Abstract
Previous studies have found inconsistent results from testing methods used to measure heterotrophic plate count (HPC) bacteria in dental unit waterline (DUWL) samples. This study used 63 samples to compare the results obtained from an in-office chairside method and 2 currently used commercial laboratory HPC methods (Standard Methods 9215C and 9215E). The results suggest that the Standard Method 9215E is not suitable for application to DUWL quality monitoring, due to the detection of limited numbers of heterotrophic organisms at the required 35°C incubation temperature. The results also confirm that while the in-office chairside method is useful for DUWL quality monitoring, the Standard Method 9215C provided the most accurate results.
The water that is supplied via dental unit waterline (DUWL) tubing to air/water syringes, handpieces, and ultrasonic scalers in a typical dental unit is fed directly from the main water supply or via a self-contained reservoir on the dental unit itself. DUWL tubing typically is 2 mm in diameter and made of either polyvinyl chloride or polyurethane. This tubing forms a complex network inside a dental unit, resulting in a high ratio of tubing surface area to water volume.1 These factors, along with the periodic pooling of stagnant water inside the tubing, facilitate an ideal environment for bacterial growth and biofilm formation (up to 50μ thick) comprised of a heterogeneous population of organisms.2,3 Microorganisms from the biofilm are continuously shed as the water flows through the DUWL tubing, resulting in microbial contamination of the patient treatment water.4
The Centers for Disease Control and Prevention (CDC) recommends that the water used in dental offices should meet the drinking water standard established by the US Environmental Protection Agency (EPA) of <500 colony forming units per milliliter (CFU/ml) for routine dental treatment output water.5,6 In order for dental practitioners to comply with these guidelines, DUWL monitoring should be performed as recommended by the dental unit manufacturers.5 Waterline monitoring can be done in-office with chairside kits or via commercial laboratories. The purpose of monitoring is to measure the heterotrophic (organisms that use a carbon source for survival) plate count (HPC) of DUWL samples.
Currently, there is only 1 in-office, chairside kit available: the HPC Sampler (EMD Millipore), consisting of a removable dip paddle contained in a plastic sampler. The dip paddle contains a 0.45μ filter and an absorbent pad with dehydrated agar medium which absorbs 1 ml of the liquid sample, facilitating the recovery of stressed (that is, partially sanitized or nutritionally starved) aerobic bacteria in 7 days. According to the manufacturer, this method can produce accurate readings up to 300 CFU/ml; all counts >300 CFU/ml are considered too numerous to count (TNTC).7 There is evidence from previous studies to show that, although the HPC Sampler underestimates bacterial counts compared with other methods, it is useful as a screening tool for regular DUWL quality monitoring in dental offices to ensure the water used in the treatment of patients meets the CDC/EPA recommendation of <500 CFU/ml.8-10
Dental offices can also utilize services offered by commercial laboratories for a more accurate assessment of water quality. Waterline samples are collected and mailed using kits that are supplied to offices by the commercial entities. Standard laboratory methods, as published in the “Standard Methods for the Examination of Water and Wastewater”(hereafter referred to as Standard Methods), are recommended by the American Public Health Association, American Water Works Association, and Water Environment Federation.11 The list includes 4 standard methods and 5 types of media for use in different combinations as appropriate for testing purposes.11
Standard Method 9215C (a spread plate method on R2A medium) has long been considered the gold standard for analysis of DUWL quality.12 This procedure, using a low nutrient R2A formulation (Becton, Dickson & Company) and room temperature incubation for 7 days, was designed for the detection of common water organisms. The disadvantages of this method are that it is time-consuming to prepare the R2A and it relies on a small volume of liquid (0.1 ml), which can become quickly absorbed if the agar dries out.11
Standard Method 9215E (SimPlate for HPC, IDEXX Laboratories, Inc.) is a user-friendly method that has been included in the list of Standard Methods in recent years.11 A proprietary enzyme substrate is mixed with the water sample, and as bacteria metabolize the substrate they fluoresce after 48 hours of incubation at 35°C. The number of fluorescent wells are counted and converted to the most probable number (MPN), using a table provided by the manufacturers. The maximum MPN/ml recorded from an undiluted sample is 73.8; for more highly contaminated water samples, 10-fold serial dilutions can be used.13 Since its introduction as a Standard Method, SimPlate for HPC has become widely used in commercial laboratories, and dental offices that use a mail-in laboratory service may be obtaining their results from this method.
A previous study by the authors found that bacterial counts were underestimated on the SimPlate for HPC compared to R2A agar.14 The purpose of this experiment was to expand on those findings and to compare bacterial counts and genera from all 3 currently available monitoring methods: the spread plate R2A (Method 9215C), the SimPlate for HPC (Method 9215E), and the in-office HPC Sampler.
Materials and methods
The experiment was designed to collect an approximately uniform distribution of water sample contamination based on 3 source types and 7 exposure durations yielding a total of 63 waterline samples. Sterile collection bottles (100 ml), each containing sodium thiosulfate to neutralize residual chlorine (IDEXX Laboratories, Inc.) were used to collect samples from the handpiece lines, the air/water syringe lines, and the source tap water in 21 randomly selected dental operatories in a teaching institution.
Each sample was cultured on HPC Sampler, R2A agar (Method 9215C), and SimPlate for HPC (Method 9215E) according to manufacturer-recommended methods. The pH of the source tap water and the residual free chlorine level (mg/l) were tested before the experiment and were found to be 7.2 and 0.5mg/l, respectively. These levels were assumed to remain constant as previous readings in the institution had shown this to be the norm.15
Sample cultures
All laboratory procedures were conducted by 1 laboratory technician. A 10-fold serial dilution of each sample was made with phosphate buffer solution.
For Method 9215C, 0.1 ml of each sample was spread on R2A plates in triplicate, incubated at room temperature, and the microbial CFU/ml was recorded after 7 days.11
For Method 9215E, 10 ml of each solution were placed in the center of the SimPlates and the manufacturer’s instructions were followed. Plates were incubated for 48 hrs at 35°C, and the MPN/ml was calculated.16 Following the calculation of MPN/ml, liquid was collected (using an inoculating loop) from randomly selected fluorescent wells, then spread on R2A plates and incubated at room temperature for 7 days to prepare isolates for molecular identification.
For HPC Sampler cultures, an undiluted 10 ml sample was placed in the outer sheath, and the dip paddle was replaced for 30 seconds until 1 ml was absorbed. The remainder of the DUWL sample was discarded and the Sampler was incubated at room temperature for 7 days, at which point CFU/ml were recorded using the comparison chart provided by the manufacturer.7
Molecular identification
A selection of HPC Samplers and R2A plates with the largest bacterial colonies was transported to the Department of Microbiology at the University of Texas Health Science Center at San Antonio (UTHSCSA) for molecular identification, and a sequence-based approach using the 16s ribosomal DNA regions as targets for the molecular identification isolates was performed.17
DNA isolation
Isolates were suspended in 600 μl cell lysis buffer (blood Maxwell LEV kit, Promega Corporation) in a 0.5 ml microfuge tube. The suspension was bead-beaten for 45 seconds to 1 minute to aid in cell wall breakdown. The suspension was then pelleted for 3 minutes at maximum speed in a microfuge according to the manufacturer’s instructions. The supernatant was transferred to the Maxwell LEV cartridge and then mounted on the automated Maxwell system, resulting in 150 ng/μl of purified bacterial DNA after a 45-minute run.
Polymerase chain reaction
Polymerase chain reactions (PCR) were performed directly on 3 μl of the DNA supernatant in a 50 μl reaction using a 5 prime PCR Extender system (Thermo Fisher Scientific, Inc.), according to the manufacturer’s instructions. 16s amplicons were obtained using primers 27F and 1525R. Amplifications were performed in a PTC-100 thermocycler (MJ Research, Inc.) using the preprogrammed, 3-step protocol as the standard program for all reactions, consisting of 35 cycles using an annealing temperature of 55°C and 1 minute extension time. A 5 μl aliquot of the PCR reaction was run on a 0.7% agarose gel and stained with ethidium bromide to confirm amplification. The remaining PCR reaction (45 μl) was run on a gel as described above, then purified using the Wizard SV Gel and PCR Clean-Up System (Promega Corporation), eluted in 30 μl sterile water according to the manufacturer’s instructions, and incubated with proteinase K at 56°C for 15 minutes.
Sequencing
DNA obtained from the PCR reaction was prepared for sequencing by cleaning with a Qiaprep Spin Miniprep Kit (Qiagen Sciences, Inc.) according to manufacturer’s instructions. The purified DNA was sequenced at the UTHSCSA Advanced Nucleic Acids Core facility. Sequences were then used to perform individual nucleotide-nucleotide searches of the ribosomal 16s region using the BLASTn algorithm at the National Center for Biotechnology Information website.18 Identifications were calculated based on a percentage made from the alignment matches obtained from the top 3 BLAST searches for the 16s region to yield a variety level identification. The 3 highest percent identities for each isolate were analyzed for bacterial identification.
Statistical analysis
For the 3 types of detection methods, all possible pairwise Pearson and/or Spearman correlation coefficients with corresponding 95% confidence intervals were performed to determine if any significant association was observed among the 3 measurement methods, with log transformations performed if appropriate. Correlations were performed for all waterline samples and, if appropriate, separately for each waterline sample source type. Statistical analyses and graphics were performed using Stata 13.0 (StataCorp LP).
Results
As expected, the R2A measures approximated an exponential distribution; however, the SimPlate for HPC values approximated a uniform distribution ranging from a minimum of 0.4 MPN/ml to the maximum 73.8 MPN/ml, followed by 12 (19%) samples with unspecified values >73.8 MPN/ml, as the corresponding 110 dilution plates did not provide any results. There were also 4 samples based on a 110 dilution that had values ranging from 112 to 440 MPN/ml and 1 handpiece sample that could not be assayed due to technical error. For the purposes of graphs and correlations, an arbitrary value of 80 MPN/ml was used to represent all SimPlate for HPC values >73.8 MPN/ml.
Results for each of the methods can be seen in Table 1. The HPC Sampler detection method showed that 46 (73%) of the dip paddle surfaces were entirely covered with TNTC small microbial colonies. For 2 handpiece samples, no HPC counts were detected; otherwise, all handpiece and air/water samples counts were TNTC. For the purposes of graphs and correlations, an arbitrary value of 1,000 CFU/ml was used to represent TNTC results, and the 2 handpiece samples for which the HPC Sampler failed to detect CFUs were excluded as having implausible results. Specific HPC counts were detected for 14 of 21 source water samples. Due to the characteristics of the distribution of HPC measures, correlations were performed for all samples and for source water samples only (Table 2).
Table 1.
HPC from DUWL samples
| R2A agar |
SimPlate |
HPC sampler |
||
|---|---|---|---|---|
| Dental unit |
Sample source | CFU/ml | MPN/ml | CFU/ml |
| 1 | Handpiece | 61,300 | 26.6 | 1000a |
| Air/water | 87,000 | 23.9 | 1000 | |
| Source water | 1,130 | 23.1 | 59 | |
|
| ||||
| 2 | Handpiece | 494,000 | 23.1 | 1000 |
| Air/water | 102,000 | 37.2 | 1000 | |
| Source water | 293 | 2.1 | 12 | |
|
| ||||
| 3 | Handpiece | 142,000 | 41.4 | 1000 |
| Air/water | 6,570 | 26.6 | 1000 | |
| Source water | 8,430 | 29.9 | 1000 | |
|
| ||||
| 4 | Handpiece | 138,000 | 31.1 | 1000 |
| Air/water | 392,000 | 47.0 | 1000 | |
| Source water | 86.7 | 0.4 | 33 | |
|
| ||||
| 5 | Handpiece | 341,000 | 55.5 | 1000 |
| Air/water | 235,000 | 44.0 | 1000 | |
| Source water | 1,470 | 1.9 | 360 | |
|
| ||||
| 6 | Handpiece | 252,000 | 68.0 | 1000 |
| Air/water | 284,000 | 50.7 | 1000 | |
| Source water | 1,850 | 27.6 | 1000 | |
|
| ||||
| 7 | Handpiece | 642,000 | 62.3 | 0 |
| Air/water | 573,000 | 73.8 | 1000 | |
| Source water | 327 | 73.8 | 1000 | |
|
| ||||
| 8 | Handpiece | 361,000 | 239.0 | 1000 |
| Air/water | 535,000 | 62.3 | 1000 | |
| Source water | 73.3 | 1.0 | 20 | |
|
| ||||
| 9 | Handpiece | 178,000 | 47.0 | 1000 |
| Air/water | 39,900 | 80.0b | 1000 | |
| Source water | 10 | 0.6 | 1 | |
|
| ||||
| 10 | Handpiece | 151,000 | 80.0b | 1000 |
| Air/water | 323,000 | 55.5 | 1000 | |
| Source water | 24,600 | 33.9 | 1000 | |
|
| ||||
| 11 | Handpiece | 243,000 | 41.4 | 1000 |
| Air/water | 176,000 | 39.2 | 1000 | |
| Source water | 27,700 | 47.0 | 1000 | |
|
| ||||
| 12 | Handpiece | 543,000 | 80.0b | 0 |
| Air/water | 47,300 | 80.0b | 1000 | |
| Source water | 133 | 15.1 | 39 | |
|
| ||||
| 13 | Handpiece | 411,000 | 55.5 | 1000 |
| Air/water | 244,000 | 80.0b | 1000 | |
| Source water | 387 | 0.2 | 86 | |
|
| ||||
| 14 | Handpiece | 795,000 | 257.0 | 1000 |
| Air/water | 722,000 | 37.2 | 1000 | |
| Source water | 213 | 1.0 | 151 | |
|
| ||||
| 15 | Handpiece | 233,000 | 73.8 | 1000 |
| Air/water | 362,000 | 62.3 | 1000 | |
| Source water | 417 | 2.6 | 73 | |
|
| ||||
| 16 | Handpiece | 8,370 | 62.3 | 1000 |
| Air/water | 139,000 | 80.0b | 1000 | |
| Source water | 76.7 | 47.0 | 39 | |
|
| ||||
| 17 | Handpiece | 115,000 | 80.0b | 1000 |
| Air/water | 101,000 | 80.0b | 1000 | |
| Source water | 2,390 | 73.8 | 600 | |
|
| ||||
| 18 | Handpiece | 33,400 | NA | 1000 |
| Air/water | 70,700 | 112.0 | 1000 | |
| Source water | 283 | 37.2 | 216 | |
|
| ||||
| 19 | Handpiece | 42,300 | 80.0b | 1000 |
| Air/water | 13,100 | 80.0b | 1000 | |
| Source water | 527 | 73.8 | 116 | |
|
| ||||
| 20 | Handpiece | 343,000 | 440.0 | 1000 |
| Air/water | 226,000 | 80.0b | 1000 | |
| Source water | 367 | 13.2 | 36 | |
|
| ||||
| 21 | Handpiece | 91,300 | 55.5 | 1000 |
| Air/water | 57,700 | 80.0b | 1000 | |
| Source water | 51,300 | 73.8 | 1000 | |
1000 CFU/ml
MPN >73.8/ml
Abbreviations: CFU, colony forming units; DUWL, dental unit waterline; HPCs, heterotrophic plate counts; MPN, most probable number; NA, not available.
Table 2.
Correlation coefficients (95% confidence interval).
| Pearson | Spearman | |
|---|---|---|
| Sample source |
R2A with SimPlate for HPC
|
|
| All | 0.607 (0.421, 0.744) | 0.475(0.256, 0.648) |
| Handpiece | 0.103 (−0.356, 0.522) | 0.174 (−0.291, 0.572) |
| Air/water | −0.068 (−0.486, 0.375) | −0.268 (−0.627, 0.185) |
| Source water | 0.481 (0.062, 0.756) | 0.521 (0.115, 0.778) |
|
Millipore with SimPlate for HPC
|
||
| All | 0.650 (0.474, 0.776) | 0.598 (0.406, 0.740) |
| Source water | 0.573 (0.188, 0.805) | 0.624 (0.263, 0.832) |
|
R2A with Millipore
|
||
| All | 0.871 (0.793, 0.921) | 0.734 (0.592, 0.832) |
| Source water | 0.795 (0.554, 0.913) | 0.797 (0.557, 0.914) |
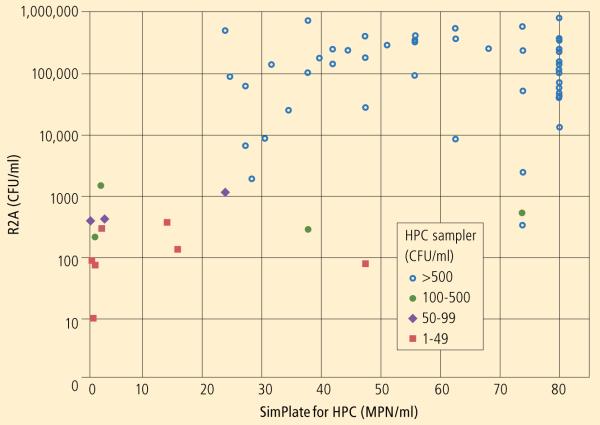
For the R2A with SimPlate for HPC, the overall Pearson correlation coefficient of 0.607 was moderate, while the corresponding Spearman rank correlation coefficient of 0.475 was lower. Correlations for each source type showed similar results for source water samples and poorer results for handpiece and air/water samples. To depict the pairwise association, a scatterplot was generated displaying the paired results for each sample with symbols indicating the source type (Chart).
Chart.
R2A with SimPlate for HPC method scatterplot, displaying legend markers indicating the range of the corresponding HPC Sampler value for each sample.
Note that 2 handpiece samples for which no CFUs were detected by HPC Sampler were excluded. All samples with R2A >1500 CFU/ml had HPC Sampler values >500 CFU/ml, while only 1 sample with R2A <1500 CFU/ml had an HPC Sampler value >500 CFU/ml.
For the HPC Sampler with SimPlate for HPC, the overall Pearson correlation coefficient of 0.650 was significantly lower than the overall Pearson correlation coefficient of 0.871 for SimPlate for HPC with R2A. Similarly, the corresponding Spearman correlation for HPC Sampler with R2A was higher than that for SimPlate with R2A, but the 2 Spearman correlations were not significantly different.
When restricted to the source water samples, the HPC Sampler with SimPlate for HPC Pearson correlation coefficient of 0.573 was significantly lower than the HPC sampler with R2A coefficient of 0.795, while the corresponding Spearman correlations were not significantly different.
Recovered microorganisms
As seen in Table 3, 16 genera of bacteria were recovered. The most commonly occurring genus was Sphingomonas, and only 2 species—Cupriavidus metallidurans and Sphingomonas parapaucimobilis—were found on all 3 culture media. Micrococcus luteus was the only gram-positive species found. All other recovered bacteria were gram-negative.
Table 3.
Bacteria recovered from DUWL samples.
| Acidovorax sp. a |
| Acidovoraxcitrulli |
| Acidovoraxtemperans |
| Afipia sp. |
| Blastomonas natatoria |
| Bradyrhizobium sp. |
| Bradyrhizobium yuanmingens |
| Caulobacter segnis |
| Cupriavidus basilensis |
| Cupriavidus metallidurans b |
| Methylobacterium extorquens |
| Methylobacterium oryzae |
| Methylobacterium populi |
| Methylobacterium radiotolerans |
| Methylobacterium rhodesianum |
| Methylobacterium thiocyanatum |
| Micrococcus luteus |
| Novosphingobium stygium |
| Pseudomonas koreen c |
| Pseudomonas libane c |
| Ralstonia sp. |
| Sphingobium sp. |
| Sphingobium amiense |
| Sphingomonas sp. |
| Sphingomonas adhaesiva |
| Sphingomonas parapaucimobilis b |
| Sphingomonas sanguinis |
| Sphingomonas trueperi |
| Sphingomonas yunnanensis |
| Sphingopyxis alaskensis |
| Sphingopyxis chilensis |
| Xenophilus aerolatus |
| Xulophilus ampelinus |
Grown on R2A and Millipore HPC Samplers
Grown on all 3 media
Grown on Millipore HPC Samplers only
No symbol: grown on R2A agar only
Discussion
This article describes an evaluation of 3 currently available methods for monitoring HPC bacteria in DUWLs. The SimPlate for HPC (Method 9215E) recovered the lowest numbers of microorganisms and the highest readings were found on spread plate R2A (Method 9215C), although it must be noted that all HPC methods enumerate only a fraction of microorganisms in any water sample and no single method will recover all genera.19 The overall results are not altogether unexpected, since the media composition and incubation parameters were specifically designed to recover different microbial populations.
Statistical analysis showed moderate correlations between Method 9215E and the other 2 methods, while Method 9215C and HPC Samplers had high correlations. Correlations based on source tap water samples involved fewer arbitrary approximation values, resulting in a decrease in Pearson correlations and an increase in Spearman correlations.
Unlike the other 2 laboratory methods, serial dilutions of samples were not done prior to culturing on HPC Samplers due to its purposeful design as an in-office, chairside monitoring device. As stated earlier, previous studies have shown that HPC Samplers underestimate bacterial counts when compared to the spread plate R2A agar method, and some have attributed this to its failure to grow certain phenotypes.20,21 The results of this study concur with those findings. However, this study also confirms the high sensitivity of the HPC Samplers, as microbial counts on the majority of the paddles were TNTC and 5 different species of bacteria were detected. For the 2 handpiece samples in which zero bacterial growth was recovered on HPC Samplers, a plausible explanation may be variation among kits, as it was unlikely due to laboratory error (based on the reliability of the standard laboratory methods employed).
The spread plate R2A agar (Method 9215C) has long been considered the gold standard for application to DUWL monitoring with the advantage of producing a true assessment of HPC contamination levels. In this study, accurate counts were obtained using serial dilutions, and 14 different genera of bacteria were detected on R2A plates.
The inclusion of SimPlate for HPC (Method 9215E) in the list of the Standard Methods endorses its use for analysis of drinking water and source water sampes.11 It is recommended as an alternative to the pour plate method (9215A), which uses high nutrient plate count agar to test for general EPA compliance monitoring; studies have demonstrated good correlation between the 2 methods.11,13,16 Both 9215A and 9215E methods require incubation periods of 48 hours at mammalian physiological incubation temperature (35°C), favoring the growth of bacteria from human and animal wastes.22 However, a previous study showed that Method 9215E showed lower microbial counts when compared to the membrane filter method (9215D), which utilizes low nutrient R2A agar and incubation periods of 48 hours at 22°C-28°C.13 Lower incubation temperature (22°C-28°C), along with a longer incubation time favor the growth of indigenous aquatic bacteria.22 SimPlate for HPC was a method designed for higher incubation temperatures, and the results of this study add to the body of existing scientific evidence showing that Method 9215E underestimates microbial contamination at 22°C-28°C.13,14,16
Significance of microorganisms recovered
Culture plates that were selected for organism identification were based on recovered colony size, so the number of recovered organisms represents a mere snapshot of the total bacterial population. Not surprisingly, due to the limited number of microorganisms isolated on SimPlate for HPC, only 2 bacterial species were identified. However, it must also be noted that this method is not designed for recovering particular organisms, as stated in the Standard Methods.11
One gram-positive organism was identified on R2A: M. luteus, which is ubiquitously found in soil, dust, air, and water. Cases of infective endocarditis due to M. luteus have been reported in the literature.23 All other microorganisms were gram-negative, which are known to have lipopolysaccharide molecules (endotoxins) in their cell wall that can trigger inflammatory responses in humans. Several studies have reported a significant association between the presence and severity of asthma and a raised concentration of airborne gram-negative bacteria in the indoor environment.24 A significant correlation between endotoxin levels and high bacterial load in DUWLs has also been reported.25
Two species of Pseudomonas isolated on the HPC Samplers in this study have previously been recovered from DUWLs and reported as the causative organisms of postoperative dental infections and respiratory infections in immunocompromised patients.26,27
Only 2 bacterial types were common to the 3 culture methods tested in the study: Cupriavidus metallidurans and Sphingomonas parapaucimobilis; these were the only bacterial species recovered on SimPlate for HPC, verifying the limitations of this culture method for detection of common water organisms. C. metallidurans belongs to the α-Proteobacteria group, known to be the predominant survivor in chlorinated water distribution systems.28,29
The most frequently isolated genera in this study were Sphingomonas, also closely aligned with the phylogenic group α-Proteobacteria, and previously found in DUWL samples and ultrapure water.2,15,29,30 A review of nosocomial infections concluded that the species S. parapaucimobilis has emerged in recent years as an opportunistic pathogen as it has been associated with many cases of bacteremia and other systemic infections in immunocompromised patients.31
Conclusion
The variety of potentially pathogenic organisms recovered from waterlines in this study reinforces the need for monitoring DUWL quality to ensure the delivery of high quality dental patient treatment water.
The study confirmed that Millipore HPC Samplers are useful for routine in-office, chairside DUWL quality monitoring when the benchmark CDC recommended level of <500 CFU/ml is used. The study also confirmed that the spread plate R2A agar method (9215C) provides the most accurate analysis of DUWL quality.
The laboratory SimPlate for HPC method (9215E) failed to detect microbial contamination of DUWL samples to the same extent as Method 9215C, most likely due to the specific design of SimPlate for HPC for the recovery of fast-growing organisms at 35°C. While Method 9215E clearly has value for application in EPA compliance monitoring, this study found that it is not acceptable for application in DUWL quality monitoring, when quantification of slow-growing water organisms at 22°C-28°C and a correct assessment of dental patient treatment water quality are required.
Dental offices can reliably use in-office, chairside Millipore HPC Samplers to screen DUWL quality, ensuring that patient treatment water is compliant with the EPA/CDC recommendation of <500 CFU/ml. However, for offices that rely on commercial laboratories to provide an accurate assessment of their DUWL quality, it is recommended that the spread plate R2A method (9215C) be requested, rather than the SimPlate for HPC method (9215E), when DUWL samples are submitted for analysis.
Manufacturers.
Becton, Dickson & Company, Franklin Lakes, NJ
888.237.2862, www.bd.com
EMD Millipore, Billerica MA
781.533.6000, www.emdmillipore.com
IDEXX Laboratories, Inc., Westbrook, ME
800.548.6733, www.idexx.com
MJ Research, Inc., St. Bruno, Quebec, Canada
450.461.6245, mj-research.com
Promega Corporation, Madison, WI
608.274.4330, www.promega.com
Qiagen Sciences, Inc., Germantown, MD
240.686.7700, www.qiagen.com
StataCorp LP, College Station, TX
800.782.8272, www.stata.com
Thermo Fisher Scientific, Inc., Waltham, MA
800.678.5599, www.thermofisher.com
Acknowledgments
Research reported in this article was supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health (NIH) under Award Number R01 DE018707-05. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Drs. Porteous and Sun are co-principal investigators and Mr. Schoolfield is a co-investigator.
The authors wish to thank Monica Herrera, MD, research associate, Department of Microbiology, UTHSCSA, for her work on molecular analysis of organisms at the time of this study.
Footnotes
Disclaimer
The authors have no financial, economic, commercial, and/or professional interests related to topics presented in this article.
Contributor Information
Dr. Nuala Porteous, Department of Comprehensive Dentistry, University of Texas Health and Science Center at San Antonio (UTHSCSA).
Yuyu Sun, Department of Chemistry, University of Massachusetts in Lowell..
Mr. John Schoolfield, Department of Periodontics..
References
- 1.Mills SE. The dental unit waterline controversy: defusing the myths, defining the solutions. J Am Dent Assoc. 2000;131(10):1427–1441. doi: 10.14219/jada.archive.2000.0054. [DOI] [PubMed] [Google Scholar]
- 2.Szymanska J. Bacterial contamination of water in dental unit reservoirs. Ann Agric Environ Med. 2007;14(1):137–140. [PubMed] [Google Scholar]
- 3.Cunningham AB, Lennox JE, Ross RJ, editors. [Accessed November 7, 2014];Biofilm growth and development. Biofilms: The Hypertextbook. Available at: http://www.biofilmbook.com.
- 4.Lenz AP, Williamson KS, Pitts B, Stewart PS, Franklin MJ. Localized gene expression in Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2008;74(14):4463–4471. doi: 10.1128/AEM.00710-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings – 2003. MMWR Recomm Rep. 2003;52(RR17):1–61. [PubMed] [Google Scholar]
- 6.United States Environmental Protection Agency [Accessed November 7, 2014];National Primary Drinking Water Regulations. Available at: http://www.epa.gov/safewater/contaminants/index.html.
- 7. [Accessed November 7, 2014];EMD Millipore [product guide] Available at: http://www.millipore.com/catalogue/module/C10712.
- 8.Momeni SS, Tomline N, Ruby JD, Dasanayake AP. Evaluation of in-office dental unit waterline testing. Gen Dent. 2012;60(3):142–147. [PubMed] [Google Scholar]
- 9.Morris BF, Vandewalle KS, Hensley DM, Bartoloni JA. Comparison of in-office dental unit waterline test kits. Mil Med. 2010;175(11):901–906. doi: 10.7205/milmed-d-10-00032. [DOI] [PubMed] [Google Scholar]
- 10.Bartoloni JA, Porteous NB, Zarzabal LA. Measuring the validity of two in-office water test kits. J Am Dent Assoc. 2006;137(3):363–371. doi: 10.14219/jada.archive.2006.0186. [DOI] [PubMed] [Google Scholar]
- 11.American Water Works Association. American Public Health Association. Water Environment Federation . Microbiological examination. In: Rice EW, Baird RB, Eaton AD, Clesceri LS, editors. Standard Methods for the Examination of Water and Wastewater. 22nd ed Washington, DC: 2012. pp. 9.49–9.52. [Google Scholar]
- 12.De Paola LG, Mangan D, Mills SE, et al. A review of the science regarding dental unit waterlines. J Am Dent Assoc. 2002;133(9):1199–206. doi: 10.14219/jada.archive.2002.0361. quiz 1260. [DOI] [PubMed] [Google Scholar]
- 13.Stillings A, Herzig D, Roll B. Comparative Assessment of the Newly-Developed Simplate™ Method With the Existing EPA-Approved Pour Plate Method for the Detection of Heterotrophic Plate Count Bacteria in Ozone-Treated Drinking Water. International Ozone Association Conference; October 1998; [Accessed November 10, 2014]. Available at: https://www.idexx.com/resource-library/water/water-reg-article8B.pdf. [Google Scholar]
- 14.Porteous N, Sun Y, Dang S, Schoolfield J. A comparison of 2 laboratory methods to test dental unit waterline water quality. Diag Microbiol Infect Dis. 2013;77(3):206–208. doi: 10.1016/j.diagmicrobio.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porteous N, Luo J, Hererra M, Schoolfield J, Sun Y. Growth and identification of bacteria in N-halamine dental unit waterline tubing using an ultrapure water source. Int J Microbiol. 2011;767314 doi: 10.1155/2011/767314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson RW, Osborne K, Barnes G, et al. Multiregional evaluation of the SimPlate heterotrophic plate count method compared to the standard plate count agar pour plate method in water. Appl Environ Microbiol. 2000;66(1):453–454. doi: 10.1128/aem.66.1.453-454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol. 2008;74(8):2461–2470. doi: 10.1128/AEM.02272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Biotechnology Information [Accessed November 10, 2014];Blast: Basic Local Alignment Search Tool. Available at: http://blast.ncbi.nlm.nih.gov/Blast.cgi.
- 19.Allen MJ, Edberg SC, Reasoner DJ. Heterotrophic plate count bacteria—what is their significance in drinking water? Int J Food Microbiol. 2004;92(3):265–274. doi: 10.1016/j.ijfoodmicro.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Smith RS, Pineiro SA, Singh R, Romberg E, Labib ME, Williams HN. Discrepancies in bacterial recovery from dental unit water samples on R2A medium and a commercial sampling device. Current Microbiol. 2004;48(4):243–246. doi: 10.1007/s00284-003-4130-5. [DOI] [PubMed] [Google Scholar]
- 21.Cohen ME, Harte JA, Stone ME, O’Connor KH, Coen ML, Cullum ME. Statistical modeling of dental unit water bacterial test kit performance. J Clin Dent. 2007;18(2):39–44. [PubMed] [Google Scholar]
- 22.Reasoner DJ. Heterotrophic plate count methodology in the United States. Int J Food Microbiol. 2004;92(3):307–315. doi: 10.1016/j.ijfoodmicro.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Miltiadous G, Elisaf M. Native valve endocarditis due to Micrococcus luteus: a case report and review of the literature. J Med Case Rep. 2011;5:251. doi: 10.1186/1752-1947-5-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rennie DC, Lawson JA, Kirychuk SP, et al. Assessment of endotoxin levels in the home and current asthma and wheeze in school-age children. Indoor Air. 2008;18(6):447–453. doi: 10.1111/j.1600-0668.2008.00543.x. [DOI] [PubMed] [Google Scholar]
- 25.Huntington MK, Williams JF, Mackenzie CD. Endotoxin contamination in the dental surgery. J Med Microbiol. 2007;56(Pt 9):1230–1234. doi: 10.1099/jmm.0.47231-0. [DOI] [PubMed] [Google Scholar]
- 26.Martin MV. The significance of the bacterial contamination of dental unit water systems. Br Dent J. 1987;163(5):152–154. doi: 10.1038/sj.bdj.4806220. [DOI] [PubMed] [Google Scholar]
- 27.Langton Hewer SC, Smyth AR. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst Rev. 2009;4 doi: 10.1002/14651858.CD004197.pub5. CD004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Rozycki T, Nies DH. Cupriavidus metallidurans: evolution of a metal-resistant bacterium. Antonie van Leeuwenhoek. 2009;96(2):115–139. doi: 10.1007/s10482-008-9284-5. [DOI] [PubMed] [Google Scholar]
- 29.Williams MM, Domingo JW, Meckes MC, Kelty A, Rochon HS. Phylogenic diversity of drinking water bacteria in a distribution system simulator. J Appl Microbiol. 2001;96(5):954–964. doi: 10.1111/j.1365-2672.2004.02229.x. [DOI] [PubMed] [Google Scholar]
- 30.Oie S, Oomaki M, Yorioka K, et al. Microbial contamination of ‘sterile water’ used in Japanese hospitals. J Hosp Infect. 1998;38(1):61–65. doi: 10.1016/s0195-6701(98)90175-x. [DOI] [PubMed] [Google Scholar]
- 31.Ryan MP, Adley CC. Sphingomonas paucimobilis: a persistent Gram-negative nosocomial infectious organism. J Hosp Infect. 2010;75(3):153–157. doi: 10.1016/j.jhin.2010.03.007. [DOI] [PubMed] [Google Scholar]