Abstract
Objective
White matter hyperintensities (WMH) are markers of brain white matter injury seen on magnetic resonance imaging. WMH increase with age and are associated with neuropsychiatric disorders. WMH progression can be slowed by controlling vascular risk factors in individuals with advanced disease. Since physical activity can decrease vascular risk factors, physical activity may slow the progression of WMH in individuals without advanced disease, thereby preventing neuropsychiatric disorders. The purpose of this systematic review was to examine the association between physical activity and WMH in individuals without advanced disease.
Methods
Articles published in English through March 18, 2014 were searched using PubMed, Web of Science, Cochrane Library and EBSCOhost.
Results
Six studies found that more physical activity was associated with less WMH, while 6 found no association. Physical activity is associated with less WMH in individuals without advanced disease when studies are longitudinal or take into consideration physical activity across the lifespan, have a younger sample of older adults, measure different types of physical activity beyond leisure or objectively measure fitness via VO2 max, measure WMH manually or semi-automatically, and control for risk factors associated with WMH.
Conclusion
More physical activity was associated with less white matter hyperintensities in individuals without advanced disease.
Keywords: Leukoaraiosis, Exercise, Magnetic resonance imaging
Highlights
-
•
White matter hyperintensities (WMHs) indicate brain white matter injury seen on MRI.
-
•
WMHs increase with age and are associated with neuropsychiatric disorders.
-
•
Slowing the progression of WMH may delay or prevent neuropsychiatric disorders.
-
•
More physical activity was associated with less WMH.
-
•
Perhaps physical activity can slow WMH, preventing neuropsychiatric disorders.
Introduction
White matter hyperintensities (WMH) are markers of brain white matter injury seen on magnetic resonance imaging (MRI). WMH increase with age (Decarli et al., 1995) and are associated with several disorders including late-onset depression (Herrmann, Le Masurier, and Ebmeier, 2008), cognitive decline, dementia, stroke, and increased risk for death (Debette et al., 2010). Reducing the risks for depression, cognitive decline, dementia and stroke is a major public health goal.
The progression of WMH can be slowed by controlling vascular risk factors. One study found that controlling blood pressure with medication slowed the progression of WMH in patients who had a stroke (Dufouil et al., 2005). Another study found vascular care composed of physical activity, diet, smoking cessation, and treatment of hypertension and dyslipidemia with medicine slowed the progression of WMH in patients with Alzheimer's disease (Richard, Gouw, Scheltens, and van Gool, 2010). In both studies, patients had advanced disease. From a health promotion perspective, slowing the progression of WMH in individuals without advanced disease may delay or prevent disorders associated with WMH such as a stroke or Alzheimer's disease.
Physical activity is any body movement produced by skeletal muscles that results in energy expenditure (Caspersen et al., 1985). The total amount of physical activity is commonly measured, which takes into account intensity, frequency, duration (Shephard, 2003), as well as settings such as occupation, transportation, household and leisure (van Poppel et al., 2010). Since physical activity improves many vascular risk factors (Nelson et al., 2007), physical activity is a lifestyle factor that could slow the progression of WMH in individuals without advanced disease. Support for this hypothesis centers on the knowledge that vigorous intensity leisure physical activity prevents age-related white matter volume decline in healthy older adults (Colcombe et al., 2006). The purpose of this systematic review was to examine the literature for evidence that physical activity can slow the progression of WMH in individuals without advanced disease such as stroke or Alzheimer's disease.
Methods
We searched the literature published in English in PubMed, Web of Science, Cochrane Library, and EBSCOhost through March 18, 2014. Inclusion criteria comprised cross-sectional, longitudinal and experimental studies of human subjects, and any measurement of physical activity or WMH. Since the focus of this study was on health promotion, exclusion criteria comprised studies focused solely on individuals with advance diseases associated with WMH, specifically depression, cognitive decline, dementia and stroke. Table 1 describes the keyword searches in each of the databases. Leukoaraiosis was included as a search term as it is a synonym for WMH. Leukoencephalopathy/leukoencephalopathies were included as search terms as they are broad term that applies to all brain white matter diseases. Two reviewers performed an independent screening of each study. Abstracts were reviewed, and full-text articles were inspected. We also examined the reference lists from full-text articles to seek additional sources.
Table 1.
Keyword searches of articles published in English through March 18, 2014.
| Databases | Search terms |
|---|---|
| PubMed | (“Leukoencephalopathies”[Mesh] OR “Leukoaraiosis”[Mesh] OR (white matter hyperintensities[All Fields] OR white matter hyperintensity[All Fields]) OR “white matter lesion*”[All Fields]) AND (“Motor Activity”[Mesh] OR “Exercise”[Mesh] OR “physical activity”[All Fields] OR (“exercise”[MeSH Terms] OR “exercise”[All Fields])) |
| Web of Science | TOPIC: (white matter hyperintensities OR white matter hyperintensity OR white matter lesions OR white matter lesion OR leukoaraiosis) AND TOPIC: (physical activity OR exercise) |
| Cochrane Library | (“white matter hyperintensities” or “white matter hyperintensity” or “white matter lesions” or “white matter lesion” or “leukoaraiosis”) AND (“physical activity” or “exercise”) |
| EBSCOhost: CINAHL Plus PsycInfo PsycARTICLES SocIndex Social Sciences |
(MH “Physical Activity”) OR “physical activity” OR (MH “Exercise”) OR (MH “Physical Fitness”) OR (MH “Physical Performance”) OR (MH “Physical Endurance”) OR (MH “Exercise Test”) OR (MH “Activity and Exercise Enhancement (Iowa NIC) (Non-Cinahl)”) OR (MH “Physical Activity (Omaha)”) OR (MH “Physical Education, Adapted”) OR (MH “Activity Therapy (Iowa NIC)”) AND DE “White Matter” OR DE “Leukoaraiosis” OR DE “Leukoencephalopathy” OR white matter hyperintensit* OR “white matter lesion*” |
Results
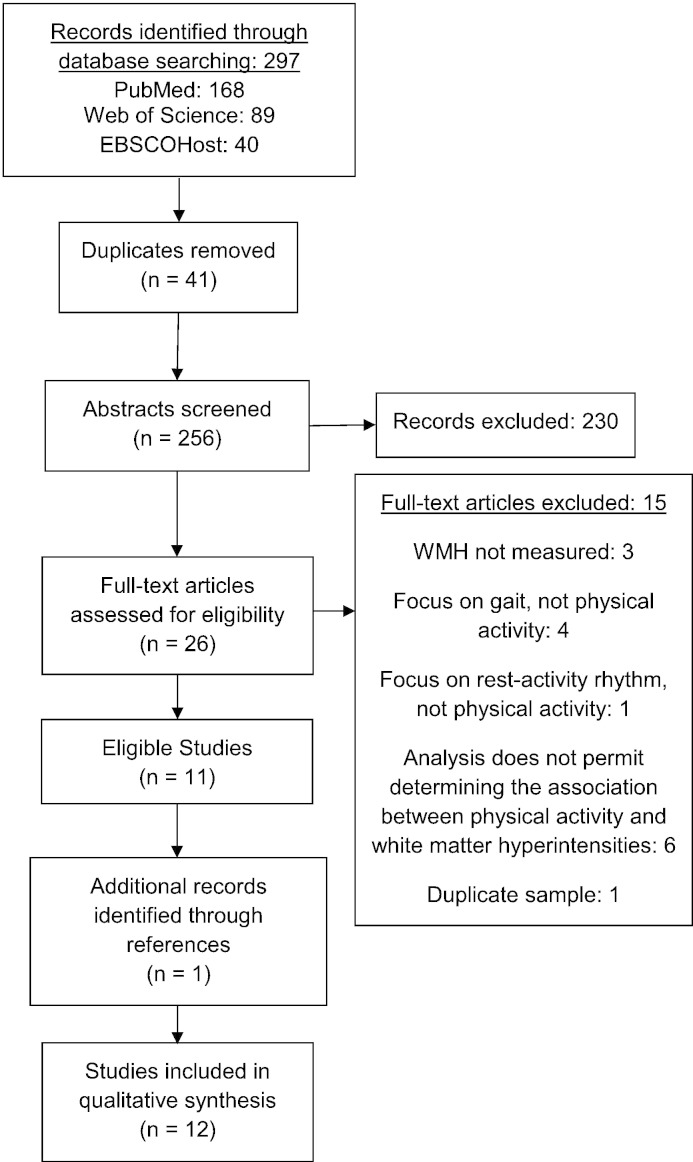
The process of inclusion of studies identified for review and analysis is shown in Fig. 1 (Liberati et al., 2009). Of the 256 non-duplicate articles found, we excluded 245 during a secondary screen. The most common reason for exclusion was that the study measured physical activity or WMH, but not both. We excluded other studies due to methodological issues, such as measuring white matter, but not WMH. Some studies measured mobility, gait, or cognitive exercises, but not physical activity. Since the purpose of this review was to determine the effects of physical activity on WMH, WMH had to be the dependent variable. Finally, we excluded studies in which the association between physical activity and WMH was undeterminable.
Fig. 1.
Flow diagram of articles published in English through March 18, 2014.
Studies included in the review (Fig. 1) represent data collected in six countries: the United States, the United Kingdom, Iceland, Finland, Austria, and Australia. Per Table 2, seven of the studies were cross-sectional (Ho et al., 2011, Rosano et al., 2010, Saczynski et al., 2008, Sen et al., 2012, Smith et al., 2009, Tseng et al., 2013, Zheng et al., 2012). Per Table 3, the five longitudinal studies (Carmelli et al., 1999, Gow et al., 2012, Podewils et al., 2007, Rovio et al., 2010, Willey et al., 2011) measured physical activity at baseline and WMH from three (Gow et al., 2012) to 25 years (Carmelli et al., 1999) later. All except one of the studies were community- or population-based (Tseng et al., 2013). The non-community/population-based study was the only one designed to examine the association between physical activity and WMH (Tseng et al., 2013). All studies focused on older adults, excluded individuals with contraindications to MRI, and excluded those missing data on variables of interest. Five studies reported no other exclusion criteria (Carmelli et al., 1999, Gow et al., 2012, Podewils et al., 2007, Rovio et al., 2010, Saczynski et al., 2008). Four studies excluded adults with cognitive impairment or dementia (Ho et al., 2011, Sen et al., 2012, Tseng et al., 2013, Zheng et al., 2012), while another study accounted for adults with cognitive impairment or dementia in the analysis (Podewils et al., 2007). Three studies excluded adults with a history of neurological disorders (Tseng et al., 2013), such as stroke (Sen et al., 2012, Willey et al., 2011). The study originally designed to examine the association between physical activity and WMH excluded a number of potential confounders—that is smoking, recreational drug use, cardiovascular or cerebrovascular diseases, dementia, and major psychiatric and neurologic disorders (Tseng et al., 2013).
Table 2.
Summary of cross-sectional studies on physical activity and white matter hyperintensities published in English through March 18, 2014.
| Citation | Sample | Physical activity | White matter hyperintensities (WMH) | Statistical analysis | Results |
|---|---|---|---|---|---|
| Ho et al. (2011) | Pittsburgh, PA, U.S., n = 226, 42% male, mean age 77.9 (SD = 3.6). Exclusion criteria: mild cognitive impairment or Alzheimer's disease. | Modified Minnesota Leisure-Time Activities Questionnaire covered two weeks prior to when MRI was obtained, estimated in kcal/week which was divided into quintiles. | Expert visual grading on a scale of 0 to 9, with no white matter findings classified as grade 0 and the most severe WMH classified as grades 8 and 9. | Kruskal–Wallis one-way ANOVA (WMH not normally distributed) | No association |
| Rosano et al. (2010) | Pittsburgh, PA, U.S., n = 27, 3% male, mean age 81 (SD = 3.4), 20 remained active and 10 remained sedentary 2 years after a pilot intervention; 18 of the active group had WMH measurements. Inclusion criteria: age of 70–89 years, sedentary lifestyle (< 20 min/week spent in structured physical activity during the past month), being able to walk 400 m within 15 min without sitting and without use of any assistive device, having a Short Physical Performance Battery score 9 (on a scale of 0–12), having completed behavioral tests related to logging health behavior, and not planning to move for at least 9 months. | The physically active group was asked if they completely stopped their regular physical activity after the pilot intervention ended, and were included if they responded “No.” The sedentary group was asked if they spent at least 20 min a week getting regular exercise after the pilot intervention ended and were included if they responded “No.” | WMH was measured with a fully deformable automatic algorithm. | t-Test | No association |
| Saczynski | Reykjavik, Iceland, population-based sample of older adults, n = 1787, 38.9% male, mean age = 75.9 (SD = 5.4). | Questionnaire, current moderate/vigorous physical activity was assessed as never (reference), rarely, 1 h/week, 1–3 h/week, 4–7 h/week, or > 7 h/week and dichotomized into the upper quartile (high leisure activity) compared with the bottom three quartiles (low leisure activity). | WMH measured with a rating scale. Individuals in the upper quartile of WMH load for either subcortical or periventricular WMH were compared with the reference group comprising those in the lower three quartiles (reference group). | Age-adjusted analysis of variance | Compared to the low WMH/high activity group, the high WMH/low activity (F = 53.9) and high WMH/high activity (F = 41.0) were more likely to be physically inactive (p < .0.05). |
| Sen et al. (2012) | Austria, community-based cohort, n = 715, 46% male, mean age 65, range 44–83. Exclusion criteria: dementia, previous strokes. |
VO2 max on cycle ergometer. | Two experienced investigators marked and outlined each WMH, which was positively skewed and log-transformed. | Linear regression; adjusted for age, sex, hypertension, body mass index, cholesterol, smoking status, diabetes, treatment with β-blockers or calcium channel blockers. | Significant association in men (β = − 0.10, p = 0.02), not women. |
| Smith | Midwest U.S., community-based white adults, n = 777, 41% male, mean age 60 (SD = 10). Inclusion criteria: essential hypertension diagnosed before age 60 years. | Self-report average # of hours/day engaged in sedentary, moderate and heavy activity. | Global WMH was obtained with a fully automated algorithm. Brain scans with cortical infarctions were excluded due to distortions in the automated segmentation algorithm. The natural logarithm (cm3) of WMH was obtained after adjustment for age, sex, and total brain volume. | Least squares linear regression; tested for association between each of the predictor variables (1649 single nucleotide polymorphisms and quantitative covariates): hypertension, BMI, pulse pressure, smoking history, coronary heart disease, serum triglycerides, creatinine, total cholesterol, high-density lipoprotein, low-density lipoprotein, & five novel vascular risk factors including C-reactive protein, homocysteine, fibrinogen, Lp(a), and LDL particle size. | No association |
| Tseng et al. (2013) | U.S., n = 20, 75% male, mean age 73 ± 5 years, free of major medical problems based on a detailed medical history and physical exams including 12-lead electrocardiogram and echocardiogram. Exclusion criteria: smoking, used recreational drugs, had clinical evidence of cardiovascular (e.g., hypertension, diabetes mellitus, hyperlipidemia) or cerebrovascular diseases (e.g., history of stroke, transient ischemic attack or the presence of cortical infarction on MRI scans), dementia, major psychiatric and neurologic disorders. | 10 master athletes: history of endurance training > 15 years, who were still engaged in endurance exercise at the time of this study. 10 sedentary older adults: not engaging in moderate or high intensity aerobic exercise for more than 30 min, 3 times/week over the past two years. Aerobic fitness was measured with maximal oxygen uptake (VO2 max) testing. | Total, periventricular, and deep WMH were obtained with a semiautomatic method. | Mann–Whitney Rank Sum Test was conducted to detect differences in WMH volume and cardiopulmonary fitness between groups. | No differences in total or periventricular WMH between groups. Masters athletes showed 83% reduction in deep WMH volume when compared with the sedentary elderly. |
| Zheng | Eastern Sydney, Australia, prospective community-dwelling cohort, n = 287, aged 70–90, 46.3% male, mean 77.8 ± 4.5 years. Exclusion criteria: Diagnosis of dementia and inability to walk 20 m without a walking aid due to a neurological, cardiovascular, or major musculoskeletal impairment. | Incidental and Planned Exercise Questionnaire (last week — 3 months preceding interview), hour/week. | A validated automatic procedure was carried out to calculate WMH volume. Regional WMH comprised deep (frontal, parietal, temporal and occipital) and periventricular regions (anterior cap, posterior cap and periventricular body). | t-Test examined differences in baseline physical activity between participants with high and low volumes of WMH (cut off at the median). | No association |
Table 3.
Summary of longitudinal studies on physical activity and white matter hyperintensities published in English through March 18, 2014.
| Citation | Design | Sample | Physical activity | White matter hyperintensities (WMH) | Statistical analysis | Results |
|---|---|---|---|---|---|---|
| Carmelli et al. (1999) | Longitudinal | U.S. World War II veterans, monozygotic white male twins, n = 74, age 68–79 at baseline. | Interviewer-administered questionnaire at baseline. | Measured over 25 years after physical activity on scanners at four study sites. Image evaluation based on a semi-automated segmentation analysis involving operator-guided removal of non-brain elements. | Univariate correlation | No association |
| Gow et al. (2012) | Longitudinal | Lothian, U.K., n = 691, 53% male, mean age 70 (SD = 0.8) at baseline. | Self-report questionnaire measured at baseline. Physical activity rated on a 6-point scale, from “moving only in connection with necessary (household) chores” to “keep-fit/heavy exercise or competitive sport several times per week.” | Measured 3 years after physical activity. WMH calculated semi-automatically fusing 2 previously aligned structural MRI sequences. Stroke lesions were extracted from total WMH, which were normalized by intracranial volume. WMH were also rated using FLAIR & T2-weighted sequences and the Fazekas scale coded for periventricular and deep WMH separately in right and left hemispheres, and subsequently combined into total WMH ranging from 0 to 6 with ↑ score = ↑WMH. | Linear regression, adjusted for age, sex, social class, IQ, dementia risk & disease history (hypertension, cardiovascular disease and stroke). | A higher level of physical activity predicted lower WMH (standardized β = 0.09, non-standardized β = 0.09, p = 0.029), when WMH was measured semi-automatically. |
| Podewils et al. (2007) | Longitudinal | U.S., population-based, n = 179; 38% male, mean age 77 (SD = 6), 59 had Alzheimer's disease, 60 had mild cognitive impairment, and 60 cognitively stable, 3 group frequency matched by 5-year age category and sex. | Modified Minnesota Leisure Time Questionnaire, covered 2 weeks prior to baseline interview. Total energy expenditure in kilocalories/week was categorized as 271–759, 760–1874 and > 1875. | Measured 6–7 years after physical activity. Periventricular and deep WMH measured with standardized semi-quantitative rating scale by a single reader blinded to physical activity and covariates. | Multiple linear regression adjusting for age, sex, ethnicity, years of education, APOE & corresponding region-specific baseline WMH score. | Physical activity > 1875 kcal/week was significantly associated with less periventricular (β = 0.85, CI = 0.32, 1.4) and deep (β = 0.69, CI = 0.01, 1.4) WMH in the cognitively stable group only. |
| Rovio et al. (2010) | Longitudinal | North Karelia & Kuopio, eastern provinces of Finland, random population-based sample, n = 75, 32 active, 43 sedentary; 21 with dementia, 23 with mild cognitive impairment & 31 normal controls; 29.7% male, mean age at midlife was 50.6 (SD = 6.0) years, and 71.6 (SD = 4.1) at re-examination. | Self-administered questionnaire at midlife: “How often do you participate in leisure time physical activity that lasts at least 20–30 min and causes breathlessness and sweating?” Active persons participated in leisure time physical activity ≥ 2 ×/week; sedentary persons participated in leisure time physical activity ≤ 1 ×/week. | Measured with a mean duration follow-up of 20.9 (SD = 4.9) years. A semi-quantitative visual rating scale was used by a single trained rater blinded to clinical data. The total score was used to address the participants belonging to the upper quintile of the distribution to one group (severe WMH) and persons belonging to all other quintiles to another group (no or mild WMH). | Logistic regression unadjusted (crude model) and after that adjusted for age, sex, diagnosis of dementia or mild cognitive impairment, years of education and follow-up time, systolic blood pressure, total serum cholesterol, BMI, APOE 4 allele carrier status, and smoking. Furthermore, all analyses were additionally adjusted for white matter volume. | Crude analysis significant (OR = 4.65, p = 0.03). No longer significant when control for socio-demographic and vascular factors (OR = 4.20, p = 0.32). |
| Willey et al. (2011) | Longitudinal | Northeast U.S., community-based cohort, n = 1238, 40% male, 65% Hispanic, mean age 70 ± 9 SD at baseline Exclusion criteria: history of stroke. |
Self-report duration and frequency of various leisure time/recreational activities for the 2 weeks prior to interview categorized by quartiles of the metabolic equivalent (MET) score; VO2 max. | Measured a mean of 6 ± 3 years after physical activity assessment. WMH was measured semi-automatic, corrected for total cranial volume, and log-transformed. Analyses were performed blind to participant identifying information. | Linear regression unadjusted and adjusted models with demographics (age, sex, race–ethnicity, and education) and vascular risk factors (systolic blood pressure, diastolic blood pressure, glomerular filtration rate, diabetes mellitus, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, moderate alcohol use, and smoking) were constructed. | No association |
Six of the twelve articles showed a statistically significant association between physical activity and WMH, with more physical activity associated with less WMH (Gow et al., 2012, Podewils et al., 2007, Rovio et al., 2010, Saczynski et al., 2008, Sen et al., 2012, Tseng et al., 2013). One article found a statistically significant association in crude analyses, but not when controlled for socio-demographic and vascular factors (Rovio et al., 2010). One article found a statistically significant association in men, not women (Sen et al., 2012), and four articles found that a higher frequency of physical activity was associated with less WMH (Gow et al., 2012, Podewils et al., 2007, Saczynski et al., 2008, Tseng et al., 2013). Five of the six articles were population- or community-based (Gow et al., 2012, Podewils et al., 2007, Rovio et al., 2010, Saczynski et al., 2008, Sen et al., 2012).
Physical activity was typically measured with a questionnaire (Carmelli et al., 1999, Gow et al., 2012, Ho et al., 2011, Podewils et al., 2007, Rovio et al., 2010, Saczynski et al., 2008, Smith et al., 2009, Willey et al., 2011, Zheng et al., 2012). Physical activity was measured at ages 50–92, with a mean age of 71.4. Physical activity was rarely measured objectively (i.e., VO2 max) (Sen et al., 2012, Tseng et al., 2013). Although physical activity was often measured over two weeks prior to the interview (Ho et al., 2011, Podewils et al., 2007, Willey et al., 2011), most studies did not specify a time frame (Carmelli et al., 1999, Gow et al., 2012, Rovio et al., 2010, Saczynski et al., 2008, Smith et al., 2009). Leisure physical activity was the most common setting measured (Ho et al., 2011, Podewils et al., 2007, Rosano et al., 2010, Rovio et al., 2010, Tseng et al., 2013, Willey et al., 2011, Zheng et al., 2012), although often a setting was not specified (Carmelli et al., 1999, Saczynski et al., 2008, Smith et al., 2009). Most studies measured duration (Ho et al., 2011, Podewils et al., 2007, Rosano et al., 2010, Rovio et al., 2010, Saczynski et al., 2008, Smith et al., 2009, Tseng et al., 2013, Willey et al., 2011, Zheng et al., 2012), frequency (Gow et al., 2012, Ho et al., 2011, Podewils et al., 2007, Rovio et al., 2010, Tseng et al., 2013, Willey et al., 2011, Zheng et al., 2012), and/or intensity (Gow et al., 2012, Ho et al., 2011, Podewils et al., 2007, Rovio et al., 2010, Saczynski et al., 2008, Smith et al., 2009, Tseng et al., 2013, Willey et al., 2011).
WMH were measured semi-automatically (Carmelli et al., 1999, Gow et al., 2012, Tseng et al., 2013, Willey et al., 2011) and automatically (Rosano et al., 2010, Smith et al., 2009, Zheng et al., 2012). Many studies used a variety of visual rating scales (Gow et al., 2012, Ho et al., 2011, Podewils et al., 2007, Rovio et al., 2010, Saczynski et al., 2008). For example, one study used a Fazekas scale (Gow et al., 2012); another used a scale of 0 to 9 with no white matter findings classified as grade 0 and the most severe WMH classified as grades 8 and 9 (Ho et al., 2011). One study divided WMH by quartiles (Saczynski et al., 2008) and another by quintiles (Rovio et al., 2010). WMH were often measured continuously (Carmelli et al., 1999, Gow et al., 2012, Ho et al., 2011, Podewils et al., 2007, Rosano et al., 2010, Saczynski et al., 2008, Sen et al., 2012, Smith et al., 2009, Tseng et al., 2013, Willey et al., 2011) and occasionally log-transformed (Sen et al., 2012, Smith et al., 2009, Willey et al., 2011). Total or global WMH were most commonly measured (Carmelli et al., 1999, Gow et al., 2012, Ho et al., 2011, Podewils et al., 2007, Rovio et al., 2010, Sen et al., 2012, Smith et al., 2009, Tseng et al., 2013, Willey et al., 2011), followed by subcortical measures (Saczynski et al., 2008), specifically periventricular (Podewils et al., 2007, Saczynski et al., 2008, Tseng et al., 2013, Zheng et al., 2012) and deep (Podewils et al., 2007, Tseng et al., 2013, Zheng et al., 2012).
Discussion
Half of the studies found more physical activity associated with less WMH. Studies were more likely to find an association between physical activity and WMH if they were conducted outside the U.S. (4 studies outside the U.S. found an association vs. 1 study outside the U.S. that did not), were more likely to be longitudinal or take into consideration physical activity across the lifespan, had a slightly younger sample (mean age 68.5 vs. 72.8), measured different types of physical activity beyond leisure or objectively measured fitness via VO2 max, measured WMH manually or semi-automatically, and performed multivariate analyses or controlled for risk factors associated with WMH (all 6 studies that found an association between physical activity and WMH vs. 2 studies that did not). There were no differences between the articles that found that more physical activity was associated with less WMH and those that did not in terms of sample size, sex, exclusion criteria, and whether WMH was transformed or measured continuously vs. dichotomously. Notably, only one study was originally designed to examine the association between physical activity and WMH.
Most of the studies measured physical activity differently, which may account for the conflicting results. Half of the studies used a standardized physical activity measurement. Three used an objective measurement of cardiorespiratory fitness, such as VO2 max. Three used established questionnaires—the Modified Minnesota Leisure-Time Activities Questionnaire and the Incidental and Planned Exercise Questionnaire. When physical activity was measured with a questionnaire, a variety of outcomes were measured: metabolic equivalent (MET) score, kcal/week, and number of hours per day or per week. Only one study measured the setting of physical activity beyond leisure, namely household, but combined both into one variable (Tseng et al., 2013). Future studies should use a standardized measurement of physical activity with standardized outcomes, such as a MET score, that take into consideration frequency, intensity, and duration and that measure more than one setting of physical activity, such as occupation, transportation, household, and leisure.
WMH were typically measured semi-automatically with a variety of visual rating scales, which may also explain the conflicting results. Unlike physical activity, there appears to be no standardized method for measuring WMH. One method to decrease the variability between studies may be to use a validated automatic segmentation technique allowing for a more precise measurement of WMH, thereby facilitating the detection of small but significant differences that may go unnoticed when relying solely on visual rating scales (Herrmann et al., 2008). While the majority of studies measured total WMH, future studies should also measure regional WMH. For example, in the only study designed to examine the association between physical activity and WMH, no association was found with total or periventricular WMH; however, an association was found between physical activity and deep WMH.
Only two studies used advanced imaging techniques such as diffusion tensor imaging (DTI) (Gow et al., 2012, Tseng et al., 2013), which is superior at assessing white matter integrity and detecting locations where WMH may directly predispose to the development of neuropsychiatric disorders, including depression (Herrmann et al., 2008), cognitive decline, dementia, and stroke (Debette et al., 2010). DTI is used to map and characterize the three-dimensional diffusion of water as a function of spatial location and is therefore a sensitive marker of neuropathology (Alexander, Lee, Lazar, and Field, 2007). Fractional anisotropy (FA) reflects the directional coherence of water molecule diffusion (Alexander et al., 2007). Given that hundreds of research studies have observed reduced FA in a broad spectrum of diseases, while increases are rarely reported (Alexander et al., 2007), it is significant that more physical activity is associated with higher FA (Gow et al., 2012, Tseng et al., 2013). FA is highly sensitive to microstructural changes, but not specific to the type of changes (Alexander et al., 2007). Mean diffusivity (MD) is a measure of the rotationally invariant magnitude of diffusion, or how tissue is changing (Alexander et al., 2007). MD increases with the increased tissue water engendered by inflammation and edema, resulting in a decrease in FA (Alexander et al., 2007), while more physical activity is associated with lower MD (Tseng et al., 2013). Vigorous PA has been shown to reduce vessel tortuosity and increase the number of small vessels (Bullitt et al., 2009), helping to improve vascular risk factors (Nelson et al., 2007) and decreasing brain ischemia (Kim, MacFall, and Payne, 2008), which may help to preserve the integrity of white matter microstructure and forestall age-related white matter degeneration (Colcombe et al., 2006).
Finally, physical activity and WMH were each measured at one time point, even in longitudinal studies. Since physical activity typically decreases and WMH increase throughout much of the life span, the information provided by one or two time point may be incomplete and lead to erroneous conclusions about patterns of age-related change in central nervous system parameters (Coleman et al., 2004), such as WMH. Physical activity and WMH should both be measured repeatedly. In addition, physical activity should be measured at multiple time points, such as childhood, adolescence, adulthood, middle age and old age. Although recommendations have been made for measuring physical activity over relatively short reporting intervals (no longer than three months), in advanced age, long-term memory may be better preserved than recent recollections (Shephard, 2003).
There are limitations to this systematic literature review. There are likely other factors, such as nutrition, that may stop or slow the progression of WMH beyond physical activity. Although several studies measured the consequence of nutrition such as glucose levels (Carmelli et al., 1999, Willey et al., 2011), cholesterol (Carmelli et al., 1999, Rovio et al., 2010, Sen et al., 2012, Smith et al., 2009) and lipid concentrations (Carmelli et al., 1999, Smith et al., 2009, Willey et al., 2011), none of the studies measured nutrition. Another limitation surrounds theses, proceedings, and textbooks that were not reviewed, nor were researchers and sponsoring organizations contacted for unpublished results. Thus, this systematic review is at risk of publication bias leading to overestimation of the association of physical activity with WMH. However, the results of this review found that only half of the eligible studies resulted in a significant inverse association between physical activity and WMH. Whereas the discussion on publication bias focuses almost exclusively on randomized controlled trials (Dwan, Gamble, Williamson, Kirkham, and Group, 2013), the current systematic review comprises observational studies, whereby examining the association between physical activity and WMH was not part of the original design for 11 of the 12 studies. This may explain the lack of evidence for publication bias in our review. Little empirical evidence exists to recommend blinding reviews of the identities of study authors, institutions, sponsorship, publication year, and journal or study results; therefore, blind peer-reviews were not used. Data combination for meta-analysis was inappropriate (Stroup et al., 2000) due to differences in how physical activity and WMH were measured, as well as variation in use of statistical measures (i.e., Mann–Whitney Rank Sum Test, t-tests, one-way analysis of variance, correlation, and linear and logistic regression). Due to these same reasons, the magnitude of the association varied greatly by study, is difficult to compare between studies, and makes it difficult to make physical activity recommendations to the public. For instance, one study found that physical activity in excess of 1875 kcal/week in the previous two weeks prior to the baseline interview was associated with less periventricular and deep WMH five years later (Podewils et al., 2007), while another study found those who had engaged in endurance training for 15 years had an 83% reduction in deep WMH compared to their sedentary counterparts (Tseng et al., 2013). Finally, factors relating to imaging protocol—field strength of magnet, number, thickness, contiguity, and orientation of slices—add to the heterogeneity of results.
Strengths of this review include a priori development of a focused clinical question, clear and concise selection criteria, and assessment of quality (Stroup et al., 2000). The quality of the studies was assessed by focusing on methodological aspects including design, generalizability, various measurements of physical activity and WMH, and potential confounders such as neuropsychiatric disorders and vascular risk factors.
Conclusion
The progression of WMH can be slowed by controlling vascular risk factors in patients who have had a stroke or have Alzheimer's disease. It is not known if it is possible to stop or slow the progression of WMH in individuals without advanced disease, thereby delaying or preventing disorders associated with WMH. This systematic literature review found that physical activity is associated with less WMH in individuals without advanced disease when studies are longitudinal or take into consideration physical activity across the lifespan, have a younger sample of older adults, measure different types of physical activity beyond leisure or objectively measure fitness via VO2 max, measure WMH manually or semi-automatically, and perform multivariate analyses or control for risk factors associated with WMH. Future studies should account for neuropsychiatric disorders and vascular risk factors; include the use of a standardized measurement of physical activity that accounts for different settings, frequency, intensity, and duration over longer time periods; measure physical activity and WMH repeatedly; standardize WMH measurements (e.g., using a validated automatic procedure); and measure total and regional WMH.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors wish to thank Laura Hogan, Kathleen Freimuth and Michael A. Holly for their editorial assistance, and Roger Brown for his statistical consult. The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The funding source has no role in the study design, collection, analysis, interpretation of data, writing of the report, or the decision to submit for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Elisa R. Torres, Email: ertorres@wisc.edu.
Emily F. Strack, Email: estrack@wisc.edu.
Claire E. Fernandez, Email: cfernandez2@wisc.edu.
Tyler A. Tumey, Email: tumey@wisc.edu.
Mary E. Hitchcock, Email: mhitchcock@library.wisc.edu.
References
- Alexander A.L., Lee J.E., Lazar M., Field A.S. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt E., Rahman F.N., Smith J.K. The effect of exercise on the cerebral vasculature of healthy aged subjects as visualized by MR angiography. Am. J. Neuroradiol. 2009;30(10):1857–1863. doi: 10.3174/ajnr.A1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmelli D., Swan G.E., Reed T., Wolf A., Miller B.L., DeCarli C. Midlife cardiovascular risk factors and brain morphology in identical older male twins. Neurology. 1999;52(6):1119–1124. doi: 10.1212/wnl.52.6.1119. [DOI] [PubMed] [Google Scholar]
- Caspersen C.J., Powell K.E., Christenson G.M. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- Colcombe S., Erickson K., Scalf P. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Coleman P., Finch C., Joseph J. The need for multiple time points in aging studies. Neurobiol. Aging. 2004;25(1):3–4. doi: 10.1016/j.neurobiolaging.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Debette S., Beiser A., DeCarli C. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41(4):600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decarli C., Murphy D.G., Tranh M. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- Dufouil C., Chalmers J., Coskun O. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112(11):1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- Dwan K., Gamble C., Williamson P.R., Kirkham J.J., Group R.B. Systematic review of the empirical evidence of study publication bias and outcome reporting bias — an updated review. PLoS One. 2013;8(7):e66844. doi: 10.1371/journal.pone.0066844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow A.J., Bastin M.E., Maniega S.M. Neuroprotective lifestyles and the aging brain activity, atrophy, and white matter integrity. Neurology. 2012;9(17):1802–1808. doi: 10.1212/WNL.0b013e3182703fd2. [DOI] [PubMed] [Google Scholar]
- Herrmann L.L., Le Masurier M., Ebmeier K.P. White matter hyperintensities in late life depression: a systematic review. J. Neurol. Neurosurg. Psychiatry. 2008;79(6):619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- Ho A., Raji C., Becker J. The effects of physical activity, education, and body mass index on the aging brain. Hum. Brain Mapp. 2011;32(9):1371–1382. doi: 10.1002/hbm.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.W., MacFall J.R., Payne M.E. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Stress Synaptic Plast. 2008;64(4):273–280. doi: 10.1016/j.biopsych.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;7:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M.E., Rejeski W.J., Blair S.N. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- Podewils L.J., Guallar E., Beauchamp N., Lyketsos C.G., Kuller L.H., Scheltens P. Physical activity and white matter lesion progression — assessment using MRI. Neurology. 2007;68(15):1223–1226. doi: 10.1212/01.wnl.0000259063.50219.3e. [DOI] [PubMed] [Google Scholar]
- Richard E., Gouw A.A., Scheltens P., van Gool W.A. Vascular care in patients with Alzheimer disease with cerebrovascular lesions slows progression of white matter lesions on MRI: the evaluation of vascular care in Alzheimer's disease (EVA) study. Stroke. 2010;41(3):554–556. doi: 10.1161/STROKEAHA.109.571281. [DOI] [PubMed] [Google Scholar]
- Rosano C., Venkatraman V.K., Guralnik J. Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65(6):639–647. doi: 10.1093/gerona/glq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovio S., Spulber G., Nieminen L.J. The effect of midlife physical activity on structural brain changes in the elderly. Neurobiol. Aging. 2010;31(11):1927–1936. doi: 10.1016/j.neurobiolaging.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Saczynski J.S., Jonsdottir M.K., Sigurdsson S. White matter lesions and cognitive performance: the role of cognitively complex leisure activity. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63A(8):848–854. doi: 10.1093/gerona/63.8.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Gider P., Cavalieri M. Association of cardiorespiratory fitness and morphological brain changes in the elderly: results of the Austrian Stroke Prevention Study. Neurodegener. Dis. 2012;10(1–4):135–137. doi: 10.1159/000334760. [DOI] [PubMed] [Google Scholar]
- Shephard R.J. Limits to the measurement of habitual physical activity by questionnaires. Br. J. Sports Med. 2003;37(3):197–206. doi: 10.1136/bjsm.37.3.197. [discussion 206] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.A., Turner S.T., Sun Y.V. Complexity in the genetic architecture of leukoaraiosis in hypertensive sibships from the GENOA Study. BMC Med. Genet. 2009;2:16. doi: 10.1186/1755-8794-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Tseng B., Gundapuneedi T., Khan M.A. White matter integrity in physically fit older adults. Neuroimage. 2013;82:510–516. doi: 10.1016/j.neuroimage.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Poppel M.N., Chinapaw M.J., Mokkink L.B., van Mechelen W., Terwee C.B. Physical activity questionnaires for adults: a systematic review of measurement properties. Sports Med. 2010;40(7):565–600. doi: 10.2165/11531930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Willey J.Z., Moon Y.P., Paik M.C. Lower prevalence of silent brain infarcts in the physically active. Neurology. 2011;76(24):2112–2118. doi: 10.1212/WNL.0b013e31821f4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J.J.J., Delbaere K., Close J.C.T. White matter hyperintensities are an independent predictor of physical decline in community-dwelling older people. Gerontology. 2012;58(5):398–406. doi: 10.1159/000337815. [DOI] [PubMed] [Google Scholar]