Abstract
Background
Many clinicians believe that statins cause muscle pain, but this has not been observed in clinical trials and the effect of statins on muscle performance has not been carefully studied.
Methods and Results
The Effect of STatins On Skeletal Muscle Function and Performance (STOMP) study assessed symptoms and measured creatine kinase (CK), exercise capacity, and muscle strength before and after atorvastatin 80 mg or placebo were administered for 6 months to 420 healthy, statin-naive subjects. No individual CK value exceeded 10 times normal, but average CK increased 20.8 ± 141.1 U/L (p<0.0001) with atorvastatin. There were no significant changes in several measures of muscle strength or exercise capacity with atorvastatin, but more atorvastatin than placebo subjects developed myalgia (19 vs 10; p = 0.05). Myalgic subjects on atorvastatin or placebo decreased muscle strength in 5 of 14 and 4 of 14 variables respectively (p = 0.69).
Conclusions
These results indicate that high-dose atorvastatin for 6 months does not decrease average muscle strength or exercise performance in healthy, previously untreated subjects. Nevertheless, this blinded, controlled trial confirms the undocumented impression that statins increase muscle complaints. Atorvastatin also increased average CK suggesting that statins produce mild muscle injury even among asymptomatic subjects. This increase in CK should prompt studies examining the effects of more prolonged, high-dose statin treatment on muscular performance.
Clinical Trial Registration Information: www.clinicaltrials.gov; Identifier: NCT00609063.
Keywords: atorvastatin, statins, muscle strength, aerobic capacity, myalgia
INTRODUCTION
Hydroxy-methyl-glutaryl (HMG) CoA reductase inhibitors or statins are the most effective medications for reducing elevated concentrations of low-density lipoprotein cholesterol (LDL-C) and produce remarkable reductions in cardiovascular events.1 Statins can produce life-threatening rhabdomyolysis, but this is rare.2 Statins are more frequently associated with mild muscle complaints including myalgia, cramps and weakness which may compromise medication compliance and quality of life. The reported incidence of myalgia during statin therapy ranges from 1% in controlled studies 3 to 25% 4 in clinical reports. Muscle weakness has also been reported with statin therapy, but muscle and exercise performance have not been carefully studied. 5
The Effect of STatins On Muscle Performance study (STOMP; NHLBI 5R01HL081893, NCT00609063) determined the incidence of statin-associated muscle complaints and examined the effect of statins on muscle performance and exercise capacity by administering atorvastatin 80 mg daily or placebo to healthy subjects for 6 months or until subjects developed myalgia.
METHODS
Study overview
STOMP was a double-blind, random-assignment clinical trial whose methods have been described.6 Equal numbers of men and women across three age ranges (20–39, 40–54, and 55+ yrs) were recruited over 4 years. Baseline lipid, liver, kidney, thyroid, and CK measurements were obtained. Subjects completed a baseline muscle symptom questionnaire and exercise testing including a maximal exercise test with gas analysis; handgrip, elbow flexor, and knee extensor strength testing; and a knee extensor endurance exercise test. Subjects were then randomly assigned in a double-blind fashion to identical placebo or atorvastatin 80mg daily (Lipitor; Pfizer, Inc., New York, NY). Atorvastatin tablets were crushed for compounding, but this does not influence relative bioavailability of the statin (Medical Information Letter 337882; Pfizer, Inc.) Subjects were called twice monthly to ascertain symptoms. Subjects performed repeat testing after 6 months or after they developed muscle symptoms meeting the study definition of statin-induced myalgia. The study was approved by the Institutional Review Boards at Hartford Hospital, University of Massachusetts, and University of Connecticut and monitored by a Data Safety and Monitoring Board.
Subject Inclusion / Exclusion Criteria
1,415 possible subjects were evaluated. Subjects were not included if they had cancer within 5 years, a baseline alanine aminotransferase (ALT) value > 2 times upper normal limits (UNL), a creatinine > 2 mg/L, an abnormal thyroid stimulating hormone level, a history of cardiovascular disease or an ischemic appearing electrocardiographic response to exercise testing, diabetes mellitus, subjective muscle complaints or weakness, or physical disabilities that would prohibit exercise testing. LDL-C was not an inclusion/exclusion criterion because many patients with cardiovascular disease or other cardiovascular risk factors presently receive statin therapy regardless of their baseline LDL values. Women who were pregnant or planned to become pregnant were not recruited and all women in the study of child-bearing age agreed to use an established method of birth control for the duration of the trial. Subjects presently or previously treated with lipid-lowering medications were excluded, as were individuals treated with medications known to affect skeletal muscle or to alter statin metabolism 7. Subjects with hypertension were recruited if their blood pressure was controlled and ≤140/90 at baseline. A total of 468 subjects were randomized to atorvastatin or placebo of whom 203 in the atorvastatin and 214 in the placbo group provided complete data for analysis. Subjects were removed from the study if their CK exceeded 10 times UNL on any occasion or if their ALT exceeded 3 times UNL on two measurements performed within one week of the first elevated value. No subject was excluded for elevated CK values, but 9 atorvastatin and 1 placebo subjects were excluded for elevated ALT levels (Figure 1).
Figure 1.
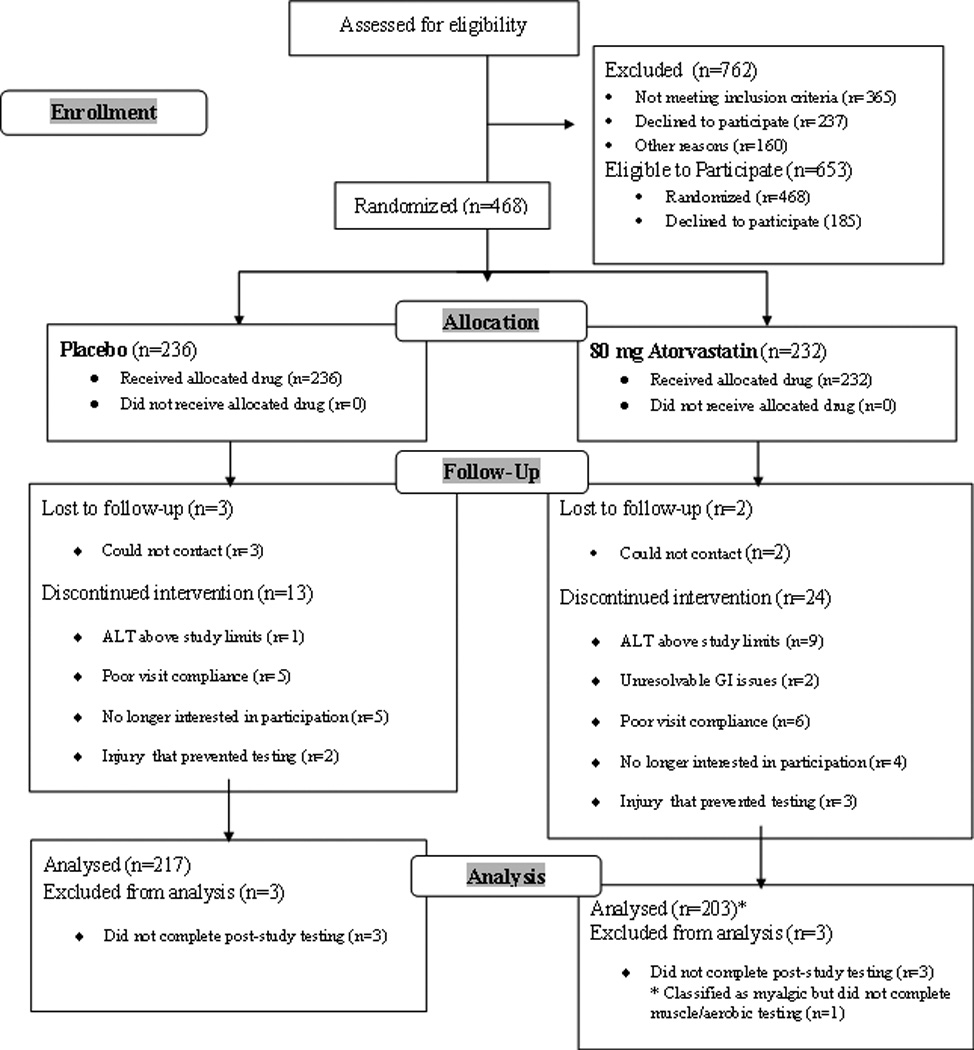
Study flow diagram detailing numbers (n) of participants who were screened, randomized, completed and analyzed in the study.
Serologic markers
Total, LDL and HDL cholesterol, triglycerides, ALT, creatinine, 25-hydroxy Vitamin D (25(OH)D (which measures both vitamin D2 and D3)), and CK levels were obtained at baseline and 6 months. Participants, their physicians and investigators were unaware of subjects’ lipid values during the study.
Study Measurements
Body anthropometrics, handgrip isometric strength of the dominant hand, elbow and knee extension/flexion isometric and isokinetic strength as well as knee endurance fatigue index 8 of the dominant limb, maximal oxygen uptake (VO2max), resting and peak respiratory gas exchange ratio (RER) and ventilatory threshold, as well as habitual self-reported and directly-measured physical activity were measured at baseline and after 6 months of drug treatment as described.6 Compliance with study medications was assessed by pill counts of unused medication at 3 months and again at 6 months.
Myalgia/Muscle Symptoms
Muscle complaints were assessed at the baseline, 3 and 6 month visits and by phone twice monthly using the Short-Form Brief Pain Inventory as described.6 Subjects met the study definition for “myalgia” if all of the following occurred: 1) They reported new or increased muscle pain, cramps, or aching, unassociated with exercise; 2) Symptoms persisted for at least 2 weeks; 3) Symptoms resolved within 2 weeks of stopping the study drug; and 4) Symptoms reoccurred within 4 weeks of restarting the study medication. Subjects who tested positive for myalgia using these criteria subsequently performed repeat serological, muscle and aerobic testing immediately after reoccurrence of muscle symptoms.
Sample Size
The primary outcome was the incidence of statin-associated myalgia, and thus sample size estimates were based on the projection that 10% of the statin group would develop myalgia. 9 Assuming a nonspecific muscle complaint rate of 2% with placebo, 162 subjects per group were needed to detect a significant difference between groups (α=0.05, power = 0.80). In addition, groups of 200 subjects each provided power to detect a 5–10% change with statin therapy in all exercise outcomes.
Statistical Analyses
Outcomes were assessed for normality using normal probability plots, histograms, and Kolmogorov test statistics. Transformations were used as necessary. All tests were two-sided with statistical significance set at alpha=0.05. Analyses were performed using SAS9.1 (SAS Institute Inc., Cary, NC).
Primary analyses were carried out on an intent-to-treat basis. We also compared outcomes excluding 23 subjects who did not reduce LDL-C by 20% or more on atorvastatin (to exclude noncompliant subjects as denoted in the original published analysis plan) but this did not alter main study findings and thus the following data include all completed subjects. To determine the incidence of statin muscle complaints, the proportion of subjects developing complaints was compared between the statin and placebo groups using a Pearson chi-square. The effect of atorvastatin on serologic markers, muscle strength, and aerobic performance was assessed with t-tests (or a Mann-Whitney U test for CK) comparing the change scores observed in the statin and placebo treated subjects pre-to-post treatment. Models were also run controlling for sex and age as categorical covariates and random site effects, which did not alter findings. Further models examined two-way and three-way interactions with ANOVA by modeling change scores in study outcomes with age, gender and drug treatment, again controlling for random site effects with non-significant interactions dropped for parsimony. Change scores according to myalgia status were also examined between treatment groups given the small number of myalgic subjects.
RESULTS
Baseline Characteristics and Lipid Changes
Subjects randomized to the atorvastatin and placebo groups were similar at baseline, although more women on atorvastatin used oral contraceptives or hormone therapy and more placebo subjects used prescription pain medication (Table 1). Compliance with treatment medication was similiar in the atorvastatin and placebo groups (94.5 ± 7.0 vs. 93.9±8.2 %). Atorvastatin subjects showed the expected reductions in LDL-cholesterol (Table 2).
Table 1.
Subject Baseline Characteristics And Medication Use By Drug Assignment For All Subjects and Those Who Developed Myalgia
| Entire Sample | Myalgic Sample | |||
|---|---|---|---|---|
| ATOR (n=203) | PL (n=217) | ATOR (n=19) | PL (n=10) | |
| Women (n) | 103 | 113 | 11 | 5 |
| Caucasian (n) | 192 | 201 | 18 | 10 |
| Age (years) | 43.6(41.4,45.8) | 44.6(42.4,46.8) | 52.5(47.1,50.9)* | 56.9±8.0 (51.9,61.9)* |
| BMI (kg/m2) | 26.3(25.6,27.0) | 26.5(25.8,27.2) | 24.7(22.8,26.6) | 25.0±3.0(23.1,26.9) |
| VO2max (mL/kg/min) | 34.6(33.3,35.9) | 33.2(31.9,34.5) | 31.0(26.7,35.3) | 30.7(26.0,35.4) |
| Resting SBP (mmHg) | 119.5(117.7,121.3) | 118.3(116.5,120.1) | 122.3(117.4,127.2) | 119.0(112.7,125.3) |
| Resting DBP (mmHg) | 75.5(74.2,76.8) | 75.1(73.8,76.4) | 76.4(73.2,79.6) | 72.6(68.9,76.3) |
| Total-C (mg/dL) | 198.7(193.3,204.1) | 194.8(189.9,199.7) | 215.3(195.9,234.7) | 212.0(195.6,228.4) |
| LDL-C (mg/dL) | 119.0(114.1,123.9) | 116.0(111.8,120.2) | 128.9(110.8,147.0) | 130.2(116.1,144.3) |
| HDL-C (mg/dL) | 57.6(55.2,60.0) | 58.6(56.3,60.9) | 68.7(61.5,75.9)* | 61.0(53.2,68.8) |
| TRIG (mg/dL) | 110.5(102.9,118.1) | 103.1(95.7,110.5) | 88.1(72.4,103.8) | 114.2(73.5,154.9) |
| Medication | ||||
| OTC Pain (n) | 21 | 21 | 3 | 2 |
| Prescription Pain (n) | 5 | 16* | 2 | 2 |
| Blood Pressure (n) | 9 | 15 | 2 | 0 |
| Thyroid (n) | 12 | 13 | 4 | 2 |
| Hormone Therapy (n) | 31 | 18* | 2 | 0 |
Data are presented as point estimates and 95% confidence intervals for atorvastatin (ATOR) vs. placebo (PL) subjects in the entire sample and only in subjects classified as myalgic. BMI = body mass index; VO2max = maximal oxygen uptake; SBP = systolic blood pressure; DBP = diastolic blood pressure; C = cholesterol; LDL = low density lipoprotein; HDL = high density lipoprotein; TRIG = triglycerides. OTC Pain = regular use of over-the-counter pain medications such as nonsteroidal anti-inflammatory drugs and acetaminophen; Prescription Pain = regular use of prescription pain medications; Blood Pressure = used a blood-pressure lowering drug; Thyroid = used a synthetic thyroid drug; hormone therapy = if female, used oral contraceptives or hormone replacement therapy.
indicates significant difference between myalgic and nonmyalgic subjects within a treatment group at p < 0.05.
Table 2.
Lipid Changes By Drug Assignment and Myalgia Status
| Total Sample | Myalgic Sample | |||
|---|---|---|---|---|
| ATOR (n=203) | PL (n=217) | ATOR (n=19) | PL (n=10) | |
| Δ Total-C (mg/dL) | −65.3(−70.1,−60.5)* | 2.7(−0.2,5.6) | −73.9(−88.0,−59.8)* | 12.2(0.0,24.4) |
| Δ LDL-C (mg/dL) | −59.0(−63.4,−54.6)* | 0.9 (−1.8,3.6) | −70.6(−83.5,−57.7)* | 10.1(−1.4,21.6) |
| Δ HDL-C (mg/dL) | −0.8(−2.0,0.4) | 0.4(−0.7,1.5) | 0.2(−4.3,4.7) | −3.4(−9.1,2.3) |
| Δ TRIG (mg/dL) | −28.3(−34.4,−22.3)* | 3.5(−3.1,10.1) | −18.2(−28.0,−8.4)* | 16.5(−12.3,45.3) |
Data are presented as point estimates and 95% confidence intervals for atorvastatin (ATOR) vs. placebo (PL)
subjects in the entire sample and only in subjects classified as myalgic. Δ = absolute change from pre-to-post
study; C = cholesterol; LDL = low-density lipoprotein; HDL = high-density lipoprotein; TRIG = triglycerides.
indicates significant change from baseline at p < 0.01; there were no differences between myalgic and nonmyalgic subjects within a treatment group.
Changes in Serological Markers
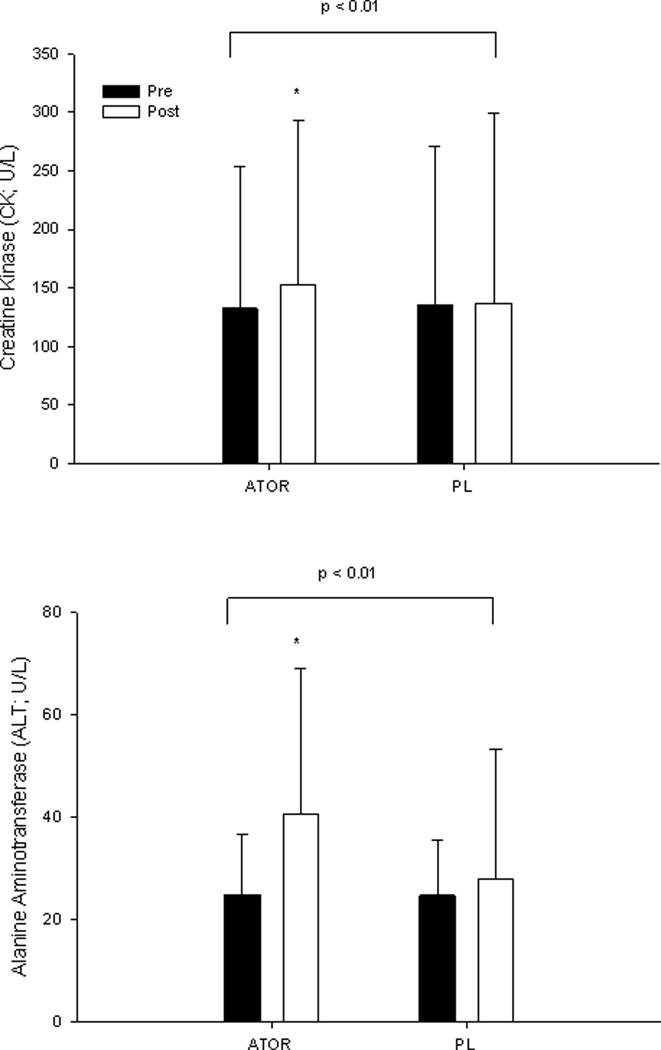
Atorvastatin produced a 20.8 ± 141.1 U/L (p<0.0001) increase in CK, although no subject demonstrated a CK value > 10 X the upper normal limit (UNL) (Figure 2). In addition, forty subjects on atorvastatin vs. 29 subjects on placebo demonstrated CK > UNL (Χ2 = 3.2; p = 0.08). Atorvastatin also increased average ALT values 15.7 ± 27.4 U/L (p< 0.0001) (Figure 2). There was no effect of 6 months atorvastatin treatment on Vitamin D (atorvastatin: 35.1 ± 12.4 to 34.2 ± 12.5 vs. placebo: 36.8 ± 14.6 to 35.1 ± 14.3 ng/mL; p = 0.94).
Figure 2.
Group mean ± SD of creatine kinase (CK; top) and alanine aminotransferase (ALT; bottom) before (Pre) and after (Post) 6 months of atorvastatin (ATOR) or placebo (PL) treatment with brackets indicating the p value for group-by-time interaction. *Significant difference between groups within a timepoint at p <0.05.
Muscle Complaints
Twenty three atorvastatin and 14 placebo subjects reported new, unexplained muscle pain. Nineteen atorvastatin and 10 placebo subjects ultimately met the study definition for myalgia (Χ2 = 3.74; p = 0.05). Myalgic subjects on atorvastatin reported predominantly leg symptoms: hip flexor, quadriceps, hamstring and/or calf aches (n=10), quadriceps or calf cramps (n=5), and/or quadriceps, hamstring and/or calf fatigue (n=6). whereas myalgic subjects on placebo reported more diverse symptoms such as whole-body fatigue (n=3), worsening of pain in previous injuries (n=3), groin pain (n=3) and foot cramping (n=1), but were otherwise similar (Table 1). Time to symptom onset was shorter in atorvastatin myalgic subjects than in myalgic subjects on placebo (35 ± 31 vs: 61 ± 33 days; p = 0.045). Myalgic vs. nonmyalgic subjects on atorvastatin or placebo did not exhibit differences in CK, ALT, lipid (Table 2) or Vitamin D changes with treatment (p > 0.19).
Pain severity and pain interference with daily life in all subjects with myalgia averaged 0.7 ± 1.2 and 0.3 ± 0.6 at baseline out of a possible 10 and increased to 2.4 ± 1.8 and to 2.0 ± 2.3 with treatment (p = 0.001) and did not differ between the atorvastatin and placebo subjects (p=0.37). Baseline pain severity and pain interference with daily life were similar in non-myalgic subjects for the atorvastatin and placebo groups(0.4 ± 0.9 and 0.2 ± 0.7, respectively) and did not change with treatment (p =0.14).
Effects of Atorvastatin on Muscle and Exercise Performance
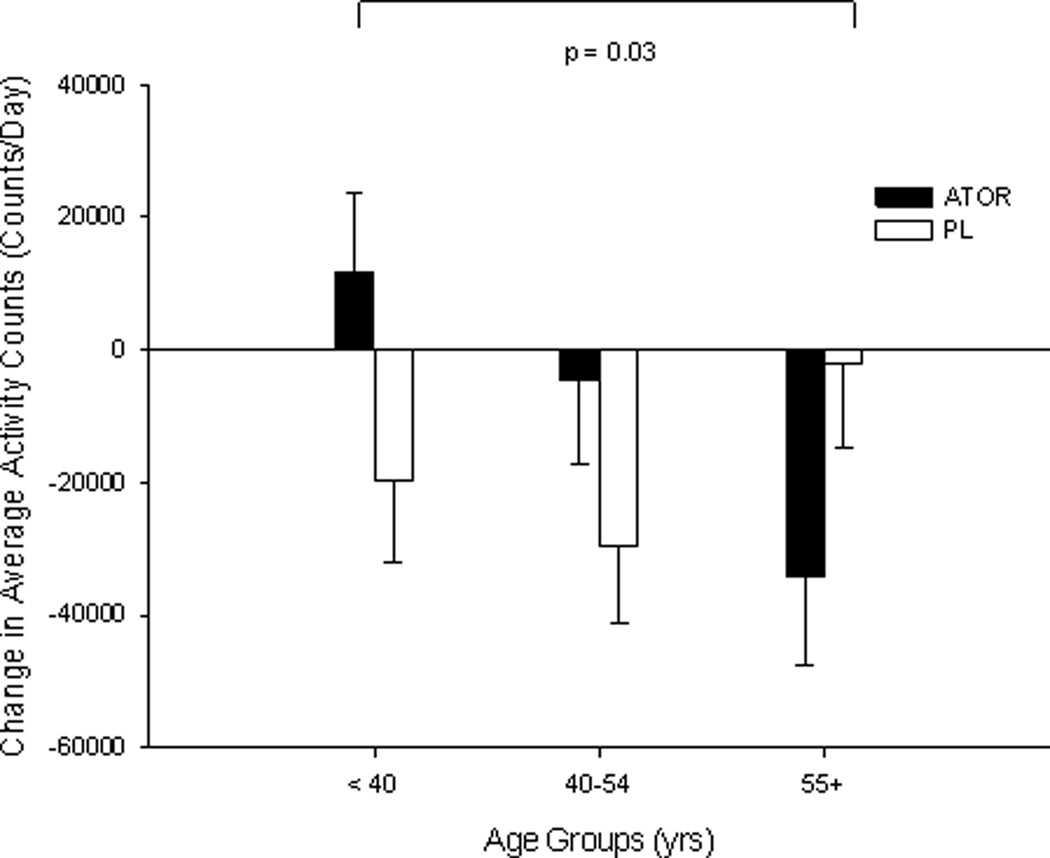
There were no baseline differences in measured strength and aerobic outcomes (all p > 0.06). There were no differential effects of atorvastatin on skeletal muscle strength and endurance, aerobic performance, or physical activity levels compared to placebo (all p values > 0.17)(Table 3). Physical activity decreased (p = 0.007) regardless of drug treatment, but the decrease in the atorvastatin group was due to a decrease in activity among the oldest tertile of study participants (p = 0.03; Figure 3).
Table 3.
Absolute (Post-Baseline) Aerobic and Strength Data by Drug Assignment
| ATOR (n=2021) | PL (n=217) | |
|---|---|---|
| Resting RER | 0.0 (−0.01,0.01) | 0.0(−0.03,0.03) |
| VO2max(mL/kg/min) | −0.8(−1.3,−0.3) | −0.8(−1.4,−0.2) |
| VT (mL/kg/min) | −0.9(−1.6,−0.2) | −0.6(−1.6,0.4) |
| Handgrip (kg) | 0.1(−0.5,0.7) | −0.6(−1.3,0.1) |
| Arm Strength (APT; N-M) | ||
| Isom Ext | 0.8(−0.2,1.8) | 0.3(−0.6,1.2) |
| Isom Flex | −0.5(−2.1,1.1) | −0.2(−1.1,0.7) |
| Isok Ext @ 60° /sec | 0.5(−0.2,1.2) | −0.2(−0.9,0.5) |
| Isok Flex @ 60° /sec | 0.0 (−0.6,0.6) | 0.0(−0.6,0.6) |
| Isok Ext @ 180° /sec | 0.5(−0.2,1.2) | 0.6(0.2,1.2) |
| Isok Flex @ 180° /sec | 0.2(−0.6,1.0) | 0.1(−0.5,0.7) |
| Leg Strength (APT; N-M) | ||
| Isom Ext | −2.1(−5.1,0.9) | −0.4(−3.4,2.6) |
| Isom Flex | −1.8(−3.2,−0.4) | −1.3(−2.6,0.0) |
| Isok Ext @ 60° /sec | 1.2(−0.8,3.2) | 0.9(−1.1,2.9) |
| Isok Flex @ 60° /sec | 0.9 (−0.5,2.3) | −0.5(−2.7,1.7) |
| Isok Ext @ 180° /sec | 3.9(2.0,5.8) | 3.8(2.3,5.3) |
| Isok Flex @ 180° /sec | 2.7 (1.5,3.9) | 2.1(1.0,3.2) |
| Knee Endurance Fatigue Index | −0.1(−1.6,1.4) | 0.2(−0.8,1.2) |
Data are presented as point estimates and 95% confidence intervals for atorvastatin (ATOR) vs. placebo (PL) subjects.. RER = Respiratory exchange ratio; VO2max = maximal oxygen uptake; VT = ventilatory threshold; APT = average peak torque; Isom = Isometric; Ext = Extension; Flex = Flexion. There were no significant differences in change scores between ATOR and PL treatment groups.
One ATOR subject with myalgia did not complete muscle and aerobic post-testing and thus was not included in analyses.
Figure 3.
Group mean ± SD of changes in physical activity (average counts/day) by age group after 6 months of atorvastatin (ATOR) or placebo (PL) treatment with brackets indicating the p value for treatment-by-age interaction.
Outcomes in Individuals with Myalgia
One atorvastatin subject with myalgia refused final testing and was not included in analyses. Compared to the asymptomatic subjects on atorvastatin, myalgic subjects had lower muscle strength in 5 of 14 measured variables (Table 4). Compared to the asymptomatic subjects on placebo, placebo myalgic subjects had significantly lower muscle strength in 4 measured variables (Χ2 = 0.16; p = 0.68).
Table 4.
Absolute (Post-Baseline) Aerobic and Strength Data by Drug Assignment in Myalgic Patients
| ATOR (n=181) | PL (n=10) | |
|---|---|---|
| Resting RER | 0.0(−0.05,0.05) | 0.1(−0.1,0.3) |
| VO2max(mL/kg/min) | −0.5(−1.3,0.3) | −1.3(−2.8,0.2) |
| VT (mL/kg/min) | −2.1(−3.9,0.3) | −2.3(−5.9,1.3) |
| Handgrip (kg) | −1.4(−3.4,0.6) | −1.0(−3.7,1.7) |
| Arm Strength (APT; N-M) | ||
| Isom Ext | −0.4(−4.2,3.4) | 1.1(−2.4,4.6) |
| Isom Flex | −3.2(−7.5,1.1) | 0.7(−3.6,5.0) |
| Isok Ext @ 60° /sec | −2.6 (−6.2,1.0) | 0.3(−1.9,2.5) |
| Isok Flex @ 60° /sec | −3.8(−7.8,0.2)* | 0.8(−1.2,2.8) |
| Isok Ext @ 180° /sec | −1.3(−4.4,1.8) | −0.4(−2.2,1.4)* |
| Isok Flex @ 180° /sec | −0.5(−4.1,3.1) | 0.2(−2.2,3.6) |
| Leg Strength (APT; N-M) | ||
| Isom Ext | −17.3 ± (−25.1,−9.5)* | −21.5(−30.6,−12.4)* |
| Isom Flex | −5.3(−10.4,−0.2) | −4.3(−10.2,1.6) |
| Isok Ext @ 60° /sec | −10.4(−20.2,−0.6)* | −6.6(−14.5,1.3) |
| Isok Flex @ 60° /sec | −3.6(−8.6,1.4)* | −4.6(−8.7,−0.5) |
| Isok Ext @ 180° /sec | −3.0(−8.9,2.9)* | −4.1(−10.1,1.9)* |
| Isok Flex @ 180° /sec | −1.0(−4.6,2.6) | −3.9(−6.8,−1.0)* |
| Knee Endurance Fatigue Index | −2.1(−4.4,0.2) | −3.6(−5.7,−1.5) |
Data are presented as point estimates and 95% confidence intervals for atorvastatin (ATOR) vs. placebo (PL) subjects in subjects classified as myalgic. RER = Respiratory exchange ratio; VO2max = maximal oxygen uptake; VT = ventilatory threshold; APT = average peak torque; Isom = Isometric; Ext = Extension; Flex = Flexion.
indicates significant difference in change scores between myalgic and nonmyalgicgroups (from Table 3) within a treatment group at p < 0.05.
One ATOR subject with myalgia did not complete muscle and aerobic post-testing and thus was not included in analyses.
DISCUSSION
STOMP is, to our knowledge, the first randomized double-blind, clinical trial to confirm the common clinical impression that statins increase the incidence of myalgia. The definition of myalgia in STOMP was predefined and required resolution of muscle symptoms promptly after stopping study medication and reappearance of symptoms on restarting the medication. STOMP also documented that statin-associated mild muscle complaints do not appear to have measurable physiologic consequences in that muscle strength was not reduced to a greater extent in myalgic participants on atorvastatin compared to placebo subjects also satisfying the study definition of myalgia. The observation that some placebo patients satisfied the myalgia definition documents the importance of using a double-blind trial to examine the incidence and characteristics of statin-associated myalgia and not relying solely on clinical characteristics. STOMP also demonstrated, we believe again for the first time, that. high-dose statin treatment increases average CK levels, suggesting that statins produce low level muscle injury in healthy subjects that occurs independent of muscle symptoms.
The reported incidence of myalgia during statin therapy has varied from 1% to 25%. 3, 10, 11 Determining the true incidence of mild muscle complaints from prior studies is difficult because these complaints are typically not solicited and because most large trials typically define myopathy as either myositis or rhabdomyolysis and require CK levels > 10 ULN. We used a standardized study protocol and definition and observed that high-dose atorvastatin doubled the incidence of reproducible muscle complaints, from 4.6 to 9.4% of subjects. STOMP enrolled equal numbers of young, middle-aged and older men and women, but the incidence of myalgia was similar to the 10.5% rate reported among 7,924 French patients.9 Observational reports suggest that statin-associated muscle complaints are more frequent in older adults and/or women.12–14 Subjects with statin myalgia in STOMP did tend to be older and almost 60% were women, but the STOMP sample size was too small to detect gender differences in incidence. Interestingly, the French study cited above 9 observed that muscle symptoms generally appeared within a month of drug initiation. This was also true in STOMP, and the time to symptom onset was significantly shorter in myalgic subjects on atorvastatin than on placebo, 1 vs. 2 months.
Clinical trials define statin-associated myopathy as CK levels > 10 ULN.4, 14 No subject in STOMP recorded a CK > 10 ULN, but 6 months of treatment with atorvastatin resulted in a small (~20 U/L) but significant (p<0.01) increase in CK. This suggests that low level muscle injury occurs with high-dose statins, although there are other possible explanations for the CK increase. Inflammatory conditions including rheumatoid arthritis and systemic lupus are associated with reduced CK levels.15 Statins reduce systemic inflammation 1 and could increase average CK by decreasing inflammation. We did not measure inflammatory markers in STOMP so cannot evaluate this possible relationship. Alternatively, hepatic macrophages participate in the clearance of CK.16 Decreasing hepatic macrophages in animal models increases CK concentrations suggesting that statins, if they decrease hepatic macrophage content, could reduce CK clearance and increase CK levels. There was no correlation between changes in CK and ALT in STOMP, but we cannot further evaluate this possibility. Both explanations seem less likely than the hypothesis that statins produce low-level muscle injury given the plethora of data linking statins with skeletal muscle damage, 17–19 even in the absence of muscle symptoms. 20
The long-term clinical consequences of this mild CK elevation are unclear, since the CK elevation was not accompanied by changes in muscle strength. Anecdotally, some patients with weakness attributed to statin therapy develop such complaints only after years of treatment.12 Therefore, it is possible that low-level muscle injury, indicated by mild elevations in CK, will produce subsequent weakness in some statin-treated subjects. Testing this hypothesis will be difficult since it requires a long-term placebo-controlled trial, an ethically difficult proposition given the documented therapeutic value of statins. Also, such a study would require sophisticated strength testing since we detected decreased strength among our myalgic subjects even though few complained of muscle weakness.
There were no significant changes in muscle strength and endurance as well as aerobic performance in the total atorvastatin STOMP population. Previous investigations have been equivocal, with some studies suggesting that statins reduce muscle strength in older individuals and that statins may shift exercise substrate utilization from fat to carbohydrate. 21–24 Most studies were small, used crude measures of strength and exercise performance and were not blinded or placebo-controlled. 5 STOMP provides reassurance that healthy adults who tolerate high-dose atorvastatin without muscle complaints experience no deleterious effects on muscle strength or performance over 6 months of treatment. We did observe that average physical activity, measured by accelerometer, decreased significantly in the oldest STOMP age group with atorvastatin therapy, but this could simply represent measurement variability since physical activity increased in the youngest atorvastatin-treated subjects.
In contrast to the total study sample, statin-treated subjects meeting the study definition of myalgia did demonstrate reductions in muscle strength in 5 of 14 measured muscle strength variables (Table 4). Several observational studies have suggested that patients with myalgia experience a decrease in strength.4, 25 However, placebo group subjects also meeting the study definition of myalgia also demonstrated muscle strength declines in 4 measured variables, suggesting that the majority of muscle strength changes with myalgia in STOMP may have been non-specific. Caution should be warranted in interpreting these data though as the analyses were secondary to major study outcomes in a very small sample of patients. Therefore, larger studies of patients with true statin myalgia—rigorously tested with a double-blind, randomized cross-over period to confirm myalgia in the initial study population—are needed to determine the effects of statin on muscle outcomes in myopathic patients.
STOMP has significant advantages over prior observational studies, but also limitations. STOMP only examined the effect of atorvastatin on muscle complaints and function so cannot evaluate if other statins at comparable doses would produce similar results. STOMP only enrolled healthy individuals so cannot address the frequency or severity of muscular complaints in patients with concomitant diseases and medications. The inclusion of younger subjects and both genders may have reduced the number of subjects with myalgia during atorvastatin treatment since subjects with statin myalgia in STOMP tended to be older and more were woman. STOMP only lasted 6 months so may underestimate the incidence of myopathic complaints during longer term statin therapy, although onset of myalgia with atorvastatin occurred an average of one month after initiation of therapy. Longer placebo-controlled trials to examine the frequency of myopathic complaints in sicker subjects will be difficult, however, given the general reluctance to assign statin-eligible subjects to placebo treatment. On the other hand, given that statins have been clinically available and widely used in the US since 1987 and the paucity of documented decreases in muscle strength among statin-treated patients, STOMP may overestimate the frequency and strength changes of statin-associated myalgia. This seems unlikely, however, given the rigor of the STOMP design.
SUMMARY
STOMP is, to our knowledge, the first randomly assigned, double-blind study designed to examine the effects of statins on muscular complaints, muscle strength and exercise performance. The results are reassuring since there was no effect of atorvastatin 80 mg daily on muscle strength or exercise performance over 6 months of treatment in healthy subjects. On the other hand, STOMP did document that atorvastatin significantly increased the frequency of myalgia. STOMP also demonstrated an increase in average CK in the atorvastatin-treated cohort suggesting that statins could produce low level muscle injury. These results should prompt additional studies examining muscular performance with long term statin treatment in both healthy patients as well as those with confirmed statin-associated myalgia.
Clinical Summary.
Many clinicians believe that statins cause muscle pain, but this has not been observed in clinical trials and the effect of statins on muscle performance has not been carefully studied. The Effect of STatins On Skeletal Muscle Function and Performance (STOMP) study assessed symptoms and measured creatine kinase (CK), exercise capacity, and muscle strength before and after atorvastatin 80 mg or placebo were administered for 6 months to 420 healthy, statin-naive subjects. No CK value exceeded 10 times normal in any subject during the trial, but average CK increased 20.8 ± 141.1 U/L (p<0.01) with atorvastatin. There were no significant changes in several measures of muscle strength or exercise capacity with atorvastatin, but more atorvastatin than placebo subjects developed myalgia (19 vs 10; p = 0.05). Myalgic subjects on atorvastatin or placebo decreased muscle strength in 6 of 14 and 4 of 14variables respectively (p = 0.43).These results indicate that 6 months of high-dose atorvastatin treatment does not decrease average muscle strength or exercise performance in healthy, previously untreated subjects. Nevertheless, this blinded, controlled trial confirms the undocumented impression that statins increase muscle complaints. Atorvastatin also increased average CK suggesting that statins produce mild muscle injury even among asymptomatic subjects. This increase in CK should prompt studies examining the effects of more prolonged, high-dose statin treatment on muscular performance.
Acknowledgments
The authors wish to acknowledge the following individuals for their participation on the Data Safety Monitoring Board: JoAnne Foody, M.D., Pamela Hartigan, Ph.D., and Ira Ockene, M.D. (chair). In addition, Gualberto Ruano, M.D., Ph.D. provided statistical and scientific assistance with data interpretation.
Funding Sources: The STOMP study is funded by NHLBI/NIH grant RO1 HL081893 (P. Thompson).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: Paul D. Thompson reports receiving research grants from the National Institutes of Health, GlaxoSmithKline, Anthera, B. Braun, Genomas, Roche, Aventis, Novartis, and Furiex; serving as a consultant for Astra Zenica, Furiex, Regeneron, Merck, Roche, Genomas, Abbott, Lupin, Runners World, Genzyme, Sanolfi, Pfizer, and GlaxoSmithKline; receiving speaker honoraria from Merck, Pfizer, Abbott, Astra Zenica, GlaxoSmithKline, and Kowa: owing stock in General Electric, JA Wiley Publishing, J&J, Sanolfi-Aventis and Abbott; and serving as a medical legal consultant on cardiac complications of exercise, statin myopathy, tobacco, ezetimibe and non-steroidals. No other authors have any financial disclosures.
References
- 1.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 2.Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med. 2002;346:539–540. doi: 10.1056/NEJM200202143460721. [DOI] [PubMed] [Google Scholar]
- 3.Physicians' Desk Reference. Montvale, NJ: Medical Economics; 2002. p. 56. [Google Scholar]
- 4.Phillips PS, Haas RH, Bannykh S, Hathaway S, Gray NL, Kimura BJ, Vladutiu GD, England JD. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581–585. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan GM, Thompson PD. The effects of statins on skeletal muscle strength and exercise performance. CurrOpinLipidol. 2010;21:324–328. doi: 10.1097/MOL.0b013e32833c1edf. [DOI] [PubMed] [Google Scholar]
- 6.Thompson PD, Parker BA, Clarkson PM, Pescatello LS, White CM, Grimaldi AS, Levine BD, Haller RG, Hoffman EP. A randomized clinical trial to assess the effect of statins on skeletal muscle function and performance: rationale and study design. PrevCardiol. 2010;13:104–111. doi: 10.1111/j.1751-7141.2009.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 8.Katsiaras A, Newman AB, Kriska A, Brach J, Krishnaswami S, Feingold E, Kritchevsky SB, Li R, Harris TB, Schwartz A, Goodpaster BH. Skeletal muscle fatigue, strength, and quality in the elderly: the Health ABC Study. J Appl Physiol. 2005;99:210–216. doi: 10.1152/japplphysiol.01276.2004. [DOI] [PubMed] [Google Scholar]
- 9.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 10.Phillips PS, Haas RH. Observations from a statin myopathy clinic. Arch Intern Med. 2006;166:1232–1233. doi: 10.1001/archinte.166.11.1232-b. [DOI] [PubMed] [Google Scholar]
- 11.El-Salem K, Ababneh B, Rudnicki S, Malkawi A, Alrefai A, Khader Y, Saadeh R, Saydam M. Prevalence and risk factors of muscle complications secondary to statins. Muscle Nerve. 2011;44:877–881. doi: 10.1002/mus.22205. [DOI] [PubMed] [Google Scholar]
- 12.Cham S, Evans MA, Denenberg JO, Golomb BA. Statin-associated muscle-related adverse effects: a case series of 354 patients. Pharmacotherapy. 2010;30:541–553. doi: 10.1592/phco.30.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am CollCardiol. 2002;40:567–572. doi: 10.1016/s0735-1097(02)02030-2. [DOI] [PubMed] [Google Scholar]
- 14.Maningat P, Breslow JL. Needed: pragmatic clinical trials for statin-intolerant patients. N Engl J Med. 2011;365:2250–2251. doi: 10.1056/NEJMp1112023. [DOI] [PubMed] [Google Scholar]
- 15.Lee YH, Choi SJ, Ji JD, Song GG. Serum creatine kinase in patients with rheumatic diseases. Clin Rheumatol. 2000;19:296–300. doi: 10.1007/s100670070049. [DOI] [PubMed] [Google Scholar]
- 16.Radi ZA, Koza-Taylor PH, Bell RR, Obert LA, Runnels HA, Beebe JS, Lawton MP, Sadis S. Increased serum enzyme levels associated with kupffer cell reduction with no signs of hepatic or skeletal muscle injury. Am J Pathol. 2011;179:240–247. doi: 10.1016/j.ajpath.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanai J, Cao P, Tanksale P, Imamura S, Koshimizu E, Zhao J, Kishi S, Yamashita M, Phillips PS, Sukhatme VP, Lecker SH. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117:3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urso ML, Clarkson PM, Hittel D, Hoffman EP, Thompson PD. Changes in ubiquitin proteasome pathway gene expression in skeletal muscle with exercise and statins. ArteriosclerThrombVasc Biol. 2005;25:2560–2566. doi: 10.1161/01.ATV.0000190608.28704.71. [DOI] [PubMed] [Google Scholar]
- 19.Parker BA, Augeri AL, Capizzi JA, Ballard KD, Troyanos C, Baggish AL, D'Hemecourt PA, Thompson PD. Effect of statins on creatine kinase levels before and after a marathon run. Am J Cardiol. 2012;109:282–287. doi: 10.1016/j.amjcard.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 20.Mohaupt MG, Karas RH, Babiychuk EB, Sanchez-Freire V, Monastyrskaya K, Iyer L,Hoppeler H, Breil F, Draeger A. Association between statin-associated myopathy and skeletal muscle damage. CMAJ. 2009;18:E11–E18. doi: 10.1503/cmaj.081785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paolisso G, Barbagallo M, Petrella G, Ragno E, Barbieri M, Giordano M, Varricchio M. Effects of simvastatin and atorvastatin administration on insulin resistance and respiratory quotient in aged dyslipidemic non-insulin dependent diabetic patients. Atherosclerosis. 2000;150:121–127. doi: 10.1016/s0021-9150(99)00352-4. [DOI] [PubMed] [Google Scholar]
- 22.Phillips PS, Phillips CT, Sullivan MJ, Naviaux RK, Haas RH. Statin myotoxicity is associated with changes in the cardiopulmonary function. Atherosclerosis. 2004;177:183–188. doi: 10.1016/j.atherosclerosis.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Mullen PJ, Zahno A, Lindinger P, Maseneni S, Felser A, Krähenbühl S, Brecht K. Susceptibility to simvastatin-induced toxicity is partly determined by mitochondrial respiration and phosphorylation state of Akt. BiochimBiophys Acta. 2011;1813:2079–2087. doi: 10.1016/j.bbamcr.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Wu JS, Buettner C, Smithline H, Ngo LH, Greenman RL. Evaluation of skeletal muscle during calf exercise by 31-phosphorus magnetic resonance spectroscopy in patients on statin medications. Muscle Nerve. 2011;43:76–81. doi: 10.1002/mus.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobkin BH. Underappreciated statin-induced myopathic weakness causes disability. Neurorehabil Neural Repair. 2005;19:259–263. doi: 10.1177/1545968305277167. [DOI] [PMC free article] [PubMed] [Google Scholar]