.75
Thus, the sequencing of IPMNs has identified genetic changes that may help classify a cyst as an IPMN, changes that may be useful for early detection and genetic changes that may be therapeutically targetable.
Mucinous cystic neoplasm
Mucinous cystic neoplasms (MCNs) of pancreas are distinctive mucin-producing neoplasms that arise almost exclusively in the body and tail of pancreas.2 Most cases occur in young or middle aged females. In contrast to IPMNs, the cysts of MCN do not communicate with the larger pancreatic ducts. The cysts are most frequently multi-loculated and distended with tenacious mucin. Grossly, the inner surfaces of the cyst walls may be smooth, they may have papillary excrescences or they may have isolated intracystic solid nodules. Microscopic examination show two characteristic components: 1) cysts lined with columnar mucinous type epithelium arranged in a flat or papillary architecture, and 2) distinctive ovarian-type stroma, the cells of which may express estrogen and progesterone receptors.2 As is true for IPMNs, the cytological atypia of the lining epithelium is graded as low, intermediate and high grade. MCNs are precursors to invasive cancer, and up to one-third of MCNs have an associated invasive carcinoma.
Molecular Genetics of MCNs
The exomes of a series of well-characterized MCNs have been sequenced, and MCNs harbor fewer mutations than do ductal adenocarcinomas.62 The KRAS, p16/CDKN2A, TP53, RNF43 and SMAD4 genes have all been reported to be targeted in MCNs. Somatic KRAS mutations are the most common and their prevalence increases as histologic grade increases.77,78 Majority (80%) of MCNs associated with invasive tumor or high grade dysplasia has KRAS mutations as compared to only 30% of MCNs with low-grade dysplasia.78 SMAD4, TP53 and p16/CDKN2A genes all appear to be targeted in higher-grade lesions.39,70,79 Approximately 40% of MCNs harbor somatic mutations in tumor suppressor RNF43, which is also targeted in IPMNs, suggesting the role of this gene in the development of mucin-producing neoplasms of pancreas.62 In contrast to IPMNs, GNAS does not appear to be mutated in MCNs.47
Solid-pseudopapillary neoplasm
Solid-pseudopapillary neoplasms (SPNs) are rare solid neoplasms of the pancreas that typically undergo cystic degeneration. The vast majority arise in young women.2 Although these lesions often carry a favorable prognosis, they are fully malignant neoplasms, and local invasion or even metastases occur in 10–15% of patients.80,81 There is no definite predilection for anatomical location within the pancreas. Grossly SPNs are well circumscribed with solid (cellular) and cystic (degenerative) areas. Microscopically, SPNs are composed of bland appearing cells with clear cell features and uniform nuclear morphology arranged in sheets and a pseudopapillary configuration. Characteristic pseudo-papillae are acquired due to degenerative changes and loss of cellular cohesion, which leaves a thin layer of neoplastic cells lining delicate vessels. Degenerative features include foam cells, hyalinization, cholesterol clefts, microcystic change and hemorrhage. Hyaline globules are frequently present.(Fig 1B) The neoplastic cells characteristically invade in the adjacent pancreatic parenchyma by insinuating themselves between non-neoplastic cells.82 SPNs may have considerable morphologic overlap with pancreatic neuroendocrine tumors. An immunolabeling panel that includes CD10 and β-catenin (nuclear expression, Fig 1C) may aid in making diagnosis of SPN.82 Characteristic dot like paranuclear (Golgi zone) immunolabeling is also reported for CD99.83 While the presence of focal degenerative atypia is not prognostically significant, the presence of a dedifferentiated component portends a significantly worse prognosis.80,84
Molecular Genetics of SPNs
The exomes of SPNs have been sequenced and SPNs have an average of only three non-synonymous mutations per tumor, the lowest number of any adult solid tumor sequenced to date.62 The genes targeted in SPNs are different from those targeted in other tumors of the pancreas. Alterations in TP53, KRAS, p16/CDKN2A, and SMAD4 are not seen. Instead, the Wnt/β-catenin signaling pathway is activated in almost all SPNs.85 Almost all SPNs (95%) have somatic activating mutations in the β-catenin gene (exon 3 of CTNNB1) leading to abnormal nuclear localization of the β-catenin protein.86,87 This aberrant nuclear localization of β-catenin can be exploited diagnostically, as nuclear labeling for the β-catenin protein can be used to distinguish SPNs from other pancreatic neoplasms. Loss of β-catenin is associated with alterations in the pattern of E-cadherin expression. Immunolabeling with antibodies to the extracellular domain of E-cadherin show a loss of membrane staining, while an aberrant nuclear pattern of labeling isseen with antibodies to the intracellular domain.88,89 Loss of the adhesion molecule E-cadherin may also explain the dyscohesive nature of the neoplastic cells in SPNs.
Pancreatic Neuroendocrine Tumors
Pancreatic neuroendocrine tumors (PanNETs) are a morphologically and genetically distinct category of pancreatic neoplasms with neuroendocrine differentiation, accounting for ~1% of diagnosed pancreatic neoplasms.2,90,91 Although less aggressive than ductal adenocarcinomas, neuroendocrine tumors (≥0.5cm) are malignant neoplasms with a five-year survival of only 65%. These neoplasms can produce hormones that can cause significant clinical symptoms (“functional tumors”) and can arise in patients with a genetic syndrome such as the multiple neuroendocrine neoplasia type 1 (MEN-1) syndrome. The clinical presentation is variable and is based on the functional status of the tumor. There is no specific anatomical predilection within the pancreas. Sporadic neoplasms are often solitary in contrast to the multi-focality of PanNETs associated with multiple endocrine neoplasia (MEN-1) syndrome.
These well-circumscribed neoplasms may have a fibrous capsule and are usually tan-yellow and solid. Some examples are hemorrhagic, while others may be cystic. Necrosis and degeneration are more common in larger tumors. The histological differentiation ranges from well to poorly differentiated, and grading is based entirely on the proliferation rate. In the World Health Organization (WHO)-2010 classification, well-differentiated tumors are classified into grade 1 (mitosis < 2/10 high power field or HPF; Ki-67 proliferation < 3%) and grade 2 (mitosis 2–20/10 HPF; Ki-67 proliferation 3–20%).92 Poorly differentiated tumors are classified as grade 3 with mitosis and Ki-67 proliferation index of greater than 20/HPF and 20% respectively. Grade 3 neuroendocrine tumors are designated carcinomas. When assessing the proliferation rate of neuroendocrine tumors, the inclusion of both the mitotic rate and Ki-67 labeling index can help refine prognostication.93
Molecular genetics of PanNETs
The genes targeted in neuroendocrine tumors differ significantly from those targeted in ductal adenocarcinomas. Whole exome sequencing shows an average of 16 nonsynonymous mutations per tumor, far fewer than in ductal adenocarcinoma.94 The genes commonly targeted in neuroendocrine tumors include MEN1, DAXX and ATRX, and genes coding for members of the mammalian target of rapamycin (mTOR) pathway.94–97 DAXX (death-domain-associated protein) and ATRX (α thalassemia/mental retardation syndrome X-linked) are chromatin remodeling genes, and one of these genes is somatically mutated in 45% of sporadic PanNETs. These genes encode nuclear proteins that mutually interact and function in chromatin remodeling at telomeric and peri-centromeric regions. Immunolabeling for the DAXX and ATRX proteins correlates with gene status.(Fig 1D) Mutations in these genes are associated with the alternative lengthening of telomeres (ALT) phenotype, a telomerase independent mechanism for telomere maintenance.98 Mutations in MEN1, DAXX and ATRX are associated with better prognosis than wild-type tumors. Somatic mutations in DAXX and ATRX are acquired late in PanNET oncogenesis as shown by their absence in microadenomas.99 Mutations in mTOR (mammalian target of rapamycin) pathway including PIK3CA, PTEN and TSC2 are present in 14% of PanNETs and may be candidate for treatment with mTOR pathway inhibitors.94,100 Although the VHL gene is not commonly altered by small somatic mutations in sporadic tumors, the VHL/HIF pathway is also important in PanNET pathogenesis.101 Pancreatic neuroendocrine microadenomas are present in >70% of patients with von Hippel Lindau syndrome. Sporadic PanNETs rarely harbor somatic VHL gene mutation, but promoter hypermethylation and deletion of VHL occur in up to 25% sporadic PanNETs and are associated with adverse prognosis.102
Acinar Cell Carcinoma
Acinar cell carcinomas (ACCs) are rare pancreatic neoplasms. They have poor prognosis and considerable morphologic overlap with pancreatoblastoma – both neoplasms have acinar differentiation, though pancreatoblastoma also displays other types of differentiation (see below). The mean survival of patients with an acinar cell carcinoma is 18–24 months and the 3 year survival rate is only 25%.103,104There is no anatomical predilection within the pancreas, and frequent presentation is in the form of a large solitary, solid and well-circumscribed mass lesion. Microscopically, they are cellular neoplasms with acinar cell differentiation, based on morphology and immunohistochemical staining. The cyanophilic acinar appearing cells consist of granular cytoplasm and centrally located nucleus with prominent nucleolus, which are arranged in sheets and trabecular pattern.2
Molecular genetics of ACCs
Recent whole exome sequencing of acinar cell carcinomas revealed a mean of 119 non-synonymous mutations, which is higher than the average number of mutations in ductal adenocarcinomas and other primary pancreatic neoplasms.105 These carcinomas display striking genomic instability, and multiple genetic aberrations are often present, including large chromosomal gains and losses and microsatellite instability. At the gene level, genes coding for members of the APC–β-catenin pathway are targeted in 20–25% of acinar cell carcinomas,106 while KRAS and TP53 are only rarely targeted. Of particular note, potentially therapeutically targetable mutations have been identified in some acinar cell carcinomas, including mutations in genes coding for members of the Fanconi anemia pathway, which would potentially be targetable with poly (ADP-ribose) polymerase (PARP) inhibitors. Other potentially targetable genetic alterations in acinar cell carcinomas include mutations in BRAF and JAK1.105,107–109
Pancreatoblastoma
Pancreatoblastomas (PBs) are very rare pancreatic neoplasms that have a bimodal age distribution with one peak in pediatric patients and the other later in adulthood.110 The prognosis is strongly influenced by the completeness of surgical resection.111 There is no location preference within the pancreas.112 The microscopic appearance of pancreatoblastomas is diverse, but at a minimum pancreatoblastomas contains cells with acinar differentiation and squamoid nests.2 Other common directions of differentiation include neuroendocrine and primitive blastemal histomorphology. The neoplastic cells are arranged in sheets,tubules, nests and acinar patterns. Presence of squamoid nests differentiates pancreatoblastomas from acinar cell carcinomas.
Molecular genetics of PBs
The genetic aberrations in pancreatoblastoma are not well-characterized because these neoplasms are so rare.105 At the genetic level, pancreatoblastomas bear closer resemblance to the infantile embryonal tumors as suggested by their reported occurrence with Beckwith-Wiedemann syndrome and chromosome 11p loss.113 Most commonly the somatic inactivating mutations involve APC / β-catenin pathway. Allelic loss on 11p15.5 is present in 86% cases.113 Infrequently there is diffuse loss of Smad4 protein expression in 22% cases. Somatic mutations in KRAS or TP53 are not reported in pancreatoblastomas.113
Conclusion
Detailed molecular and genetic analyses of pancreatic neoplasms show that each tumor type has its own unique set of genetic aberrations (Table 1), which are important for molecular sub-classification, screening, prognostication and prospective targeted treatment. Some of the mutations occur in very early precursor lesions, suggesting that they may be useful targets in early detection tests, while others are late events, suggesting they may be more suitable for targeted therapies. We are confident that an improved understanding of the important molecular and genetic changes of pancreatic neoplasms will ultimately help improve the diagnosis and management of patients with these tumors.
Acknowledgments
Supported by the NIH SPORE (Specialized Programs of Research Excellence) in Gastrointestinal Cancer Grant P50-CA-62924
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA. Cancer. J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Hruban RH, Pitman MB, Klimstra DS, et al. Tumors of the pancreas. Washington, DC: American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology; 2007. [Google Scholar]
- 3.Hermann PC, Trabulo SM, Sainz B, Jr, et al. Multimodal Treatment Eliminates Cancer Stem Cells and Leads to Long-Term Survival in Primary Human Pancreatic Cancer Tissue Xenografts. PloS One. 2013;8:e66371. doi: 10.1371/journal.pone.0066371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binkley CE, Zhang L, Greenson JK, et al. The molecular basis of pancreatic fibrosis: common stromal gene expression in chronic pancreatitis and pancreatic adenocarcinoma. Pancreas. 2004;29:254–263. doi: 10.1097/00006676-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa T, Klöppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. Int. J. Pathol. 2005;447:794–799. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 6.Murphy SJ, Hart SN, Lima JF, et al. Genetic alterations associated with progression from pancreatic intraepithelial neoplasia to invasive pancreatic tumor. Gastroenterology. 2013;145:1098.e1–1109.e1. doi: 10.1053/j.gastro.2013.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanda M, Matthaei H, Wu J, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730.e9–733.e9. doi: 10.1053/j.gastro.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanda M, Sadakari Y, Borges M, et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin. Gastroenterol. Hepatol. 2013;11:719.e5–730.e5. doi: 10.1016/j.cgh.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hruban RH, Goggins M, Parsons J, et al. Progression Model for Pancreatic Cancer. Clin. Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 10.Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am. J. Surg. Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goggins M, Offerhaus GJ, Hilgers W, et al. Pancreatic adenocarcinomas with DNA replication errors (RER+) are associated with wild-type K-ras and characteristic histopathology. Poor differentiation, a syncytial growth pattern, and pushing borders suggest RER+ Am. J. Pathol. 1998;152:1501–1507. [PMC free article] [PubMed] [Google Scholar]
- 14.Ying H, Kimmelman AC, Lyssiotis CA, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le A, Rajeshkumar NV, Maitra A, et al. Conceptual framework for cutting the pancreatic cancer fuel supply. Clin. Cancer Res. 2012;18:4285–4290. doi: 10.1158/1078-0432.CCR-12-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le A, Cooper CR, Gouw AM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son J, Lyssiotis CA, Ying H, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeNicola GM, Karreth FA, Humpton TJ, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Commisso C, Davidson SM, Soydaner-Azeloglu RG, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S, Wang X, Contino G, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 22.Shen R, Wang Q, Cheng S, et al. The biological features of PanIN initiated from oncogenic Kras mutation in genetically engineered mouse models. Cancer Lett. 2013;339:135–143. doi: 10.1016/j.canlet.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Collins MA, Bednar F, Zhang Y, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J. Clin. Invest. 2012;122:639–653. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 25.Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 26.Mills LD, Zhang Y, Marler RJ, et al. Loss of the transcription factor GLI1 identifies a signaling network in the tumor microenvironment mediating KRAS oncogene-induced transformation. J. Biol. Chem. 2013;288:11786–11794. doi: 10.1074/jbc.M112.438846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 28.Stromnes IM, Brockenbrough JS, Izeradjene K, et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut. 2014 doi: 10.1136/gutjnl-2013-306271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le DT, Jaffee EM. Harnessing immune responses in the tumor microenvironment: all signals needed. Clin. Cancer Res. 2013;19:6061–6063. doi: 10.1158/1078-0432.CCR-13-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellegata NS, Sessa F, Renault B, et al. K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res. 1994;54:1556–1560. [PubMed] [Google Scholar]
- 31.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat. Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 32.Maitra A, Hruban RH. Pancreatic cancer. Annu. Rev. Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharpless NE. INK4a/ARF: a multifunctional tumor suppressor locus. Mutat. Res. 2005;576:22–38. doi: 10.1016/j.mrfmmm.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 34.Wilentz RE, Geradts J, Maynard R, et al. Inactivation of the p16 (INK4A) tumorsuppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res. 1998;58:4740–4744. [PubMed] [Google Scholar]
- 35.Caldas C, Hahn SA, Costa LT da, et al. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat. Genet. 1994;8:27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- 36.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 37.Iacobuzio-Donahue CA, Song J, Parmiagiani G, et al. Missense mutations of MADH4: characterization of the mutational hot spot and functional consequences in human tumors. Clin. Cancer Res. 2004;10:1597–1604. doi: 10.1158/1078-0432.ccr-1121-3. [DOI] [PubMed] [Google Scholar]
- 38.Wilentz RE, Su GH, Dai JL, et al. Immunohistochemical labeling for dpc4 mirrors genetic status in pancreatic adenocarcinomas: a new marker of DPC4 inactivation. Am. J. Pathol. 2000;156:37–43. doi: 10.1016/S0002-9440(10)64703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tascilar M, Skinner HG, Rosty C, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2001;7:4115–4121. [PubMed] [Google Scholar]
- 40.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J. Clin. Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin. Cancer Res. 2009;15:4674–4679. doi: 10.1158/1078-0432.CCR-09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heek NT van, Meeker AK, Kern SE, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am. J. Pathol. 2002;161:1541–1547. doi: 10.1016/S0002-9440(10)64432-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong S-M, Heaphy CM, Shi C, et al. Telomeres are shortened in acinar-to-ductal metaplasia lesions associated with pancreatic intraepithelial neoplasia but not in isolated acinar-to-ductal metaplasias. Mod. Pathol. 2011;24:256–266. doi: 10.1038/modpathol.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod. Pathol. 2002;15:1087–1095. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- 45.Molin M Dal, Matthaei H, Wu J, et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann. Surg. Oncol. 2013;20:3802–3808. doi: 10.1245/s10434-013-3096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann. Surg. 2010;251:470–476. doi: 10.1097/SLA.0b013e3181cf8a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci. Transl. Med. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calhoun ES, Jones JB, Ashfaq R, et al. BRAF and FBXW7 (CDC4, FBW7, AGO, SEL10) mutations in distinct subsets of pancreatic cancer: potential therapeutic targets. Am. J. Pathol. 2003;163:1255–1260. doi: 10.1016/S0002-9440(10)63485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilentz RE, Goggins M, Redston M, et al. Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas: A newly described and characterized entity. Am. J. Pathol. 2000;156:1641–1651. doi: 10.1016/S0002-9440(10)65035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winter JM, Ting AH, Vilardell F, et al. Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin. Cancer Res. 2008;14:412–418. doi: 10.1158/1078-0432.CCR-07-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong S-M, Li A, Olino K, et al. Loss of E-cadherin expression and outcome among patients with resectable pancreatic adenocarcinomas. Mod. Pathol. 2011;24:1237–1247. doi: 10.1038/modpathol.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimura W, Nagai H, Kuroda A, et al. Analysis of small cystic lesions of the pancreas. Int. J. Pancreatol. 1995;18:197–206. doi: 10.1007/BF02784942. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X-M, Mitchell DG, Dohke M, et al. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–553. doi: 10.1148/radiol.2232010815. [DOI] [PubMed] [Google Scholar]
- 54.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. quiz e14–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR. Am. J. Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jong K de, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin. Gastroenterol. Hepatol. 2010;8:806–811. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 57.Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatol. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am. J. Surg. Pathol. 2001;25:26–42. doi: 10.1097/00000478-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann. Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adsay NV, Conlon KC, Zee SY, et al. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62–77. doi: 10.1002/cncr.10203. [DOI] [PubMed] [Google Scholar]
- 62.Wu J, Jiao Y, Molin M Dal, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc. Natl. Acad. Sci. U. S. A. 2011;108:21188–21193. doi: 10.1073/pnas.1118046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kitago M, Ueda M, Aiura K, et al. Comparison of K-ras point mutation distributions in intraductal papillary-mucinous tumors and ductal adenocarcinoma of the pancreas. Int. J. Cancer. J. Int. Cancer. 2004;110:177–182. doi: 10.1002/ijc.20084. [DOI] [PubMed] [Google Scholar]
- 64.Amato E, Molin MD, Mafficini A, et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J. Pathol. 2014;233:217–227. doi: 10.1002/path.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Satoh K, Shimosegawa T, Moriizumi S, et al. K-ras mutation and p53 protein accumulation in intraductal mucin-hypersecreting neoplasms of the pancreas. Pancreas. 1996;12:362–368. doi: 10.1097/00006676-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 66.Lubezky N, Ben-Haim M, Marmor S, et al. High-throughput mutation profiling in intraductal papillary mucinous neoplasm (IPMN) J. Gastrointest. Surg. 2011;15:503–511. doi: 10.1007/s11605-010-1411-8. [DOI] [PubMed] [Google Scholar]
- 67.Schönleben F, Qiu W, Remotti HE, et al. PIK3CA, KRAS, and BRAF mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/C) of the pancreas. Langenbecks Arch. Surg. 2008;393:289–296. doi: 10.1007/s00423-008-0285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawahira H, Kobayashi S, Kaneko K, et al. p53 protein expression in intraductal papillary mucinous tumors (IPMT) of the pancreas as an indicator of tumor malignancy. Hepatogastroenterology. 2000;47:973–977. [PubMed] [Google Scholar]
- 69.Chadwick B, Willmore-Payne C, Tripp S, et al. Histologic, immunohistochemical, and molecular classification of 52 IPMNs of the pancreas. Appl. Immunohistochem. Mol. Morphol. 2009;17:31–39. doi: 10.1097/PAI.0b013e31817c02c6. [DOI] [PubMed] [Google Scholar]
- 70.Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, et al. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am. J. Pathol. 2000;157:755–761. doi: 10.1016/S0002-9440(10)64589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weinstein LS, Liu J, Sakamoto A, et al. Minireview: GNAS: normal and abnormal functions. Endocrinology. 2004;145:5459–5464. doi: 10.1210/en.2004-0865. [DOI] [PubMed] [Google Scholar]
- 72.Gaujoux S, Salenave S, Ronot M, et al. Hepatobiliary and Pancreatic neoplasms in patients with McCune-Albright syndrome. J. Clin. Endocrinol. Metab. 2014;99:E97–E101. doi: 10.1210/jc.2013-1823. [DOI] [PubMed] [Google Scholar]
- 73.Kanda M, Knight S, Topazian M, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut. 2013;62:1024–1033. doi: 10.1136/gutjnl-2012-302823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shinada K, Tsukiyama T, Sho T, et al. RNF43 interacts with NEDL1 and regulates p53-mediated transcription. Biochem. Biophys. Res. Commun. 2011;404:143–147. doi: 10.1016/j.bbrc.2010.11.082. [DOI] [PubMed] [Google Scholar]
- 75.Jiang X, Hao H-X, Growney JD, et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc. Natl. Acad. Sci. U. S. A. 2013;110:12649–12654. doi: 10.1073/pnas.1307218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koo B-K, Spit M, Jordens I, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 77.Yanagisawa A, Kato Y, Ohtake K, et al. c-Ki-ras point mutations in ductectatic-type mucinous cystic neoplasms of the pancreas. Jpn. J. Cancer Res. Gann. 1991;82:1057–1060. doi: 10.1111/j.1349-7006.1991.tb01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jimenez RE, Warshaw AL, Z’graggen K, et al. Sequential accumulation of K-ras mutations and p53 overexpression in the progression of pancreatic mucinous cystic neoplasms to malignancy. Ann. Surg. 1999;230:501–509. doi: 10.1097/00000658-199910000-00006. discussion 509–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sorio C, Capelli P, Lissandrini D, et al. Mucinous cystic carcinoma of the pancreas: a unique cell line and xenograft model of a preinvasive lesion. Virchows Arch. Int. J. Pathol. 2005;446:239–245. doi: 10.1007/s00428-004-1167-1. [DOI] [PubMed] [Google Scholar]
- 80.Tang LH, Aydin H, Brennan MF, et al. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am. J. Surg. Pathol. 2005;29:512–519. doi: 10.1097/01.pas.0000155159.28530.88. [DOI] [PubMed] [Google Scholar]
- 81.Reddy S, Cameron JL, Scudiere J, et al. Surgical management of solid-pseudopapillary neoplasms of the pancreas (Franz or Hamoudi tumors): a large single-institutional series. J. Am. Coll. Surg. 2009;208:950–957. doi: 10.1016/j.jamcollsurg.2009.01.044. discussion 957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meriden Z, Shi C, Edil BH, et al. Hyaline globules in neuroendocrine and solid-pseudopapillary neoplasms of the pancreas: a clue to the diagnosis. Am. J. Surg. Pathol. 2011;35:981–988. doi: 10.1097/PAS.0b013e31821a9a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo Y, Yuan F, Deng H, et al. Paranuclear dot-like immunostaining for CD99: a unique staining pattern for diagnosing solid-pseudopapillary neoplasm of the pancreas. Am. J. Surg. Pathol. 2011;35:799–806. doi: 10.1097/PAS.0b013e318219c036. [DOI] [PubMed] [Google Scholar]
- 84.Kim SA, Kim M-S, Kim M-S, et al. Pleomorphic solid pseudopapillary neoplasm of the pancreas: degenerative change rather than high-grade malignant potential. Hum. Pathol. 2014;45:166–174. doi: 10.1016/j.humpath.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 85.Abraham SC, Klimstra DS, Wilentz RE, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am. J. Pathol. 2002;160:1361–1369. doi: 10.1016/s0002-9440(10)62563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Min Kim S, Sun CD, Park KC, et al. Accumulation of beta-catenin protein, mutations in exon-3 of the beta-catenin gene and a loss of heterozygosity of 5q22 in solid pseudopapillary tumor of the pancreas. J. Surg. Oncol. 2006;94:418–425. doi: 10.1002/jso.20509. [DOI] [PubMed] [Google Scholar]
- 87.Tanaka Y, Kato K, Notohara K, et al. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61:8401–8404. [PubMed] [Google Scholar]
- 88.Chetty R, Serra S. Loss of expression of E-cadherin in solid pseudopapillary tumors of the pancreas. Pancreas. 2009;38:338. doi: 10.1097/MPA.0b013e318183d74a. author reply 338–339. [DOI] [PubMed] [Google Scholar]
- 89.Audard V, Cavard C, Richa H, et al. Impaired E-cadherin expression and glutamine synthetase overexpression in solid pseudopapillary neoplasm of the pancreas. Pancreas. 2008;36:80–83. doi: 10.1097/mpa.0b013e318137a9da. [DOI] [PubMed] [Google Scholar]
- 90.Yao JC, Eisner MP, Leary C, et al. Population-based study of islet cell carcinoma. Ann. Surg. Oncol. 2007;14:3492–3500. doi: 10.1245/s10434-007-9566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Falconi M, Plockinger U, Kwekkeboom DJ, et al. Well-differentiated pancreatic nonfunctioning tumors/carcinoma. Neuroendocrinology. 2006;84:196–211. doi: 10.1159/000098012. [DOI] [PubMed] [Google Scholar]
- 92.Bosman FT, editor. World Health Organization, International Agency for Research on Cancer. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- 93.McCall CM, Shi C, Cornish TC, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am. J. Surg. Pathol. 2013;37:1671–1677. doi: 10.1097/PAS.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chiang HC, O’Dorisio TM, Huang SC, et al. Multiple hormone elevations in Zollinger-Ellison syndrome. Prospective study of clinical significance and of the development of a second symptomatic pancreatic endocrine tumor syndrome. Gastroenterology. 1990;99:1565–1575. doi: 10.1016/0016-5085(90)90459-e. [DOI] [PubMed] [Google Scholar]
- 96.Jonkers YMH, Ramaekers FCS, Speel EJM. Molecular alterations during insulinoma tumorigenesis. Biochim. Biophys. Acta. 2007;1775:313–332. doi: 10.1016/j.bbcan.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 97.Hessman O, Lindberg D, Einarsson A, et al. Genetic alterations on 3p, 11q13, and 18q in nonfamilial and MEN 1-associated pancreatic endocrine tumors. Genes. Chromosomes Cancer. 1999;26:258–264. [PubMed] [Google Scholar]
- 98.Heaphy CM, Wilde RF de, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilde RF de, Heaphy CM, Maitra A, et al. Loss of ATRX or DAXX expression and concomitant acquisition of the alternative lengthening of telomeres phenotype are late events in a small subset of MEN-1 syndrome pancreatic neuroendocrine tumors. Mod. Pathol. 2012;25:1033–1039. doi: 10.1038/modpathol.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Speisky D, Duces A, Bièche I, et al. Molecular profiling of pancreatic neuroendocrine tumors in sporadic and Von Hippel-Lindau patients. Clin. Cancer Res. 2012;18:2838–2849. doi: 10.1158/1078-0432.CCR-11-2759. [DOI] [PubMed] [Google Scholar]
- 102.Schmitt AM, Schmid S, Rudolph T, et al. VHL inactivation is an important pathway for the development of malignant sporadic pancreatic endocrine tumors. Endocr. Relat. Cancer. 2009;16:1219–1227. doi: 10.1677/ERC-08-0297. [DOI] [PubMed] [Google Scholar]
- 103.Rosa S La, Adsay V, Albarello L, et al. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: insights into the morphology and immunophenotype and search for prognostic markers. Am. J. Surg. Pathol. 2012;36:1782–1795. doi: 10.1097/PAS.0b013e318263209d. [DOI] [PubMed] [Google Scholar]
- 104.Klimstra DS, Heffess CS, Oertel JE, et al. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am. J. Surg. Pathol. 1992;16:815–837. doi: 10.1097/00000478-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 105.Jiao Y, Yonescu R, Offerhaus GJA, et al. Whole-exome sequencing of pancreatic neoplasms with acinar differentiation. J. Pathol. 2014;232:428–435. doi: 10.1002/path.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abraham SC, Wu T-T, Hruban RH, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am. J. Pathol. 2002;160:953–962. doi: 10.1016/s0002-9440(10)64917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, et al. Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol. Cancer Ther. 2011;10:3–8. doi: 10.1158/1535-7163.MCT-10-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Williamson CT, Kubota E, Hamill JD, et al. Enhanced cytotoxicity of PARP inhibition in mantle cell lymphoma harbouring mutations in both ATM and p53. EMBO Mol. Med. 2012;4:515–527. doi: 10.1002/emmm.201200229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salman B, Brat G, Yoon Y-S, et al. The diagnosis and surgical treatment of pancreatoblastoma in adults: a case series and review of the literature. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2013;17:2153–2161. doi: 10.1007/s11605-013-2294-2. [DOI] [PubMed] [Google Scholar]
- 111.Bien E, Godzinski J, Dall’igna P, et al. Pancreatoblastoma: a report from the European cooperative study group for paediatric rare tumours (EXPeRT) Eur. J. Cancer Oxf. Engl. 1990. 2011;47:2347–2352. doi: 10.1016/j.ejca.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 112.Esfahani H, Olad E, Dehghan A, et al. Infantile Extrapancreatic Pancreatoblastoma: A Report on a Rare Infantile Abdominal Mass. J. Pediatr. Hematol. Oncol. 2013 doi: 10.1097/MPH.0b013e3182a0627f. [DOI] [PubMed] [Google Scholar]
- 113.Abraham SC, Wu TT, Klimstra DS, et al. Distinctive molecular genetic alterations in sporadic and familial adenomatous polyposis-associated pancreatoblastomas: frequent alterations in the APC/beta-catenin pathway and chromosome 11p. Am. J. Pathol. 2001;159:1619–1627. doi: 10.1016/s0002-9440(10)63008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]