What’s in a name? the history of medicine is replete with eponyms saluting a scientist’s discovery of a disease. However, it is unusual that the strength of this association results in the use of a pioneer’s name for a contrasting disease. Perhaps this singular distinction has been achieved through the continued use of the eponymous ‘Hodgkin’ for two types of lymphomas: Hodgkin and non-Hodgkin. The first of this two-part tale of lymphomas is devoted to the original category, which was named Hodgkin’s disease, and is today called Hodgkin lymphoma (HL). It all began with a basic dilemma: was this disease an infection, inflammation or tumour?
The Pioneers
In 1666, Marcell Malpighi was one of the first physicians to be credited with describing HL, observed during the autopsy of an 18-year-old female, in his publication: De viscerum structura exercitatio anatomica.1,2 Centuries later, in 1832, a paper was read to the Medical and Chirurgical Society in London, UK, on behalf of Thomas Hodgkin. It described seven patients with painless enlargement of the lymph nodes and included autopsy findings and illustrations from one of the patients given to the author by Robert Carswell.1,3 The author, Thomas Hodgkin (1798–1866) of Guy’s Hospital, London, went on to publish a paper entitled: On some morbid cases of the absorbent glands and spleen.
At this time, autopsies were an irreplaceable source of knowledge for understanding diseases; it is no wonder that in his appointment as ‘Inspector of the Dead’ Hodgkin was an astounding contributor to the Museum at Guy’s Hospital.4 Interest in this disease grew when Richard Bright published Observations on abdominal tumours and intumescence illustrated by cases of disease of the spleen in 1838, linking the spleen to the disease beyond its primary nodal origins.2
In 1856, Samuel Wilks observed similar cases and later, in 1865, published Cases of lardaceous diseases and some allied affections with remarks.5 In the true spirit of peer recognition, he honoured Hodgkin by eponymously referring to this disease as Hodgkin’s lymphoma.6 It is notable that neither Hodgkin nor Wilks supported the diagnosis of this new disease by its microscopic appearance, something that would be unthinkable today when the diagnostic world demands no less proof than morphology, immunohistochemistry and, in many cases, molecular genetics. From the archival tissues of Hodgkin’s original cases available today, only two were authenticated by immunophenotyping.1
In 1872 and 1878, Langhan and Greenfield, respectively, provided microscopic descriptions of HL; however the cells that came to typify the diagnosis, Reed-Sternberg (R-S) cells, were attributed to observations and illustrations made by Carl Sternberg in 1898 and Dorothy Reed in 1902.7,8 Despite Reed’s unique diagnostic contribution to pathology, as well as her work in distinguishing Hodgkin disease from tuberculosis, Reed went on to become a paediatrician. Her prospects of joining the academic ranks in pathology at Johns Hopkins School of Medicine as a woman were not in keeping with the prevailing attitudes of the time!9
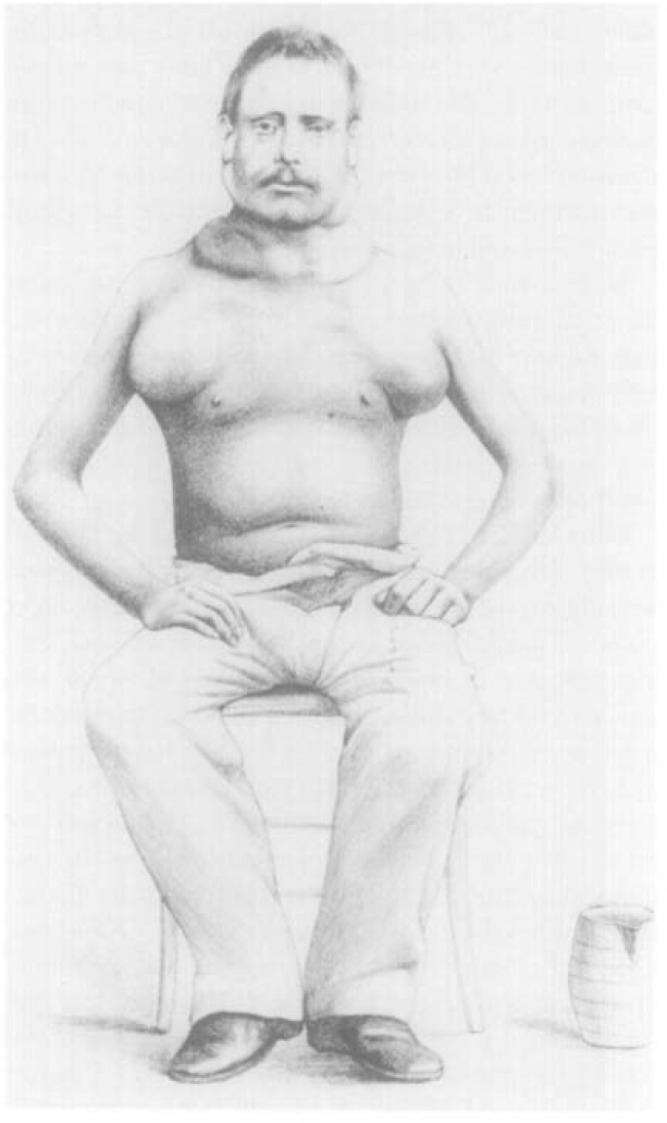
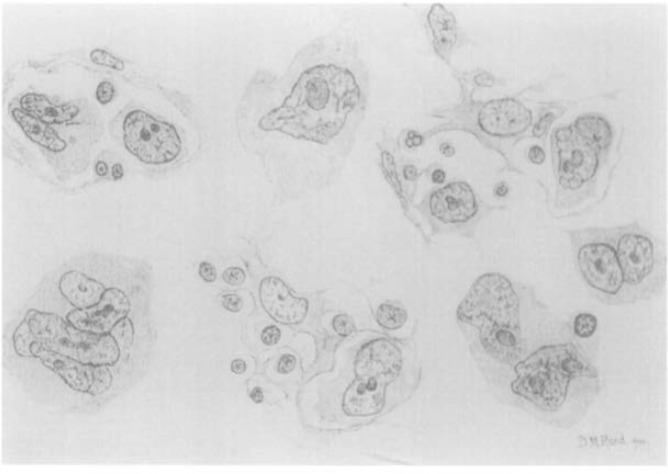
Microscopy, at that time, became the gold standard for diagnosis as reviews of reported cases based on macroscopy alone showed that some cases may have been caused by other lymphomas or other prevalent infections like tuberculosis or syphilis. The dramatic illustration of the bulky lymphadenopathy in a prototype soldier-patient, and the original drawings of the R-S cells by Reed were sourced from an article in the Annals of Diagnostic Pathology entitled The Original Illustrations of Hodgkin’s Disease.9,10 These images remind us of the painstaking methods adopted to preserve medical knowledge only a little over a century ago [Figures 1 and 2].10–12
Figure 1:
An original illustration of a patient with Hodgkin’s disease.10,11
Figure 2:
Dorothy Reed’s drawings of what came to be referred to as Reed-Sternberg cells.10,12
More than a hundred years after these microscopic recordings, generations of pathologist physicians have been avid hunters of the large cells: Hodgkin, R-S or their variants. The pathology lexicon offered handy descriptors of these cells, like ‘popcorn’, ‘owl-eye’, ‘mirror-image’, ‘lacunar’ or ‘mummified’, based on nuclear configurations. At a time when cut and dried combinations of lineage-specific immunohistochemical markers and molecular genetics have become the cornerstone of haematolymphoid disease diagnosis, these time-honoured epithets have yet to be relegated to extinction.
Cell of Origin, Classifications and Cause
The neoplastic nature of HL was suggested by Gall and Mallory (1942), but scientifically grounded through cytogenetics and clonality in 1967 and 1975 by Boecker, Seif and their collaborators.13–15 In 1999 Cossmann and colleagues, through a detailed genomic analysis, narrowed down the origin of the R-S cell to germinal centre B cell type.16
Once a disease is identified, the classification-conscious scientific mind wastes no time in devising categories to separate clinically-relevant disease subsets based on appearance and outcome. In 1947, after a lag period of nearly half a century since HL came to be recognised as an entity, the first classification, the Jackson and Parker classification, appeared. This classification identified three groups: Hodgkin’s paragranuloma, granuloma and sarcoma. Paragranulomas represented cases with few atypical cells and an indolent course, granulomas described cases with usual appearance and progression, and sarcomas represented bizarre cytology and aggressive behaviour.17 Lukes’ and Butler’s initial classification recommended six groups: lymphocytic and/or histiocytic, nodular; lymphocytic and/or histiocytic, diffuse; nodular sclerosis; mixed; diffuse fibrosis, and reticular.18 These classifications were later streamlined to four categories: lymphocyte predominance; nodular sclerosis; mixed cellularity, and lymphocytic depletion.19 In 1994, in a dramatic immunophenotype-based approach to haematolymphoid malignancies, the Revised European-American Lymphoma classification separated nodular lymphocyte-predominant HL (nLPHL) from classical HL (cHL).20 More recently, in 2001 and 2008, the World Health Organization classification has endorsed a category of a lymphocyte-rich cHL.21,22
Much of the evolution in terminology and classification originates from gradual revelation of the lymphoma’s cell of origin. Unlike most other malignancies, HL-affected nodes show an interesting admix of several types of inflammatory and lymphoid cells. In the last four decades there has been clarification in the form of the exponential growth of cluster designate (CD) markers, which identify antigen-based lineage of haematolymphoid cells, and gene expression profiling. Thus far, the fulcrum rests on a follicular centre B cell origin for the nLPHL, while the subsets of cHL are linked to post-germinal centre B cells.23 It is intriguing to note that these transformed B cells have actually lost their capacity to differentiate or respond to antigenic stimuli. The attendant combination of granulocytes (neutrophils, eosinophils) confer a polymorphic microscopic cocktail. They have been attributed to the secretion of chemoattractant cytokines by the neoplastic cells. Inflammation also explains the ‘B’ symptoms that often bring the patient to the hospital in the first place.24
The systematic evolution of understanding pathobiology has shaped clinicopathologic entities and outcome determination in HL, with mixed cellularity HL and lymphocyte depletion HL being the worst. Current prognostication, however, relies more emphatically on the disease stage, B symptoms and concurrent association with other conditions, such as immunodeficiency.25 It is certain that the last word on classification has yet to be written, with the spectre of ‘grey-zone’ lymphomas, overlapping HL and non-HL, providing fodder for researchers.25 Genome sequencing may provide new historical landmarks for future medical generations.
Epstein-Barr virus (EBV) DNA was first reported in the R-S cells of a proportion of HL cases in 1987. Since 1990, the identification of EBV-encoded ribonucleotides (RNAs) 1 and 2 is the gold standard for its demonstration in clinical tissues.16 The association gained further momentum from the fact that HL associated with human immunodeficiency virus infection has a high frequency of detection of EBV RNAs in the R-S cells.
Surgery-Radiology Set the Stage for Staging
An important basis of rational treatment was set in motion in the Rye (1966) and Ann Arbor (1971) expert meetings of the Committee of Staging and Classification of Hodgkin’s Disease, when staging systems and pathological categories determined treatment options and prognoses. Initially, radical surgery such as splenectomy, liver biopsy and retroperitoneal lymph node sampling provided proof of infradiaphragmatic involvement. This was further expanded by lymphangiography. Only a few decades later, these substantive explorations appear unnecessary and invasive, with the advent of computerised tomography (CT) scans and positron emission tomography (PET).
Treatment: Empirical to targeted
In 1832, Hodgkin tried to treat one of his patients with cascarilla, soda and iodine with an obvious lack of success, since this patient along with six others became the subject of an autopsy.1 In 1894, Fowler’s solution, which originally contained potassium arsenite when it was concocted in 1786 by Thomas Fowler, demonstrated some success,26 and earned its place in Osler’s Textbook of Medicine.2 In the 1940s, nitrogen mustard, the gas that gained notoriety for its wartime usage, was included as a therapeutic option by Goodman et al., following its successful use for other haematolymphoid malignancies.27
Though radiotherapy was first used for this disease in 1902,28 it was in 1932 that Chevalier and Bernard recommended radiotherapy for palliation.29 This became popular in the 1940s and reached a pinnacle in the 1960s.29 The important concept that Hodgkin’s disease involved lymph nodes by contiguity was proposed by radiotherapists Rene Gilbert (Switzerland) and Vera Peters (Canada) in the 1950s, providing the opportunity to offer planned, targeted coverage to the affected nodes.28
In 1955, Henry Kaplan, working at Stanford University Medical Center, used a six million volt medical linear accelerator for the first time on a child with retinoblastoma. Following its success, he turned his attention to using it for Hodgkin’s disease.30 His pioneering efforts with radiotherapy not only changed the cancer landscape but transformed Hodgkin’s disease from invariably fatal to near-curable.
The dominance of radiotherapy continued until chemotherapy entered the cancer arena and Hodgkin’s disease became the ‘poster boy’ for success in cancer treatment. The ground was laid through the discoveries of individual drugs, chlorambucil and vincristine. Soon enough the movement was galvanised by the concept of combination chemotherapy to achieve maximum benefit. In 1964, a four-drug combination of mustine hydrochloride, vincristine, procarbazine and prednisone (MOPP) was tested at the National Cancer Institute. By 1970, MOPP was shown to achieve cure rates of 70%, even in patients with advanced stage disease.31,32 Patients lived longer, and the long-term side-effects became apparent. Sterility due to procarbazine and second malignancies due to nitrogen mustard threatened to offset the gains achieved. In 1981, another four-drug combination of doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD), designed at the Istituto Nazionale dei Tumori in Milan, Italy, relegated MOPP to history.33 The cure rates were higher with ABVD and the long-term side-effects were fewer. Such was the success that the 10–30% failure rate was considered a major challenge.
The last 30 years have seen efforts to refine treatment through the identification of prognostic factors. Besides the prognostic factors at presentation,34 early metabolic response to chemotherapy assessed by PET-CT scan has changed the landscape.35 It is now possible to achieve durable responses and high cure rates through strategies such as reduction of doses, drugs and number of cycles of chemotherapy for good prognostic groups.36 On the other hand, more extensive combinations, such as bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone from the German Hodgkin Study Group and the Stanford V regimen from the Eastern Cooperative Oncology Group have helped to improve the cure rates in poor prognostic groups.37,38 Even patients who do not achieve a complete remission at the end of treatment, as well as those who relapse after achieving a complete remission, still stand a chance of being cured. High-dose chemotherapy and autologous stem cell transplantation have been shown to produce lasting remissions in 10–60% of patients.39 Optimisation of treatment has continued to evolve both in search of less toxic treatment for good prognostic patients and additional treatment for the poor prognostic patients. Examples of history-in-the-making molecules include anti-CD20 antibody rituximab for nLPHL,40 and the chemoimmunoconjugate, brentuximab vedotin, for cHL and a variety of other CD 30-positive tumours.41
Lessons from the History of Hodgkin Lymphoma
The centuries stand witness to the fascinating contributions of physicians, pathologists, radiotherapists and geneticists rising to the challenge of conquering this haematolymphoid malignancy. From clinical manifestations to interesting cell appearances, they are an acknowledgement of the times when discerning physicians used basic tools of observation and analysis to define diseases. They also tell a story of unprecedented forays in cell biology (molecular genetics), opportunity (nitrogen mustard), success (radiotherapy) and cure (chemotherapy)—each a reflection of the rapid evolution in science and technology that is the hallmark of the last two centuries. It is also a promising example of the concept that many cancers are now considered curable or chronic rather than fatal diseases.
This tale concludes by urging the reader to learn more about the persona, character, beliefs and global influence of Thomas Hodgkin, in a brilliant, personalised narrative of his life and times, delivered at his bicentennial in 1998.42 His life is epitomised by the epitaph on his grave:
Humani nihil a se alienum putabat (Nothing of humankind was foreign to him).
References
- 1.Banerjee D. Recent insights into the biology of Hodgkin’s lymphoma. From: cdn.intechopen.com/pdfs-wm/33669.pdf Accessed: Jan 2015.
- 2. LymphomaInfo.net Hodgkin’s Disease: Historical timeline. From: www.lymphomainfo.net/hodgkins/timeline.html Accessed: Jan 2015.
- 3.Hodgkin T. On some morbid appearances of the absorbent glands and spleen. Med Chir Trans. 1832;17:68–114. doi: 10.1177/095952873201700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daws JJ. Thomas Hodgkin and the museum at Guy’s hospital. Cancer Treat Rev. 1999;25:145–50. doi: 10.1053/ctrv.1998.0110. [DOI] [PubMed] [Google Scholar]
- 5.Hummel M. World Health Organization and beyond: New aspects in the pathology of an old disease. Hematol Oncol Clin North Am. 2007;21:769–86. doi: 10.1016/j.hoc.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Wilks S. Cases with enlargement of the lymphatic glands and spleen (or Hodgkin’s disease) with remarks. Guys Hosp Rep. 1865;1:57. [Google Scholar]
- 7.Sternberg C. Über eine eigenartige unter dem Bilde der Pseudoleukämie verlaufende Tuberculose des lymphatischen Apparates Ztschr Heilk. 1898;19:21–90. [Google Scholar]
- 8.Reed DM. On the pathological changes in Hodgkin’s disease, with special reference to its relation to tuberculosis. John Hopkins Hosp Rep. 1902;10:133–96. [Google Scholar]
- 9.Dawson PJ. Whatever happened to Dorothy Reed? Ann Diagn Pathol. 2003;7:195–203. doi: 10.1016/S1092-9134(03)00020-0. [DOI] [PubMed] [Google Scholar]
- 10.Dawson PJ. The original illustrations of Hodgkin’s disease. Ann Diagn Pathol. 1999;3:386–93. doi: 10.1016/S1092-9134(99)80018-5. [DOI] [PubMed] [Google Scholar]
- 11.Porter JH. Two cases of Hodgkin’s disease of the glands: Leucocythaemia; Lymphadenosis. Trans Path Soc Lond. 1878;29:335–41. [Google Scholar]
- 12.Reed DM. On the pathological changes in Hodgkin’s disease, with special reference to its relation to tuberculosis. Johns Hopkins Hosp Rep. 1902;10:113–96. [Google Scholar]
- 13.Gall EA, Mallory TB. Malignant lymphoma: A clinicopathologic survey of 618 cases. Am J Pathol. 1942;18:381–429. [PMC free article] [PubMed] [Google Scholar]
- 14.Seif GS, Spriggs AI. Chromosome changes in Hodgkin’s disease. J Natl Cancer Inst. 1967;39:557–70. doi: 10.1093/jnci/39.3.557. [DOI] [PubMed] [Google Scholar]
- 15.Boecker WR, Hossfeld DK, Gallmeier WM, Schmidt CG. Clonal growth of Hodgkin cells. Nature. 1975;258:235–6. doi: 10.1038/258235a0. [DOI] [PubMed] [Google Scholar]
- 16.Kapatai G, Murray P. Contribution of the Epstein Barr virus to the molecular pathogenesis of Hodgkin lymphoma. J Clin Pathol. 2007;60:1342–9. doi: 10.1136/jcp.2007.050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson H, Parker F. Hodgkin’s Disease and Allied Disorders. New York, USA: Oxford University Press; 1974. [Google Scholar]
- 18.Lukes RJ, Butler J, Hicks EB. [The prognosis of Hodgkin’s disease according to the histologic type and the clinical stage. Role of the reactions of the host] Nouv Rev Fr Hematol. 1966;6:15–22. [PubMed] [Google Scholar]
- 19.Lukes RJ, Butler JJ. The pathology and nomenclature of Hodgkin’s disease. Cancer Res. 1966;26:1063–83. [PubMed] [Google Scholar]
- 20.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–92. [PubMed] [Google Scholar]
- 21.Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization classification of tumours: Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Ann Oncol. 2002;13:490–1. doi: 10.1093/annonc/mdf146. [DOI] [Google Scholar]
- 22.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. [Google Scholar]
- 23.Mani H, Jaffe ES. Hodgkin lymphoma: An update on its biology with newer insights into classification. Clin Lymphoma Myeloma. 2009;9:206–16. doi: 10.3816/CLM.2009.n.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allemani C, Sant M, De Angelis R, Marcos-Gragera R, Coebergh JW, Eurocare Working Group Hodgkin disease survival in Europe and the U.S: Prognostic significance of morphologic groups. Cancer. 2006;107:352–60. doi: 10.1002/cncr.21995. [DOI] [PubMed] [Google Scholar]
- 25.Harris NL. Shades of gray between large B-cell lymphomas and Hodgkin lymphomas: Differential diagnosis and biological implications. Mod Pathol. 2013;26:S57–70. doi: 10.1038/modpathol.2012.182. [DOI] [PubMed] [Google Scholar]
- 26.Waxman S, Anderson KC. History of the development of arsenic derivatives in cancer therapy. Oncologist. 2001;6:S3–10. doi: 10.1634/theoncologist.6-suppl_2-3. [DOI] [PubMed] [Google Scholar]
- 27.Goodman LS, Wintrobe MM, Dameshek W, Goodman MJ, Gilman A, Mclennan MT. Nitrogen mustard therapy: Use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J Am Med Assoc. 1946;132:126–32. doi: 10.1001/jama.1946.02870380008004. [DOI] [PubMed] [Google Scholar]
- 28.DeVita VT., Jr A selective history of the therapy of Hodgkin’s disease. Br J Haematol. 2003;122:718–27. doi: 10.1046/j.1365-2141.2003.04541.x. [DOI] [PubMed] [Google Scholar]
- 29.Chevalier P, Bernard J. La Maladie de Hodgkin (Lymphogranulomatose Maligne) Paris. France: Mason et Cie; 1932. [Google Scholar]
- 30.Jacobs CD. Henry Kaplan and the Story of Hodgkin’s Disease Redwood City. California, USA: Stanford University Press; 2010. [Google Scholar]
- 31.DeVita VT, Jr, Serpick AA, Carbone PP. Combination chemotherapy in the treatment of advanced Hodgkin’s disease. Ann Intern Med. 1970;73:881–95. doi: 10.7326/0003-4819-73-6-881. [DOI] [PubMed] [Google Scholar]
- 32.DeVita VT, Jr, Simon RM, Hubbard SM, Young RC, Berard CW, Moxley JH, 3rd, et al. Curability of advanced Hodgkin’s disease with chemotherapy: Long-term follow-up of MOPP-treated patients at the National Cancer Institute. Ann Intern Med. 1980;92:587–95. doi: 10.7326/0003-4819-92-5-587. [DOI] [PubMed] [Google Scholar]
- 33.Bonadonna G, Zucali R, Monfardini S, De Lena M, Uslenghi C. Combination chemotherapy of Hodgkin’s disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer. 1975;36:252–9. doi: 10.1002/1097-0142(197507)36:1<252::AIDCNCR2820360128>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339:1506–14. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 35.Hutchings M, Loft A, Hansen M, Pedersen LM, Buhl T, Jurlander J, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52–9. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
- 36.Engert A, Plütschow A, Eich HT, Lohri A, Dörken B, Borchmann P, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640–52. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 37.Diehl V, Franklin J, Hasenclever D, Tesch H, Pfreundschuh M, Lathan B, et al. BEACOPP: A new regimen for advanced Hodgkin’s disease. German Hodgkin’s Lymphoma Study Group. Ann Oncol. 1998;9:S67–71. doi: 10.1093/annonc/9.suppl_5.S67. [DOI] [PubMed] [Google Scholar]
- 38.Horning SJ, Hoppe RT, Breslin S, Bartlett NL, Brown BW, Rosenberg SA. Stanford V and radiotherapy for locally extensive and advanced Hodgkin’s disease: Mature results of a prospective clinical trial. J Clin Oncol. 2002;20:630–7. doi: 10.1200/JCO.20.3.630. [DOI] [PubMed] [Google Scholar]
- 39.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: A randomised trial. Lancet. 2002;359:2065–71. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 40.Horning SJ, Bartlett NL, Breslin S, Advani RH, Hoppe RT, Ekstrand BC, et al. Results of a prospective phase II trial of limited and extended rituximab treatment in nodular lymphocyte predominant Hodgkin’s disease (NLPHD) Blood. 2007;110:644. [Google Scholar]
- 41.Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–21. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 42.Kass AM. Thomas Hodgkin remembered. Cancer Treat Rev. 1999;25:133–143. doi: 10.1053/ctrv.1998.0108. [DOI] [PubMed] [Google Scholar]