Abstract
This article is the second part of a review that addresses the role of damage-associated molecular patterns (DAMPs) in human diseases by presenting examples of traumatic (systemic inflammatory response syndrome), cardiovascular (myocardial infarction), metabolic (type 2 diabetes mellitus), neurodegenerative (Alzheimer’s disease), malignant and infectious diseases. Various DAMPs are involved in the pathogenesis of all these diseases as they activate innate immune machineries including the unfolded protein response and inflammasomes. These subsequently promote sterile autoinflammation accompanied, at least in part, by subsequent adaptive autoimmune processes. This review article discusses the future role of DAMPs in routine practical medicine by highlighting the possibility of harnessing and deploying DAMPs either as biomarkers for the appropriate diagnosis and prognosis of diseases, as therapeutics in the treatment of tumours or as vaccine adjuncts for the prophylaxis of infections. In addition, this article examines the potential for developing strategies aimed at mitigating DAMPs-mediated hyperinflammatory responses, such as those seen in systemic inflammatory response syndrome associated with multiple organ failure.
Keywords: Innate Immunity; Receptors, Pattern Recognition; Inflammation; Adaptive Immunity; Autoimmunity
In part i of this review, the role of damage-associated molecular patterns (DAMPs) in mounting inflammation and shaping adaptive immunity was briefly described by defining various classes of DAMPs that activate and orchestrate several innate immune machineries including inflammasomes and the unfolded protein response (UPR).1 In brief, DAMPs are intracellularly sequestered molecules and are hidden from recognition by the immune system under normal physiological conditions. However, under conditions of cellular stress/tissue injury, these molecules can either be actively secreted by stressed immune cells; exposed on stressed cells, for example, in terms of neo-antigens binding to natural immunoglobulin M (IgM) antibodies, or they can be passively released into the extracellular environment from dying cells or the damaged extracellular matrix.2–6 DAMPs are recognised by pattern recognition receptor (PRR)-bearing cells of the innate immune system, including macrophages, leukocytes and dendritic cells (DCs) as well as vascular cells, fibroblasts and epithelial cells, to promote pro-inflammatory and profibrotic pathways.
Various definitions and interpretations of DAMPs can be found in the literature and may confuse a new reader in this field. Thus, in this article, for didactic reasons only and without covering all possible DAMPs, they are divided into five partially overlapping classes. In the current article only three classes are covered: classes I, II and V. Class I DAMPs are recognised by physically binding to PRRs, while class II DAMPs are those sensed without directly binding to PRRs. Particularly addressed in this article are class V DAMPs, or dyshomeostasis-associated molecular patterns (see part I).1 Class V DAMPs, in terms of homeostatic danger signals, have recently been reported as an emerging class of DAMPs in terms of defining an altered pattern of molecules reflecting perturbations in the steady state of the intracellular and/or extracellular microenvironment.3 Such homeostatic DAMPs, when associated with endoplasmic reticulum (ER) stress, may be sensed by three sensor molecules of the UPR: the protein kinase-like eukaryotic initiation factor 2α kinase (PERK), the inositol-requiring transmembrane kinase/endoribonuclease 1α (IRE1α) and the activating transcription factor 6 (ATF6).7–9
The various classes of sterile inflammation-promoting DAMPs, as true for infectious inflammation-evoking pathogen-associated molecular patterns (PAMPs), are sensed by a variety of distinct PRRs, thereby promoting an inflammatory response. They are not reviewed here since PRRs and their triggered signalling pathways have recently been the subjects of excellent review articles, including those covering Toll-like receptors (TLRs),10 receptors for advanced glycation end-products (RAGE),11 nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs),11 C-type lectin receptors (CLRs),12 retonic acid inducible gene-I (RIG-I)-like receptors (RLRs)13–15 and DNA sensors, including absent in melanoma 2 protein (AIM2)-like receptors (ALRs). The recently discovered cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) synthase (cGAS) has also been covered in recent reviews.15–17
In part I of this review, the five classes of DAMPs were shown to synergistically operate in instigating (auto)inflammatory and adaptive (auto)immune pathologies as manifested by many human diseases.1 Two examples of autoimmune diseases, systemic lupus erythematosus (SLE) and rheumatoid arthritis, were discussed in order to represent a typical paradigm of the intimate interplay between innate and adaptive immune responses. This second part of the review addresses the role of DAMPs in human diseases where the involvement of immune processes (in terms of adaptive immune processes) were almost unconsidered in the past but are now clearly recognised in terms of dysregulated innate immune processes.
Traumatic Diseases
The field of trauma impressively reflects the inherently ambivalent role of injury-induced DAMPs in medicine as their controlled beneficial function instigates the whole machinery of inflammation/fibrosis-mediated wound healing following any kind of small or moderate trauma.18 On the other hand, their uncontrolled detrimental action in the case of severe trauma/polytrauma can lead to the catastrophe of a systemic inflammatory response syndrome (SIRS) associated with multiple organ failure (MOF).19,20
Typically, the generation of DAMPs correlates with the degree of severity of accidental insults in traumatic diseases ranging from small cuts to blunt-force trauma and bone fractures or severe large-scale physical or thermal injuries.21 Following all these injurious lesions, DAMPs, such as high-mobility group box 1 (HMGB1) and heat shock proteins (HSPs), not only induce an acute inflammatory response but are also responsible for subsequent tissue repair. Inflammation after tissue injury is certainly a critical component of wound repair. Innate immune inflammatory cells migrate to the wound and promote tissue regeneration by removing cellular debris, killing and phagocytosing potential invading pathogens, and producing cytokines that promote collagen production, cellular migration, wound epithelialization and angiogenesis. In fact, any post-injury profibrotic and angiogenic response, for example after surgery or accidental trauma, is mediated by DAMPs-activated PRRs-expressing innate immune cells such as fibroblasts, epithelial cells, macrophages and vascular cells.18,22,23 It is the DAMPs and their triggered pathways, together with the surrounding cytokine and growth factor milieu, that ultimately determine whether or not these post-injury innate cellular responses cause mild acute inflammation and wound healing,22,24 or subchronicly on-going inflammation and fibrosis.25 In very severe trauma, when DAMPs are produced in very high concentrations and systemically released, they can cause acutely occurring SIRs that may be accompanied by MOF.25
In severe trauma in humans, HMGB1 has been found to be systemically released within 30–60 minutes, peaking two to six hours after injury. Remarkably, patients who develop organ dysfunction and non-survivors of severe trauma have been observed to have very high levels of this DAMP. Moreover, HMGB1 levels were found to be predictive of outcome of traumatic lesions as shown, for example, in patients with traumatic brain injury.26–28 Moreover, in extensive trauma, increasing attention has been devoted to the crucial role of mitochondria-derived DAMPs, since they have been shown to be markedly elevated in severely injured patients.29 This category of DAMPs mainly includes circular DNA strands containing C-phosphate-G (CpG) DNA repeats, N-formylated peptides and mitochondrial DNA (mtDNA) itself.30 Interestingly, it has been found that mtDNA, observed to directly activate neutrophils after binding to TLR9, are released under various traumatic conditions including shock and severe traumatic brain injury.31,32 Recent clinical studies in massively injured human subjects provided the first observational evidence that plasma mtDNA DAMPs are associated with the evolution of SIRS, MOF and mortality.33 One of the most interesting reported findings was that a determination of mtDNA DAMPs levels made within eight hours of hospital admission allowed a differentiation between survivors and non-survivors.33
Interestingly, the humoral part of the innate immune system, which is likely to be induced by class IV DAMPs in terms of injury-induced neo-antigens, namely the complement system, is activated immediately after trauma as well. In fact, severe, sterile, injury-induced, systemic intravascular activation of the complement cascade (in particular, the mannose-binding lectin-mediated pathway) reportedly can promote MOF by contributing to a fulminant inflammatory response associated with disseminated intravascular coagulation and comprised microcirculation.34
Cardiovascular Diseases
Most cardiovascular diseases (CVDs) develop on the basis of atherosclerosis. For many years, atherosclerosis was simply regarded as a consequence of the accumulation of lipids in the vessel walls. Today, the picture has completely changed. Current notions in vascular biology hold that vessel wall injury-induced DAMPs elicit pro-inflammatory, profibrotic and adaptive autoimmune responses to promote atherogenesis as the underlying disorder of CVDs, the most common being coronary artery and cerebrovascular diseases.35–37
Atherosclerosis
A large variety of stressful stimuli and inciting events to the arterial wall, including hypertension, diabetes, hyperlipidaemia, drugs and chemical toxins, can lead to vascular injury associated with the creation of various DAMPs. Remarkably, most of these injuries are mediated by the generation of reactive oxygen species (ROS), a typical example being hypertension, an insult-mediating injurious factor that has been already described for SLE.1,38 Accordingly, numerous studies have identified oxidative stress-induced DAMPs as the major activators of innate immune-mediated vascular inflammation promoting atherosclerosis.35–37
Low-density lipoprotein (LDL) is a key DAMP that accumulates in the subendothelium in the form of oxidised LDL (oxLDL) and minimally oxidised LDL. Both DAMPs activate vascular cells via recognition of their cognate receptors, lectin-like oxLDL receptor 1 (LOX-1) and TLR4. Further oxidation-specific epitopes, for example those derived from oxLDL, appear to play a prominent atherogenic role by forming a distinct family of DAMPs consisting of various categories of oxidative reactions. Other injury-induced DAMPs, such as HSPs and HMGB1, known to bind to TLR2, TLR4 and RAGE on vascular cells, in particular on vascular macrophages, are also involved in establishing a vascular pro-inflammatory/profibrotic cascade contributing to atherogenesis.35–37,39–41 More recently, other types of DAMPs released from severely damaged cells, such as S100A8/A9, sensed by TLR4 and RAGE, and mtDNA, sensed by TLR9, have increasingly been recognised to contribute to vascular innate immunity-mediated inflammatory pathways involved in atherogenesis.1,42
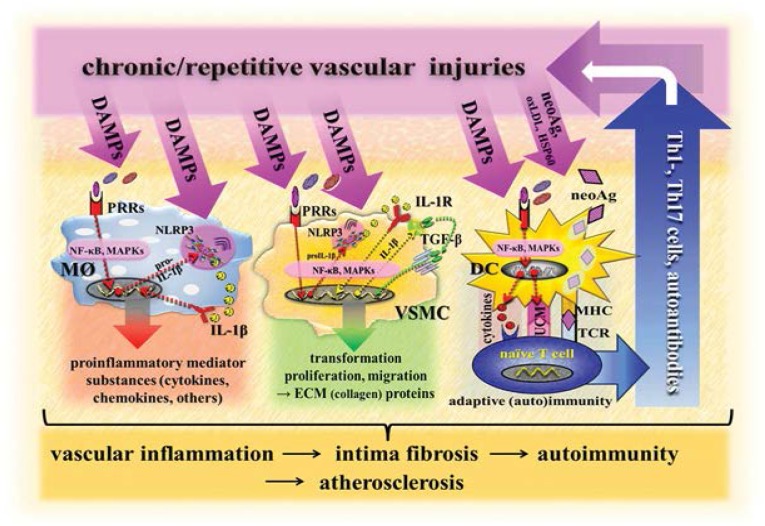
Of note, the NLR-containing pyrin domains (NLRP3) inflammasome, located in vascular macrophages and smooth muscle cells (SMCs), is reportedly also involved in atherogenesis via promotion of pro-inflammatory and profibrotic responses. In particular, studies on murine and human phagocytes and in in vivo settings revealed that crystals of cholesterol, operating as DAMPs, activate the NLRP3 inflammasome required for atherogenesis [Figure 1].36,43
Figure 1:
Scenario model of vascular DAMPs-induced innate and adaptive immune responses involved in atherogenesis.
DAMPs = damage-associated molecular patterns; neoAg = neo-antigens (altered-self antigens); oxLDL = oxidised low-density lipoprotein; HSP60 = heat shock protein 60; Th1 = T helper 1 subset of CD4+ cells; TH17 = T helper 17 subset of CD4+ cells; PRRs = pattern recognition receptors; IL-1R = interleukin-1 receptor; NLRP3 = nucleotide-binding oligomerization domain (NOD)-like receptor-containing pyrin domain 3; NF-κB = nuclear factor kappa B; MAPKs = mitogen-activated protein kinases; proIL-1β = prointerleukin-1-beta; IL-1β = interleukin-1-beta; TGF-β = transforming growth factor-beta; DC = dendritic cell; MØ = macrophage; VSMC = vascular smooth muscle cell; UCM = upregulation of costimulatory molecules; MHC = major histocompatibility complex; ECM = extracellular matrix; TCR = T cell receptor.
At a later stage of the disease, immunostimulatory DCs in the arterial wall, activated after recognition of DAMPs through PRRs, engulf and process stress/injury-induced neo-antigens in terms of altered/modified self-proteins generated in early atherosclerotic lesions such as the oxidatively modified apolipoprotein B100 component of LDL, HSPs and others. The vascular autostimulatory DCs then present these altered self-proteins as peptide/major histocompatibility complex (MHC) complexes to naïve autoreactive T cells in secondary lymphoid tissues of the host, leading to an adaptive T cell autoimmune response. In a vicious cycle, cytotoxic effector T cells then migrate into arterial lesions where they cause further vascular injury, leading to the induction of DAMPs that again initiate pro-inflammatory and/or profibrotic innate immune pathways [Figure 1].35–37
It is of note that homeostatic danger signals, denoted here as class V DAMPs, can initiate an UPR in endothelial cells (ECs), SMCs and vascular macrophages. In fact, multiple local stressors in the arterial wall, including to the presence of ROS and oxidised lipids, shear stress and increased homocysteine-/cholesterol-mediated stress, have been shown to cause ER stress in vessel cells during the initiation and progression of atherosclerosis. As highlighted in a recent review, the activation of the various UPR signalling pathways displays a temporal pattern of activation at different stages of the disease.44 Thus, the ATF6 and IRE1α pathways are activated in ECs in athero-susceptible regions of pre-lesional arteries whereas the PERK pathway is activated in SMCs and macrophages in early lesions.1 With the progression of atherosclerosis, the extended duration and increased intensity of ER stress in lesions lead to prolonged and enhanced UPR signalling. Under this circumstance, the PERK pathway induces the expression of death effectors and, possibly, IRE1α activates apoptosis signalling pathways. This leads to the apoptosis of macrophages, ECs and SMCs in advanced lesions. The subsequent unavoidable elicitation of other classes of DAMPs, then, may promote what is now UPR-independent vascular inflammation. It is likely this occurs in terms of a crosstalk with the NLRP3 inflammasomes located in macrophages, thereby contributing to the clinical progression of atherosclerosis.45
Myocardial Infarction
In regard to CVDs, a myocardial infarction (MI) is a classic example of an atherosclerosis-associated acute disease. Its pathophysiology is a typical example of consequences in the course of ischaemia plus postischaemic reperfusion injury (IRI), here to the myocardium mediated by a burst of ROS—in particular, mitochondria-derived ROS.46,47 Notably, experimental data suggest that up to 50% of the final infarct size may be related to IRI.48 Again, DAMPs are predominantly involved in this pathogenetic scenario.49 Induced by IRI and subsequently recognised by PRR-bearing cells (for example, neutrophils), DAMPs elicit a sterile inflammatory response following primary coronary artery occlusion. Thus, DAMPs such as HMGB1, adenosine triphosphate (ATP) and S100A8/A9 have been shown to be locally released following a MI. Recognition of these DAMPs by PRRs such as TLRs (e.g. TLR3 and TLR4) trigger innate immune pathways to evoke an inflammatory response that aggravates the primary IRI to the myocardium.42,50–53 Of note, activation of the NLRP3 inflammasome by DAMPs, such as ATP, plays an eminent role in the creation of a myocardial inflammatory response that, as stressed above, increases the infarction size.53–55
Moreover, the patient’s long-term outcome after a MI event is influenced by innate immune responses as well. Thus, post-MI, a controlled inflammatory/fibrotic innate immune response can lead to the clearance of injured tissue, angiogenesis and the proliferation of fibroblasts, eventually resulting in scar formation and infarct healing. However, uncontrolled dysregulation of the response, as shown in mice, and under involvement of the NLRP3 inflammasome may result in DAMPs-driven continued cardiomyocyte loss. This results in the overshooting of fibrosis beyond the limits of the infarcted area, reactive hypertrophy and chamber dilatation. This process is termed adverse cardiac remodelling and is known to lead to functional compromise and heart failure.54,56
Metabolic Diseases
In the case of metabolic diseases, class V DAMPs play a crucial role. As mentioned above, this class of DAMPs can be generated by intracellular stress in non-dying cells. This can occur by the slightest metabolic perturbations of the homeostasis within the intra/extracellular microenvironment. Such a scenario can be observed in diseases such as type 2 diabetes (T2D) and metabolic syndrome. Additionally, in obesity, as in T2D, primary perturbations of the ER provoke a chain of different classes of DAMPs that, via recognition by PRR-bearing cells, promote innate immune tissue inflammation resulting in cell/organ dysfunction. That metabolism and innate immunity are linked is perhaps not surprising as both systems involve recognition of exogenous stressors. But proper handling, in general, leads to the maintenance of homeostasis. In fact, recent studies have revealed intriguing molecular associations between these two processes which could give rise to substantial new insights into the pathogenesis of inflammatory diseases, as briefly described below using the example of T2D.57
T2D represents a prototypical innate immune disease where DAMPs-induced, PRR-triggered sterile autoinflammatory processes lead to β cell dysfunction and ultimately cell death (pyroptosis).58–61 Current notions hold that metabolic insults such as insulin resistance, prolonged hyperglycaemia and increased free fatty acid levels (mechanistically explained by depleting ER calcium levels) leads to excessive stimulation of insulin production in the β cells that are associated with protein (proinsulin) accumulation in the ER.1
The increasing protein (proinsulin) overload, however, leads to a disruption of ER homeostasis which results in the accumulation of newly synthesised unfolded/misfolded proteins in the ER lumen, which can be regarded as class V DAMPs.62–64 This scenario elicits a metabolic perturbation of the ER, which becomes exhausted, thereby causing ER stress that is usually associated with oxidative stress.65,66 As noted in part one of this review, this kind of ER stress/oxidative stress activates signalling pathways of the UPR whereby the three branches of the UPR—PERK, IRE1α and ATF6—sense those accumulating misfolded proteins via their function as recognition receptors.1 Consequently, it is the prolonged or excessive function of the β cell UPR that provokes a local inflammatory response in terms of a crosstalk with other members of the innate immune system that, via aggravation of insulitis, finally contributes to β cell dysfunction and death in T2D.1,59,63,67
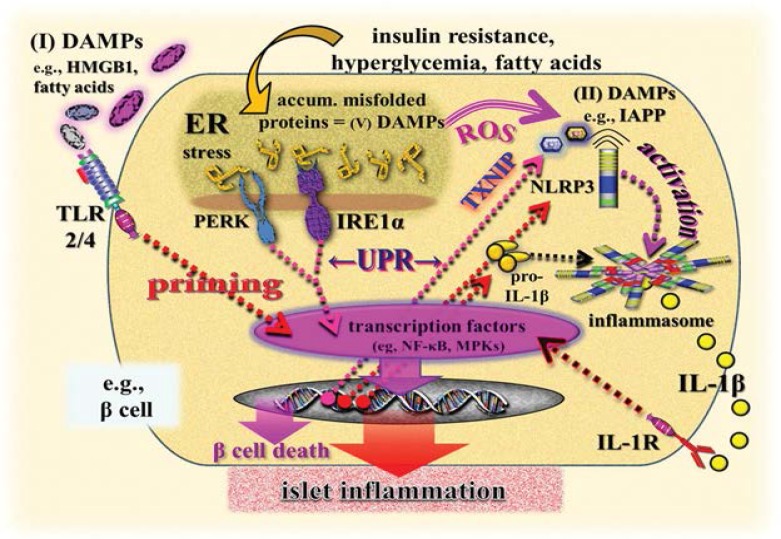
At this point, class I and II DAMPs come into play by activating, in islet cells and resident islet macrophages, the NLRP3 inflammasome as well as other NLRP3-related and NLRP6-dependent pathways.60,61,68–73 The priming step of NLRP3 inflammasome activation is reportedly believed to be instigated by systemic and/or islet tissue-derived class I DAMPs, including HMGB1, HSP70, fatty acids (palmitate) and islet amyloid polypeptide; the last of these is also discussed as an NLRP3 activator. These DAMPs can stimulate TLR2 and TLR4 expressed in islets and pancreatic macrophages to trigger transcriptional pathways, leading to the activation of nuclear factor kappa β (NF-kβ) and mitogen-activated protein kinases (MAPKs).59–61,72,74,75 Of note, for the first time, UPR-derived class II DAMPs, namely thioredoxin-interacting protein (TXNIP), have been found to initiate the post-translational activation step of the NLRP3 inflammasome in T2D. In fact, recent evidence suggests that TXNIP is a critical link between ER stress, NLRP3 inflammasome activation, islet inflammation and programmed β cell death. In the course of hyperactivation of the UPR to irremediable ER stress, TXNIP becomes rapidly activated by ER stressors via induction by the PERK and IRE1α pathway to trigger interleukin(IL)-1β (IL-1β) production, thereby contributing to local sterile islet inflammation [Figure 2].64,75,76
Figure 2:
Simplified illustration of a scenario modelling the role of DAMPs in UPR-mediated and NLRP3 inflammasome-promoted islet inflammation and programmed β cell death in type 2 diabetes mellitus.
DAMPs = damage-associated molecular pattern molecules; UPR = unfolded protein response; NLRP3 = (NOD)-like receptor (NLR)-containing pyrin domain 3; HMGB1 = high-mobility group box 1; ER = endoplasmic reticulum; ROS = reactive oxygen species; IAPP = islet amyloid polypeptide; TLR = Toll-like receptor; PERK = protein kinase-like eukaryotic initiation factor 2α kinase; IRE1α = inositol-requiring transmembrane kinase/endoribonuclease 1α; TXNIP = thioredoxin-interacting protein; pro-IL-1β = pro-interleukin-1β; NF-κβ = nuclear factor kappa β; MPKs = mitogen-activated protein kinases; IL-1β = interleukin-1β; IL-1R = interleukin 1 receptor.
Of note, as also discussed elsewhere, intersection and crosstalk between the two tools of the innate immune system, the ER stress/UPR-signalling and the inflammasome machinery, appear to regulate the quality, intensity and duration of innate immune pro-inflammatory and proapoptotic responses.77,78 This reflects a new quality of DAMPs’ role in terms of a ‘DAMPs axis’—the consecutively operating ‘DAMPs axis’ composed of class V DAMPs (misfolded proteins in the ER) → class I DAMPs (for example, HSPs) → class II DAMPs (TXNIP) which leads to islet inflammation in T2D and contributes to β cell failure. Clearly, future studies are needed to determine if the proposal of such a ‘DAMPs axis’ reflects an innate immune pathway that, in principle, contributes to the pathogenesis of metabolic inflammatory diseases or even neurodegenerative diseases.
Neurodegenerative Diseases
The phenomenon of ER stress in association with inflammasome-mediated inflammation is also encountered in neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis and prion-related diseases. All of these have diverse clinical manifestations but all involve, besides neuroinflammation, the scenario of a ‘perturbed proteostasis’, or the accumulation of misfolded pathological proteins. Notably, this fact has led to their classification as protein misfolding disorders.79 For example, the hallmark lesions in the pathology of AD, which are extracellular deposits of amyloid β (Aβ) peptides derived from cleavage of the amyloid precursor protein (APP) as well as neurofibrillary tangles composed of the hyperphosphorylated Tau protein, both arise from protein misfolding in the form of oligomers.80
On the other hand, neuroinflammatory processes characterised by the activation of astrocytes and microglia and the release of pro-inflammatory mediator substances are also recognised as aetiologic events in AD evolution. In fact, AD represents a prototypical neurodegenerative disease where DAMP-induced PRR-triggered sterile autoinflammatory processes are associated with neuronal cell dysfunction finally leading to neuron death (apoptosis/pyroptosis).
To date, there are several competing hypotheses that attempt to explain which comes first, and what drives what in governing AD pathogenesis, including the Aβ cascade, Tau protein, oxidative stress and inflammation hypotheses.81 However, according to the danger/injury model, any stress activates the innate immune system which then reacts with an inflammatory response. Thus, in the current review article, oxidative stress due to the overproduction of ROS caused by a genetically determined age-dependent decline in mitochondrial function is here proposed to be the ‘head of the snake’ of the AD-typical pathologic cascade, phrased here as the ‘mitochondrial cascade hypothesis’.
In fact, increasing evidence suggests that dysfunctioning mitochondria mutually cause oxidative stress that is associated with the production of accumulating APP-derived Aβ peptide and hyperphosphorylated Tau proteins.81 In addition, it is the intraneuronal overload of these proteins that, as similarly discussed in T2D, leads to ER stress. In fact, ER stress with a subsequent UPR may also play a direct role in the aetiopathogenesis of sporadic AD.82–84 Thus, intraneuronal ER stress in AD is well documented and is proposed to be primarily caused by mitochondrial dysfunction-mediated production of ROS leading to the accumulation of Aβ and Tau proteins. A reverse causality is also hypothesised as ER stress is primarily caused by accumulating Aβ, subsequently promoting oxidative stress [Figure 3].81,85–88 Further, as has been discussed elsewhere, the additional promotion of ER stress in AD is provided by ongoing chronic mitochondrial dysfunction, resulting in continuously greater oxidative stress associated with accumulating Aβ oligomers, as well as contributing to calcium dyshomeostasis and DNA alterations in the form of oxidised mtDNA.83,87,89,90 Finally, all of these intraneuronally accumulating molecules induce permanent ER stress, promoting an UPR which reportedly has been activated in postmortem brain samples from AD patients.82–84,91,92 In other words, these ER stress-inducing molecules operate as class V DAMPs that are recognised by the three stress sensors: PERK, IRE1 and ATF6. This promotes the UPR signalling network. Interestingly, a growing body of evidence suggests that DAMPs-induced UPR signalling events may actually control the expression of diverse AD-related proteins as well as early steps of APP maturation and processing.82,83
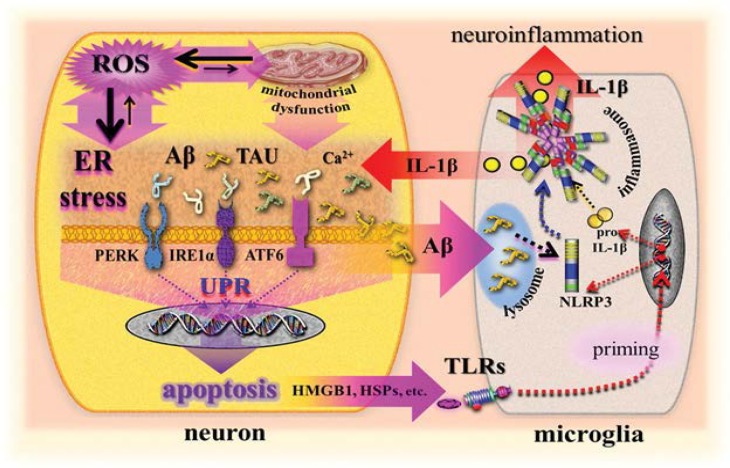
Figure 3:
Simplified illustration of a scenario modelling the role of DAMPs in ER stress/UPR-mediated, NLRP3-promoted neuroinflammation and neuronal cell death in Alzheimer’s disease.
ER = endoplasmic reticulum; UPR = unfolded protein response; NLRP3 = nucleotide binding oligomerization domain (NOD)-like receptor (NLR)-containing pyrin domain 3; ROS = reactive oxygen species; IL-1β = interleukin-1β; Aβ = amyloid β; TAU = Tau protein; Ca2+ = calcium ion; PERK = protein kinase-like eukaryotic initiation factor 2-alpha kinase; IRE1α = inositol-requiring transmembrane kinase/endoribonuclease 1α; ATF6 = activating transcription factor 6; proIL-1β = prointerleukin-1β; TLRs = Toll-like receptors; HMGB1 = high-mobility group box 1; HSPs = heat shock proteins.
Again, as has been similarly proposed for T2D, a DAMPs-driven innate immune crosstalk between ER-stress/UPR and NLRP3 activation can be discussed for AD that may contribute to pro-inflammatory and proapoptotic responses, as pathognomonically observed in AD. According to current notions, however, this crosstalk does not take place in a single cell but between two cell types: neurons (ER stress-UPR) and microglia/astrocytes (NLRP3 inflammasome) [Figure 3].
In fact, inflammasome-dependent pathways appear to play an emerging role in the pathogenesis of neuroinflammation, including AD.93 In particular, the NLRP3 inflammasome has recently gained increasing attention.94 In vivo studies, cell experiments and investigations on transgenic APP/PS1 mice have shown that fibrillar Aβ, obviously acting as a class II DAMP, activates the NLRP3 inflammasome to produce microglial IL-1β. Phagocytosis of Aβ and subsequent lysosomal damage associated with the release of cathepsin B were identified to initiate NLRP3 inflammasome activation promoting neuroinflammation.1,95,96 It is conceivable that stress- or apoptosis-derived class I DAMPs may promote priming of the NLRP3 inflammasome as transcriptionally triggered by TLRs. Thus, class I DAMPs, such as neuronal stress-induced HSP72 as well as TLRs of the microglia including TLR2, 4 and 9, have been discussed and reported to be involved in AD-associated neuroinflammation.80,97–100 In turn, microglial NLRP3 inflammasome products, such as IL-1β, promote AD pathology via contributions to intraneuronal amyloidogenesis and the formation of neurofibrillary tangles. This results in an innate vicious immune circle of pathogenic pathways in AD [Figure 3].93,94,96,101
Taken all together, a wealth of information has recently emerged that links ER stress/UPR and NLRP3 inflammasome signalling to AD pathogenesis. However, the precise roles of these innate immune pathways in promoting and modulating AD remain elusive. Still, AD must be regarded as a complex neurodegenerative disease with an unclear aetiology.
Malignant Diseases: Anticancer therapy
The role of DAMPs in malignant diseases is a therapeutic one—to attempt to eradicate tumours via the elicitation of DAMPs. Notably, the field of anticancer therapy has recently experienced a significant paradigm shift. An expanding body of evidence now indicates that antineoplastic agents do not mediate their therapeutic effects due to their capacity to directly kill malignant cells but rather actively stimulate adaptive anti-tumour immune responses via the induction of DAMPs.
In general, apoptotic cell death, as characterised by a morphologically homogenous entity, has been considered essentially non-immunogenic—that is, intrinsically tolerogenic. Thus, cancer cells undergoing a kind of physiological apoptosis cause an induction of tolerance towards cancer antigens. The already low immunogenic cancer cells are further cleared up ‘silently’ by phagocytes without evoking inflammation and anti-tumour immunity, a phenomenon called tolerogenic cell death.
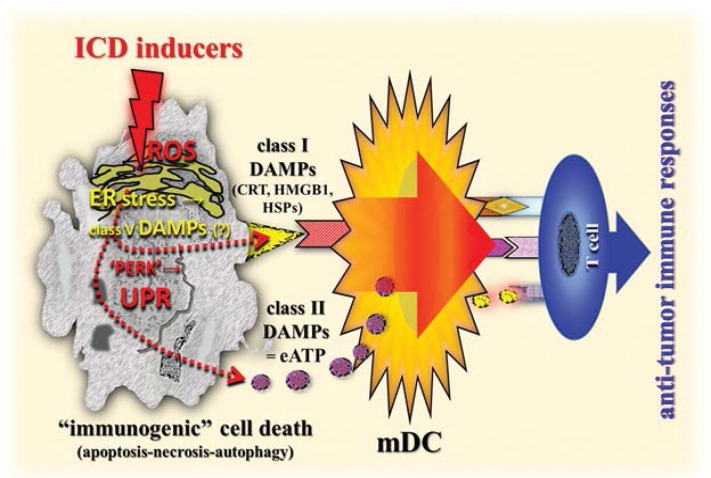
However, growing evidence indicates that certain chemotherapeutics, radiotherapy and photodynamic therapy can induce a functionally distinct type of apoptosis in cancer cells that is associated with the generation of immunogenicity-promoting DAMPs. Notably, these DAMPs assist in initiating an adaptive anti-tumour immune response, which is a phenomenon called immunogenic cell death (ICD) induced by ICD inducers.102–104 Of note, it is the spatiotemporally-defined generation of those DAMPs—the pre-, early-, mid- or late apoptotic emission of DAMPs—that are sensed by PRR-bearing cells of the innate immune system, thereby keeping the immune system alert in a pro-inflammatory state.102,105 Key DAMPs generated, trafficked and emitted by dying cancer cells that are found to be crucial for cancer immunogenicity include ER-derived calreticulin (CRT) and HSP70 exposed at the cell surface. Additionally, ATP extracellularly secreted in a complex mechanistic manner, nucleic acids and HMGB1 in a special redox modification are released from dying cells. 103,105–107 For example, during ICD, secreted extracellular ATP (eATP) mainly binds the P2X purinoceptor 7 receptors causing activation of the NLRP3 inflammasome which in turn leads to caspase-1-mediated processing and secretion of active IL-1β.1,108
Interestingly, the phenomenon described above is also at work in all scenarios of ICD; emerging molecular links between ROS-based ER stress, UPR signalling, DAMPs and anti-tumour immunity have recently been revealed.102,105 In fact, the efficient emission of DAMPs from dying cancer cells relies on the joint induction of ROS and ER stress, which governs the trafficking of those DAMPs. For example, chemotherapy-induced pre-apoptotic CRT translocation to the cell surface has been found to be mediated by co-interaction with PERK-induced eukaryotic initiation factor 2α phosphorylation, ER-to-Golgi transport and the classical secretory pathway.1 Similarly, the pre-apoptotic secretion of the class II DAMP eATP is mediated by secretory pathways including the classical and PERK-regulated proximal secretory pathway [Figure 4].1,97,106,109,110 Hence, a robust ER stress response, preferably accompanied by or induced by ROS production, is a salient biochemical prerequisite for the generation of homeostatic danger signals/class V DAMPs that are sensed by branches of the UPR, thereby activating the UPR in the scenario of ICD. In other words, the induction of as-yet-unknown class V DAMPs, via a complex interplay between ER stress and ROS production, initiate signalling pathways to emit secondary class I and II DAMPs such as pre-apoptotic CRT, early apoptotic eATP, and mid/late apoptotic HMGB1 and HSPs, leading to an ICD-induced adaptive anti-tumour immune response [Figure 4].
Figure 4:
Simplified diagram of a schematic illustration of the role of DAMPs in the elicitation of adaptive anti-tumour immune responses. Compare also Figure 4 in part 1 of this review.1
DAMPs = damage-associated molecular patterns; ICD = immunogenic cell death; ROS = reactive oxygen species; ER = endoplasmic reticulum; CRT = calreticulin; HMGB1 = high-mobility group box 1; HSPs = heat shock proteins; PERK = protein kinase-like eukaryotic initiation factor 2α kinase; UPR = unfolded protein response; eATP = extracellular adenosine triphosphate; mDC = mature dendritic cell.
In fact, these class I and II DAMPs in the company of cancer cell antigens cause maturation of DCs, which ultimately activate an anti-tumour cluster of differentiation (CD) 4+/CD8+ T cell immune response.1,105 Indeed, it currently appears that the immunogenic characteristics of dying cells (in the form of apoptotic, autophagic, necroptotic and pyroptotic cell death) are mainly mediated by DAMPs. The DAMPs, via induction of immunostimulatory tumour antigen-presenting DCs, elicit pathways leading to the development of an innate/adaptive immune defense response against tumours. This occurs following ICD induction, thereby contributing to the immune-mediated eradication of tumours.102,105,111
Infectious Diseases
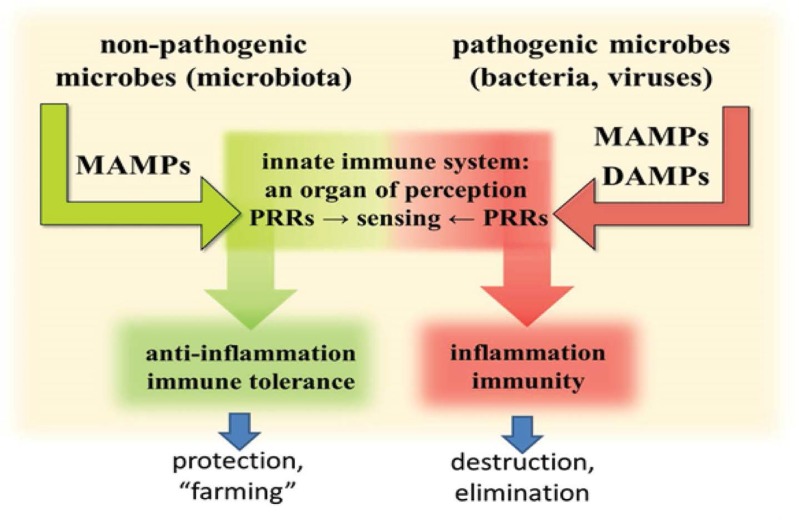
The dominating role of DAMPs in human diseases is strikingly, but perhaps unexpectedly, reflected by their participation in infectious disorders. In fact, their inflammation-amplifying effect in infectious diseases is already well known. However, there is growing evidence in support of the notion that DAMPs are the real players in instigating and mounting a vigorous injurious inflammatory response against invading pathogens, resulting in an adaptive anti-pathogen immune response.112–115 In particular, overwhelming evidence in support of this theory has come from recent insights into the function of the mammalian gut’s innate immune system’s ability to discriminate, under the control of DCs and regulated by innate immune PRRs, between harmless commensal bacteria to induce immune tolerance and harmful pathogenic bacteria to induce inflammation and immunity.112–115 In other words, commensals, although possessing PAMPs, do not cause inflammation and adaptive immunity. This is a notion that has led to the creation of the more precise term, microbe-associated molecular patterns (MAMPs), given the fact that the microbial ligands sensed by PRRs are not necessarily confined to pathogens, but are also present in commensal bacteria. In fact, on a molecular level, it appears unlikely that the innate immune system possesses the ability to distinctly discriminate between the myriads of non-pathogenic commensals within the gut microbiota and those that operate as injuring pathogenic microbes. Rather, this function may be achieved by recognising pathogen-induced DAMPs in addition to MAMPs, which do not emit a danger signal per se, that is, by sensing altered patterns of molecules associated with cell/tissue damage caused by pathogenic microbes [Figure 5]. In the following section, a few examples of the role of DAMPs in cooperation with MAMPs in pathogen-induced infectious inflammation are briefly touched upon.
Figure 5:
This model shows commensal microbes expressing MAMPs which are protected by immunotolerance. Pathogenic microbes, expressing MAMPs and causing injury-induced DAMPs, are eliminated by inflammation and immunity.
MAMPS = microbe-associated molecular patterns; DAMPs = damage-associated molecular pattern molecules; PRRs = pattern recognition receptors.
As mentioned above, any perturbation of physical or homeostatic conditions within the cell reflects the presence of class V DAMPs. This seems also to be true for cell stress as provoked by viral or bacterial infection, as has been discussed elsewhere.116 For example, virus entry requires membrane and cytoskeletal perturbation/disruption, and both membrane fusion or actin-depolymerising agents alone are able to activate antiviral genes. Accordingly, recent studies using virus-like particles have supported this hypothesis.117 In addition, viruses cause cellular stress and change the cellular environment. In particular, viruses provoke oxidative stress or ER stress accompanied with oxidative stress, thereby inducing ‘homeostatic’ class V DAMPs. Even simpler, both viruses and intracellular bacteria cause cell stress through their replication alone. Broad changes to the cellular environment, including host translational inhibition and overexpression of viral proteins could cause ER stress associated with the generation of DAMPs. Collectively, these DAMPs-induced pathways lead to ampification of PRR-triggered antiviral signalling, converging, for example, on the activation of the transcription factor interferon regulatory factor 3.85,116
In addition to ER stress-provoked class V DAMPs, class I and II DAMPs can be exposed or released from bacteria- or virus-infected cells. Thus, in studies on mice, the DAMP, S100A9, was identified as an activator of TLR signalling during influenza A virus (IAV) infection.118 S100A9 was found to be released from undamaged IAV-infected cells and extracellular S100A9 acted as a critical host-derived molecular pattern to regulate inflammatory response outcomes and disease during infection by exaggerating the pro-inflammatory response, cell death and virus pathogenesis. Furthermore, the inflammatory activity of extracellular S100A9 was mediated by activation of the TLR4-MyD88 pathway.118
In accordance with these observations in mice are studies on a bacterial high-grade sepsis model in non-human primates that allowed quantification of DAMPs and PAMPs after bacterial challenges of increasing clinical severity.119 These studies allowed a definition for the contribution of bacterial PAMPs and endogenous DAMPs to clinical organ dysfunction in septic and sterile SIRS.119 Interestingly, the experiments showed that the degree of clinical severity of the bacterial sepsis reflecting the tissue/organ injury correlated with the concentration of the circulating mtDNA DAMP better than with bacterial DNA acting as a PAMP. This indicates that DAMPs from septic injury, rather than PAMPs, determine the clinical course of bacterial sepsis. In particular, the study showed that following a lethal bacterial challenge, bacterial DNA only transiently increased while mtDNA levels remained elevated until death, suggesting on-going tissue damage long after the bacteria were cleared. It is of note that the clinical relevance of the role of DAMPs, as assessed by findings from this non-human primate sepsis model, has been stressed by a recently performed clinical study showing that the mtDNA DAMP was elevated in the blood of patients suffering from severe sepsis.120
Outlook
Without a doubt, DAMPs will have a considerate impact on routine practical medicine in the future. They could be used as either biomarkers for the proper diagnosis and prognosis of diseases, or as therapeutics in the treatment of tumours or in vaccines for prophylaxis of infections. The use of DAMPs as biomarkers is indeed emerging; thus, in view of the possibility of three different types of injury-induced innate immune responses, clinicians are eager to know at an early stage what pathogenic pathways will be induced by a given injury in a patient.Will the injury have a controlled response, leading to smooth wound healing and scar formation? Will the injury undergo symptomless infarct healing after a MI? Or will the injury result in a catastrophically uncontrolled acute hyperinflammatory/chronic overshooting reparative response? Will SIRS follow polytrauma or cardiac dilative remodelling after a MI, leading to functional compromise and heart failure? Indeed, the measurement of DAMPs in terms of biomarkers in such situations will be helpful in assessing the degree of the underlying lesions, ensuring the right diagnosis, making personalised DAMPs-based therapeutic decisions and assigning valid outcome prognoses. For example, interfering with DAMPs-induced innate immune pathways such as NLRP3-mediated pathways may be a good treatment option to prevent SIRS or dilative cardiac remodelling.
In addition, the induction of DAMPs by special treatment modalities to eradicate tumours is also emerging. In fact, the new insights into the role of DAMPs in successfully eliciting anti-tumour immune responses have opened modern perspectives for the development of treatment modalities aiming to cure cancer. Accordingly, it would be desirable to identify class V DAMPs evoked by ER stress or other processes such as autophagy that instigate UPR signalling in cancer cells leading to exposure and/or secretion of class I and II DAMPs, thereby eliciting adaptive anti-tumour T cell immunity.
In contrast to such treatment of tumours, namely to promote ER stress-associated class V DAMP formation to instigate an UPR, future treatment strategies in metabolic and neurodegenerative diseases should include efforts to interfere with the ER stress-associated generation of dyshomeostatic class V DAMPs. In regard to elucidating mechanisms involved in both pathways, future research will have to concentrate on efforts to explore the precise mechanism involved in the network of ER stress ↔ generation of class V DAMPs → UPR signalling ↔ induction of class I and II DAMPs.
Finally, with respect to the detrimental role of DAMPs in amplifying infectious or sterile injury-induced inflammation, another attractive therapeutic modality emerges at the horizon—to develop strategies which specifically inhibit or at least mitigate DAMPs-mediated hyperinflammatory responses without compromising the anti-pathogen innate/adaptive immune response. In fact, such a possibility may help to improve the clinical management of infection- or injury-evoked hyperinflammatory diseases such as SIRS.
Conclusion
This article is part II of a review addressing the role of DAMPs in human diseases, focusing on traumatic, cardiovascular, metabolic, neurodegenerative, malignant and infectious diseases. Available research in this area shows that there is certainly a future role for DAMPs in routine practical medicine as they could be used as either biomarkers for the proper diagnosis and prognosis of diseases, as therapeutics in the treatment of tumours or in vaccines for prophylaxis of infections. Using DAMPs as biomarkers would be advantageous in assessing the degree of underlying lesions, ensuring the right diagnosis and assigning valid outcome prognoses. In addition, the ‘dampening’ of DAMPs could also help to improve the clinical management of infection- or injury-evoked hyperinflammatory diseases such as SIRS. Research has also shown there is a plausible role for DAMPs in eliciting anti-tumour immune responses, which could lead to groundbreaking developments in treatment modalities aiming to cure cancer.
References
- 1.Land WG. The role of damage associated molecular patterns in human diseases: Part I: Promoting inflammation and immunity. Sultan Qaboos Univ Med J. 2015;15:9–21. [PMC free article] [PubMed] [Google Scholar]
- 2.Land WG. Emerging role of innate immunity in organ transplantation part II: Potential of damage-associated molecular patterns to generate immunostimulatory dendritic cells. Transplant Rev (Orlando) 2012;26:73–87. doi: 10.1016/j.trre.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Gallo PM, Gallucci S. The dendritic cell response to classic, emerging, and homeostatic danger signals: Implications for autoimmunity. Front Immunol. 2013;4:138. doi: 10.3389/fimmu.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenceslau CF, McCarthy CG, Szasz T, Spitler K, Goulopoulou S, Webb RC, Working Group on DAMPs in Cardiovascular Disease et al. Mitochondrial damage-associated molecular patterns and vascular function. Eur Heart J. 2014;35:1172–7. doi: 10.1093/eurheartj/ehu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang M, Carroll MC. Natural IgM-mediated innate autoimmunity: A new target for early intervention of ischemiareperfusion injury. Expert Opin Biol Ther. 2007;7:1575–82. doi: 10.1517/14712598.7.10.1575. [DOI] [PubMed] [Google Scholar]
- 6.Binder CJ. Naturally occurring IgM antibodies to oxidation-specific epitopes. Adv Exp Med Biol. 2012;750:2–13. doi: 10.1007/978-1-4614-3461-0_1. [DOI] [PubMed] [Google Scholar]
- 7.Uchida K. Natural antibodies as a sensor of electronegative damage-associated molecular patterns (DAMPs) Free Radic Biol Med. 2014;72:156–61. doi: 10.1016/j.freeradbiomed.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–67. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 11.Lee EJ, Park JH. Receptor for advanced glycation endproducts (RAGE), its ligands, and soluble RAGE: Potential biomarkers for diagnosis and therapeutic targets for human renal diseases. Genomics Inform. 2013;11:224–9. doi: 10.5808/GI.2013.11.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong Y, Kinio A, Saleh M. Functions of NOD-like receptors in human diseases. Front Immunol. 2013;4:333. doi: 10.3389/fimmu.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond RA, Brown GD. Signalling C-type lectins in antimicrobial immunity. PLoS Pathog. 2013;9:e1003417. doi: 10.1371/journal.ppat.1003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jounai N, Kobiyama K, Takeshita F, Ishii KJ. Recognition of damage-associated molecular patterns related to nucleic acids during inflammation and vaccination. Front Cell Infect Microbiol. 2013;2:168. doi: 10.3389/fcimb.2012.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol. 2014;32:461–88. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- 16.Unterholzner L. The interferon response to intracellular DNA: Why so many receptors? Immunobiology. 2013;218:1312–21. doi: 10.1016/j.imbio.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Ratsimandresy RA, Dorfleutner A, Stehlik C. An update on PYRIN domain-containing pattern recognition receptors: From immunity to pathology. Front Immunol. 2013;4:440. doi: 10.3389/fimmu.2013.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewar D, Moore FA, Moore EE, Balogh Z. Postinjury multiple organ failure. Injury. 2009;40:912–8. doi: 10.1016/j.injury.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Hirsiger S, Simmen HP, Werner CM, Wanner GA, Rittirsch D. Danger signals activating the immune response after trauma. Mediators Inflamm. 2012;2012:315941. doi: 10.1155/2012/315941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoecklein VM, Osuka A, Lederer JA. Trauma equals danger-damage control by the immune system. J Leukoc Biol. 2012;92:539–51. doi: 10.1189/jlb.0212072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Straino S, Di Carlo A, Mangoni A, De Mori R, Guerra L, Maurelli R, et al. High-mobility group box 1 protein in human and murine skin: Involvement in wound healing. J Invest Dermatol. 2008;128:1545–53. doi: 10.1038/sj.jid.5701212. [DOI] [PubMed] [Google Scholar]
- 23.Yang S, Xu L, Yang T, Wang F. High-mobility group box-1 and its role in angiogenesis. J Leukoc Biol. 2014;95:563–74. doi: 10.1189/jlb.0713412. [DOI] [PubMed] [Google Scholar]
- 24.Zampell JC, Yan A, Avraham T, Andrade V, Malliaris S, Aschen S, et al. Temporal and spatial patterns of endogenous danger signal expression after wound healing and in response to lymphedema. Am J Physiol Cell Physiol. 2011;300:C1107–21. doi: 10.1152/ajpcell.00378.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–76. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen MJ, Brohi K, Calfee CS, Rahn P, Chesebro BB, Christiaans SC, et al. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: Role of injury severity and tissue hypoperfusion. Crit Care. 2009;13:R174. doi: 10.1186/cc8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peltz ED, Moore EE, Eckels PC, Damle SS, Tsuruta Y, Johnson JL, et al. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock. 2009;32:17–22. doi: 10.1097/SHK.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang KY, Yu GF, Zhang ZY, Huang Q, Dong XQ. Plasma high-mobility group box 1 levels and prediction of outcome in patients with traumatic brain injury. Clin Chim Acta. 2012;413:1737–41. doi: 10.1016/j.cca.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–64. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock. 2010;34:55–9. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
- 32.Walko TD, 3rd, Bola RA, Hong JD, Au AK, Bell MJ, Kochanek PM, et al. Cerebrospinal fluid mitochondrial DNA: A novel DAMP in pediatric traumatic brain injury. Shock. 2014;41:499–503. doi: 10.1097/SHK.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, et al. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013;258:591–6. doi: 10.1097/SLA.0b013e3182a4ea46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber-Lang M, Kovtun A, Ignatius A. The role of complement in trauma and fracture healing. Semin Immunol. 2013;25:73–8. doi: 10.1016/j.smim.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–12. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 36.Land WG. Chronic allograft dysfunction: A model disorder of innate immunity. Biomed J. 2013;36:209–28. doi: 10.4103/2319-4170.117622. [DOI] [PubMed] [Google Scholar]
- 37.Witztum JL, Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol. 2014;9:73–102. doi: 10.1146/annurev-pathol-020712-163936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobrin PB. Mechanical factors associated with the development of intimal and medial thickening in vein grafts subjected to arterial pressure. A model of arteries exposed to hypertension. Hypertension. 1995;26:38–43. doi: 10.1161/01.HYP.26.1.38. [DOI] [PubMed] [Google Scholar]
- 39.Land WG. Interaction of Damage-Associated Molecular Patterns and Pathogen-Associated Molecular Patterns with Pattern Recognition Receptor-Bearing Vascular Cells and Myofibroblasts and its Consequences for the Development of Chronic Allograft Dysfunction. In: Land WG, editor. Innate Alloimmunity. Part II. Innate immunity and rejection. Ankara, Lengerich: Başkent University-Pabst Science Publishers; 2011. pp. 518–65. [Google Scholar]
- 40.Weismann D, Binder CJ. The innate immune response to products of phospholipid peroxidation. Biochim Biophys Acta. 2012;1818:2465–75. doi: 10.1016/j.bbamem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Souza AW, Westra J, Limburg PC, Bijl M, Kallenberg CG. HMGB1 in vascular diseases: Its role in vascular inflammation and atherosclerosis. Autoimmun Rev. 2012;11:909–17. doi: 10.1016/j.autrev.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Schiopu A, Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm. 2013;2013:828354. doi: 10.1155/2013/828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding Z, Liu S, Wang X, Dai Y, Khaidakov M, Deng X, et al. LOX-1, mtDNA damage and NLRP3 inflammasome activation in macrophages: Implications in atherogenesis. Cardiovasc Res. 2014;103:619–28. doi: 10.1093/cvr/cvu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou AX, Tabas I. The UPR in atherosclerosis. Semin Immunopathol. 2013;35:321–32. doi: 10.1007/s00281-013-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menu P, Mayor A, Zhou R, Tardivel A, Ichijo H, Mori K, Tschopp J. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 2012;3:e261. doi: 10.1038/cddis.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res. 2014;114:524–37. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouyang YB, Stary CM, White RE, Giffard RG. The use of microRNAs to modulate redox and immune response to stroke. Antioxid Redox Signal. 2015;22:187–202. doi: 10.1089/ars.2013.5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 49.de Haan JJ, Smeets MB, Pasterkamp G, Arslan F. Danger signals in the initiation of the inflammatory response after myocardial infarction. Mediators Inflamm. 2013;2013:206039. doi: 10.1155/2013/206039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding HS, Yang J, Chen P, Yang J, Bo SQ, Ding JW, et al. The HMGB1-TLR4 axis contributes to myocardial ischemia/reperfusion injury via regulation of cardiomyocyte apoptosis. Gene. 2013;527:389–93. doi: 10.1016/j.gene.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 51.Lu C, Ren D, Wang X, Ha T, Liu L, Lee EJ, et al. Toll-like receptor 3 plays a role in myocardial infarction and ischemia/reperfusion injury. Biochim Biophys Acta. 2014;1842:22–31. doi: 10.1016/j.bbadis.2013.10.006. doi: 1016/j.bbadis.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandanger Ø, Ranheim T, Vinge LE, Bliksøen M, Alfsnes K, Finsen AV, et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemiareperfusion injury. Cardiovasc Res. 2013;99:164–74. doi: 10.1093/cvr/cvt091. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi M. NLRP3 inflammasome as a novel player in myocardial infarction. Int Heart J. 2014;55:101–5. doi: 10.1536/ihj.13-388. [DOI] [PubMed] [Google Scholar]
- 54.Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–30. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mezzaroma E, Marchetti C, Toldo S. Letter by Mezzaroma, et al regarding article, “NLRP3 inflammasome as a therapeutic target in myocardial infarction”. Int Heart J. 2014;55:379. doi: 10.1536/ihj.14-140. [DOI] [PubMed] [Google Scholar]
- 56.Liaudet L, Rosenblatt-Velin N. Role of innate immunity in cardiac inflammation after myocardial infarction. Front Biosci (Schol Ed) 2013;5:86–104. doi: 10.2741/S359. [DOI] [PubMed] [Google Scholar]
- 57.Cheng SC, Joosten LA, Netea MG. The interplay between central metabolism and innate immune responses. Cytokine Growth Factor Rev. 2014;25:707–13. doi: 10.1016/j.cytogfr.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 58.Donath MY, Böni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009;24:325–31. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- 59.Eizirik DL, Miani M, Cardozo AK. Signalling danger: Endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia. 2013;56:234–41. doi: 10.1007/s00125-012-2762-3. [DOI] [PubMed] [Google Scholar]
- 60.Jin C, Flavell RA. Innate sensors of pathogen and stress: Linking inflammation to obesity. J Allergy Clin Immunol. 2013;132:287–94. doi: 10.1016/j.jaci.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 61.Westwell-Roper C, Nackiewicz D, Dan M, Ehses JA. Toll-like receptors and NLRP3 as central regulators of pancreatic islet inflammation in type 2 diabetes. Immunol Cell Biol. 2014;92:314–23. doi: 10.1038/icb.2014.4. [DOI] [PubMed] [Google Scholar]
- 62.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–17. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oslowski CM, Urano F. The binary switch that controls the life and death decisions of ER stressed β cells. Curr Opin Cell Biol. 2011;23:207–15. doi: 10.1016/j.ceb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16:250–64. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landau G, Kodali VK, Malhotra JD, Kaufman RJ. Detection of oxidative damage in response to protein misfolding in the endoplasmic reticulum. Methods Enzymol. 2013;526:231–50. doi: 10.1016/B978-0-12-405883-5.00014-4. [DOI] [PubMed] [Google Scholar]
- 66.Lu J, Holmgren A. The thioredoxin superfamily in oxidative protein folding. Antioxid Redox Signal. 2014;21:457–70. doi: 10.1089/ars.2014.5849. [DOI] [PubMed] [Google Scholar]
- 67.Liu YP, Zeng L, Tian A, Bomkamp A, Rivera D, Gutman D, et al. Endoplasmic reticulum stress regulates the innate immunity critical transcription factor IRF3. J Immunol. 2012;189:4630–9. doi: 10.4049/jimmunol.1102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim S, Joe Y, Jeong SO, Zheng M, Back SH, Park SW, et al. Endoplasmic reticulum stress is sufficient for the induction of IL-1β production via activation of the NF-κB and inflammasome pathways. Innate Immun. 2014;20:799–815. doi: 10.1177/1753425913508593. [DOI] [PubMed] [Google Scholar]
- 70.Grant RW, Dixit VD. Mechanisms of disease: Inflammasome activation and the development of type 2 diabetes. Front Immunol. 2013;4:50. doi: 10.3389/fimmu.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernandes-Alnemri T, Kang S, Anderson C, Sagara J, Fitzgerald KA, Alnemri ES. Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol. 2013;191:3995–9. doi: 10.4049/jimmunol.1301681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Masters SL. Specific inflammasomes in complex diseases. Clin Immunol. 2013;147:223–8. doi: 10.1016/j.clim.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Shenderov K, Riteau N, Yip R, Mayer-Barber KD, Oland S, Hieny S, et al. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1β in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J Immunol. 2014;192:2029–33. doi: 10.4049/jimmunol.1302549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012;15:518–33. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 75.Oslowski CM, Hara T, O’Sullivan-Murphy B, Kanekura K, Lu S, Hara M, et al. Thioredoxin- interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012;16:265–73. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anthony TG, Wek RC. TXNIP switches tracks toward a terminal UPR. Cell Metab. 2012;16:135–7. doi: 10.1016/j.cmet.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 77.Martinon F, Glimcher LH. Regulation of innate immunity by signaling pathways emerging from the endoplasmic reticulum. Curr Opin Immunol. 2011;23:35–40. doi: 10.1016/j.coi.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 80.Guillot-Sestier MV, Town T. Innate immunity in Alzheimer’s disease: A complex affair. CNS Neurol Disord Drug Targets. 2013;12:593–607. doi: 10.2174/1871527311312050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swerdlow RH. Alzheimer’s disease pathologic cascades: Who comes first, what drives what. Neurotox Res. 2012;22:182–94. doi: 10.1007/s12640-011-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cornejo VH, Hetz C. The unfolded protein response in Alzheimer’s disease. Semin Immunopathol. 2013;35:277–92. doi: 10.1007/s00281-013-0373-9. [DOI] [PubMed] [Google Scholar]
- 83.Hetz C, Mollereau B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci. 2014;15:233–49. doi: 10.1038/nrn3689. [DOI] [PubMed] [Google Scholar]
- 84.Li JQ, Yu JT, Jiang T, Tan L. Endoplasmic reticulum dysfunction in Alzheimer’s disease. Mol Neurobiol. 2015;51:383–95. doi: 10.1007/s12035-014-8695-8. [DOI] [PubMed] [Google Scholar]
- 85.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sutherland GT, Chami B, Youssef P, Witting PK. Oxidative stress in Alzheimer’s disease: Primary villain or physiological by-product? Redox Rep. 2013;18:134–41. doi: 10.1179/1351000213Y.0000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta. 2014;1842:1240–7. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Z, Zhong C. Oxidative stress in Alzheimer’s disease. Neurosci Bull. 2014;30:271–81. doi: 10.1007/s12264-013-1423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gibson GE, Chen HL, Xu H, Qiu L, Xu Z, Denton TT, Shi Q. Deficits in the mitochondrial enzyme α-ketoglutarate dehydrogenase lead to Alzheimer’s disease-like calcium dysregulation. Neurobiol Aging. 2012;33:e13–24. doi: 10.1016/j.neurobiolaging.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bradley-Whitman MA, Timmons MD, Beckett TL, Murphy MP, Lynn BC, Lovell MA. Nucleic acid oxidation: An early feature of Alzheimer’s disease. J Neurochem. 2014;128:294–304. doi: 10.1111/jnc.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoozemans JJ, van Haastert ES, Nijholt DA, Rozemuller AJ, Scheper W. Activation of the unfolded protein response is an early event in Alzheimer’s and Parkinson’s disease. Neurodegener Dis. 2012;10:212–5. doi: 10.1159/000334536. [DOI] [PubMed] [Google Scholar]
- 92.Abisambra JF, Jinwal UK, Blair LJ, O’Leary JC, 3rd, Li Q, Brady S, et al. Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation. J Neurosci. 2013;33:9498–507. doi: 10.1523/JNEUROSCI.5397-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu L, Chan C. The role of inflammasome in Alzheimer’s disease. Ageing Res Rev. 2014;15:6–15. doi: 10.1016/j.arr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tan MS, Yu JT, Jiang T, Zhu XC, Tan L. The NLRP3 inflammasome in Alzheimer’s disease. Mol Neurobiol. 2013;48:875–82. doi: 10.1007/s12035-013-8475-x. [DOI] [PubMed] [Google Scholar]
- 95.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–65. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–8. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamos JE, Oblas B, Pulaski-Salo D, Welch WJ, Bole DG, Drachman DA. Expression of heat shock proteins in Alzheimer’s disease. Neurology. 1991;41:345–50. doi: 10.1212/WNL.41.3.345. [DOI] [PubMed] [Google Scholar]
- 98.Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer’s disease: Amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol. 2009;87:181–94. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 99.Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, et al. TLR2 is a primary receptor for Alzheimer’s amyloid β peptide to trigger neuroinflammatory activation. J Immunol. 2012;188:1098–107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- 100.Trotta T, Porro C, Calvello R, Panaro MA. Biological role of Toll-like receptor-4 in the brain. J Neuroimmunol. 2014;268:1–12. doi: 10.1016/j.jneuroim.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 101.Shi JQ, Zhang CC, Sun XL, Cheng XX, Wang JB, Zhang YD, et al. Antimalarial drug artemisinin extenuates amyloidogenesis and neuroinflammation in APPswe/PS1dE9 transgenic mice via inhibition of nuclear factor-κB and NLRP3 inflammasome activation. CNS Neurosci Ther. 2013;19:262–8. doi: 10.1111/cns.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 103.Krysko O, Løve Aaes T, Bachert C, Vandenabeele P, Krysko DV. Many faces of DAMPs in cancer therapy. Cell Death Dis. 2013;4:e631. doi: 10.1038/cddis.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garg AD, Dudek AM, Agostinis P. Cancer immunogenicity, danger signals, and DAMPs: What, when, and how? Biofactors. 2013;39:355–67. doi: 10.1002/biof.1125. [DOI] [PubMed] [Google Scholar]
- 105.Garg AD, Martin S, Golab J, Agostinis P. Danger signalling during cancer cell death: Origins, plasticity and regulation. Cell Death Differ. 2014;21:26–38. doi: 10.1038/cdd.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inoue H, Tani K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. 2014;21:39–49. doi: 10.1038/cdd.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dodd K, Nance S, Quezada M, Janke L, Morrison JB, Williams RT, Beere HM. Tumor- derived inducible heat-shock protein 70 (HSP70) is an essential component of anti-tumor immunity. Oncogene. 2015;34:1312–22. doi: 10.1038/onc.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 109.Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008;15:1499–509. doi: 10.1038/cdd.2008.67. [DOI] [PubMed] [Google Scholar]
- 110.Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19:1880–91. doi: 10.1038/cdd.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hou W, Zhang Q, Yan Z, Chen R, Zeh HJ, III, Kang R, et al. Strange attractors: DAMPs and autophagy link tumor cell death and immunity. Cell Death Dis. 2013;4:e966. doi: 10.1038/cddis.2013.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carvalho FA, Aitken JD, Vijay-Kumar M, Gewirtz AT. Toll-like receptor-gut microbiota interactions: Perturb at your own risk! Annu Rev Physiol. 2012;74:177. doi: 10.1146/annurevphysiol-020911-153330. [DOI] [PubMed] [Google Scholar]
- 113.Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–35. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 114.Kamdar K, Nguyen V, DePaolo RW. Toll-like receptor signaling and regulation of intestinal immunity. Virulence. 2013;4:207–12. doi: 10.4161/viru.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Strober W. The impact of the gut microbiome on mucosal inflammation. Trends Immunol. 2013;34:423–30. doi: 10.1016/j.it.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Collins SE, Mossman KL. Danger, diversity and priming in innate antiviral immunity. Cytokine Growth Factor Rev. 2014;25:525–31. doi: 10.1016/j.cytogfr.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 117.Roberts AP, Abaitua F, O’Hare P, McNab D, Rixon FJ, Pasdeloup D. Differing roles of inner tegument proteins pUL36 and pUL37 during entry of herpes simplex virus type 1. J Virol. 2009;83:105–16. doi: 10.1128/JVI.01032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsai SY, Segovia JA, Chang TH, Morris IR, Berton MT, Tessier PA, et al. DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: Role of DDX21-TRIF-TLR4-MyD88 pathway. PLoS Pathog. 2014;10:e1003848. doi: 10.1371/journal.ppat.1003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sursal T, Stearns-Kurosawa DJ, Itagaki K, Oh SY, Sun S, Kurosawa S, Hauser CJ. Plasma bacterial and mitochondrial DNA distinguish bacterial sepsis from sterile systemic inflammatory response syndrome and quantify inflammatory tissue injury in nonhuman primates. Shock. 2013;39:55–62. doi: 10.1097/SHK.0b013e318276f4ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yamanouchi S, Kudo D, Yamada M, Miyagawa N, Furukawa H, Kushimoto S. Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: Time course and the association with clinical status. J Crit Care. 2013;28:1027–31. doi: 10.1016/j.jcrc.2013.05.006. [DOI] [PubMed] [Google Scholar]