Abstract
Targeting respiration and ATP synthesis has received strong interest as a new strategy for combatting drug-resistant Mycobacterium tuberculosis. Mycobacteria employ a respiratory chain terminating with two branches. One of the branches includes a cytochrome bc1 complex and an aa3-type cytochrome c oxidase while the other branch terminates with a cytochrome bd-type quinol oxidase. In this communication we show that genetic inactivation of cytochrome bd, but not of cytochrome bc1, enhances the susceptibility of Mycobacterium smegmatis to hydrogen peroxide and antibiotic-induced stress. The type-II NADH dehydrogenase effector clofazimine and the ATP synthase inhibitor bedaquiline were bacteriostatic against wild-type M. smegmatis, but strongly bactericidal against a cytochrome bd mutant. We also demonstrated that the quinone-analog aurachin D inhibited mycobacterial cytochrome bd at sub-micromolar concentrations. Our results identify cytochrome bd as a key survival factor in M. smegmatis during antibiotic stress. Targeting the cytochrome bd respiratory branch therefore appears to be a promising strategy that may enhance the bactericidal activity of existing tuberculosis drugs.
Mycobacterium tuberculosis is the causative agent of tuberculosis disease (TB). In 2013 there were 1.5 million TB-related deaths worldwide and 9 million people were newly infected with TB1. Despite the introduction of efficient antibiotics in the 1950 s, TB treatment remains challenging, largely due to the emergence of drug-resistant strains2,3. Additionally, its metabolic flexibility allows the pathogen to exist in different states, ranging from actively replicating to dormant persisting4,5. The dormant population is difficult to eradicate and has the potential to cause active tuberculosis after resuscitation, which is especially threatening for immune-compromised patients suffering from HIV6. Therefore, drugs with novel mechanisms of action are urgently needed to adequately kill the heterogeneous population of bacteria and to counter multi-drug resistant (MDR) and extensively-drug resistant (XDR) tuberculosis strains. Since basal energy requirements and redox balance are essential for both replicating and persisting bacteria, components of the oxidative phosphorylation pathway are regarded as promising drug targets7,8,9,10,11.
The respiratory chain enzyme complexes that are part of the oxidative phosphorylation pathway establish a proton motive force across the bacterial cytoplasmic membrane and ATP synthase utilizes the energy of the proton motive force for synthesis of ATP. Mycobacterial ATP synthase has been validated as target of bedaquiline (BDQ), the lead compound of the diarylquinoline class of drugs, which selectively inhibits this enzyme in a variety of mycobacterial strains12,13,14,15,16. BDQ has received accelerated approval by the US Food & Drug Administration (FDA) and the European Medicines Agency (EMA) for treatment of MDR-TB17,18. Moreover, components of the respiratory chain such as the type-II NADH dehydrogenase (NDH-2) and the cytochrome bc1 complex are targeted by small-molecule compounds that are currently in clinical development19,20,21,22,23,24. Mycobacteria have a branched electron transport chain. Electrons from the menaquinone pool can be passed on either to the cytochrome bc1 complex, which forms a supercomplex with the cytochrome aa3 oxidase, or alternatively to the cytochrome bd-type quinol oxidase9,10,20 (Fig. 1). Both branches transfer the electrons onto molecular oxygen, yielding H2O, but they differ in the efficiency of energy conservation. The cytochrome bc1/aa3 branch establishes a higher proton motive force as compared with the cytochrome bd branch and consequently is energetically more efficient. Therefore, this respiratory branch is mainly utilized during aerobic, replicating conditions25,26.
Figure 1. The branched respiratory chain in mycobacteria.
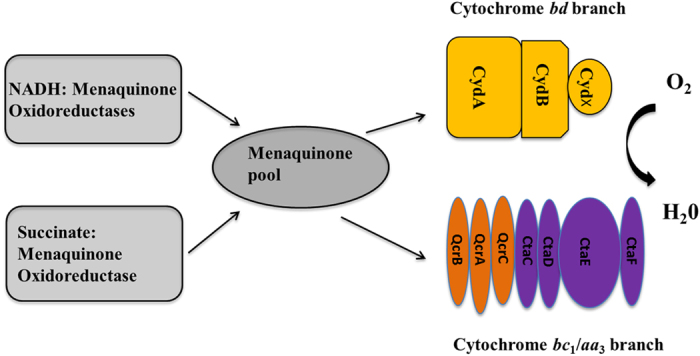
Cyd: cytochrome bd-type quinol oxidase subunits, Qcr: cytochrome bc1 complex subunits, Cta: subunits of aa3-type cytochrome c oxidase. Note that M. smegmatis does not have a soluble cytochrome c. Instead QcrC is a di-heme cytochrome c, which transfers electrons between the cytochrome bc1 complex and the aa3-type cytochrome c oxidase.
Genetic knock-out of the cytochrome bc1 complex in M. smegmatis substantially decreased the growth rate of the bacteria under aerobic growth conditions, while knock-out of cytochrome bd did not25,27. The cytochrome bc1 complex has also been validated as target of the imidazopyridine class of drugs22,24, whereas no antibacterials targeting cytochrome bd have been reported yet. These findings point towards cytochrome bc1/aa3 as the more promising drug target of the two respiratory chain branches in mycobacteria. However, the proteins of the cytochrome bc1/aa3 branch are down regulated during hypoxia and chronic infection in a mouse model, while these conditions induced the expression of cytochrome bd, suggesting an important role for this enzyme in respiration during hypoxia26. Additionally, cytochrome bd was induced when the cytochrome bc1 complex was impaired due to deletion mutations25, upon inhibition by small-molecule drugs28 or when cytochrome c maturation was disturbed29, suggesting that the cytochrome bd branch may (partially) be able to compensate for lack of function of the cytochrome bc1/aa3 branch of the respiratory chain25,28,29.
Cytochrome bd can also play a role in protection against different types of stress30,31,32. In Escherichia coli, exposure to exogenous hydrogen peroxide and nitric oxide induced expression of cytochrome bd and strains lacking cytochrome bd were found hyper-sensitive to peroxide and nitrosative stress33,34,35,36 as well as to low iron concentrations37. In M. tuberculosis, cytochrome bd expression in the mouse lung is upregulated during chronic infection26. During an inflammatory reaction, macrophages in the host can produce reactive oxygen species (ROS) to kill engulfed bacteria. Overexpression of cytochrome bd in M. tuberculosis is associated with increased peroxide resistance29. Upregulation of cytochrome bd may represent a protection mechanism to survive the host’s immune response. These data point towards cytochrome bd as an important contributor to stress resistance in (myco-) bacteria.
In this study, the role of the two mycobacterial respiratory chain branches in response to stress elicited by peroxides and antimicrobials was investigated. For this aim we challenged strains of M. smegmatis lacking cytochrome bd or the cytochrome bc1 complex in vitro with these stress factors to elucidate the importance of each respiratory chain branch in protection against them.
Results
Bioenergetic parameters of Mycobacterium smegmatis strains with inactivated respiratory chain branches
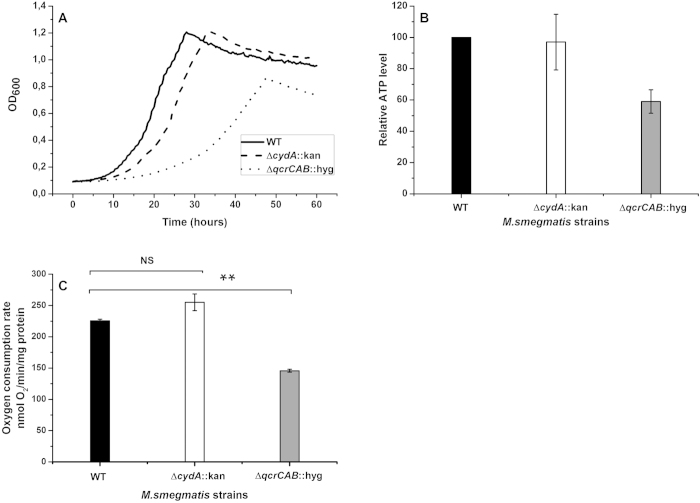
The role of two respiratory chain branches in mycobacteria was investigated using mutant strains impaired in one of the two branches. These strains maintain either only the cytochrome bd branch (strain ΔqcrCAB :: hyg) or the cytochrome bc1/aa3 branch (strain ΔcydA :: kan) (Fig. 1). The growth rate of the ΔcydA :: kan strain was comparable to that of the wild-type, whereas growth of the ΔqcrCAB :: hyg strain was substantially lower (Fig. 2A), confirming previous data25,27. We then extended the earlier reported microbiological characterization of the mutant strains and determined central bioenergetic parameters for the two mutants. Cellular ATP levels were unaltered in the ΔcydA :: kan mutant as compared with the wild-type, but were decreased by ~40% in the ΔqcrCAB :: hyg strain (Fig. 2B). Similarly, oxygen consumption rates in inverted membrane vesicles isolated form aerobically grown cells were almost unchanged in the ΔcydA :: kan mutant versus wild-type, but lower in ΔqcrCAB :: hyg (Fig. 2C). These results reflect the higher respiratory efficiency of the cytochrome bc1/aa3 branch. Based on growth rate and bioenergetic characterization the cytochrome bc1/aa3 branch can be regarded as the more promising target pathway of the two branches.
Figure 2. Bioenergetic properties of M. smegmatis strains lacking the cytochrome bd or the cytochrome bc1 complex.
(A) Wild-type (WT) and mutant strains with knocked-out cytochrome bc1 complex (ΔqcrCAB :: hyg) or cytochrome bd (ΔcydA :: kan) were grown overnight, sub-cultured in fresh medium and incubated at 37 °C for 60 h. The optical density at 600 nm was measured in 20 min intervals. Data are representative of two independent experiments, each done in triplicate. (B) Cellular ATP levels in WT and mutant M. smegmatis as determined by the Luciferase method. (C) Oxygen consumption rates of inverted membrane vesicles from wild-type and mutant M. smegmatis strains using NADH as substrate. Data represent average plus standard error of the mean (SEM) for one experiment done threefold. One-way ANOVA was used for statistical analysis, NS: not significant (P value > 0.05), ** represent P value < 0.01.
Sensitivity for hydrogen peroxide stress
Next, we investigated the importance of the two respiratory chain branches in response to peroxide stress. Exponentially growing M. smegmatis cells were exposed to hydrogen peroxide (20 mM, final conc.) for various time intervals and colony-forming units were enumerated. Incubation with hydrogen peroxide had a bacteriostatic effect on wild-type M. smegmatis and for the ΔqcrCAB :: hyg mutant a minor decrease in viability was found (Fig. 3). For the ΔcydA :: kan mutant, a 99% decline in cell viability was observed after 60 min exposure (Fig. 3). These results suggest that cytochrome bd plays a protective role during oxidative stress in M. smegmatis, whereas the cytochrome bc1 complex is of minor importance for survival under these conditions.
Figure 3. Sensitivity for hydrogen peroxide of Mycobacterium smegmatis respiratory chain mutants.
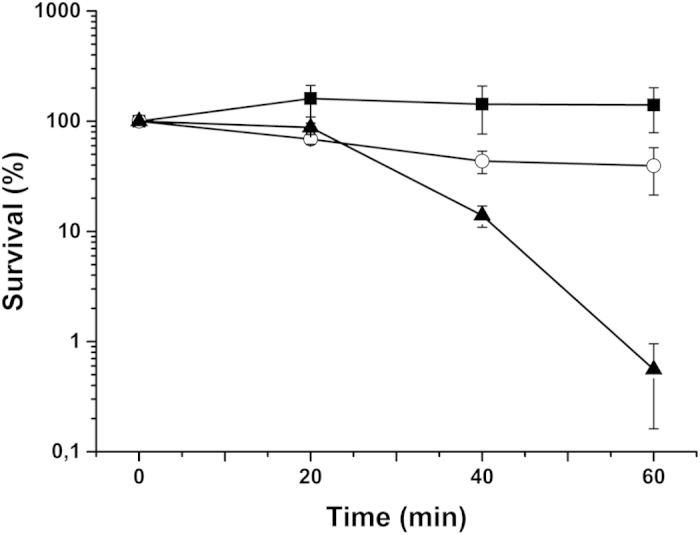
The effect of hydrogen peroxide (20 mM) on the survival of exponentially growing M. smegmatis is shown: wild-type (filled squares), ΔcydA :: kan (filled triangles), and ΔqcrCAB :: hyg (open circle). Results represent means of two independent experiments with standard error of the mean (SEM).
Sensitivity for the NDH-2 effector clofazimine
We hypothesized that mycobacteria with impaired respiratory chain branches may also be more sensitive to antimicrobials that cause production of reactive oxygen species. Clofazimine (CFZ) is a front-line anti-leprosy drug that presently is repurposed for usage against tuberculosis. CFZ is an electron carrier that interferes with the type II NADH dehydrogenase (NDH-2) in mycobacteria19. As such, it can transfer electrons from NDH-2 directly to oxygen, thereby producing ROS19. First, we confirmed that CFZ caused time-dependent development of ROS by inverted membrane vesicles from the M. smegmatis wild-type strain used in our laboratory (Supplementary Figure S1). Subsequently we investigated if either cytochrome bd or the cytochrome bc1 complex can protect M. smegmatis against CFZ. For this purpose the bacteria were incubated for 72 hours in liquid culture with varying concentrations of the drug. CFZ was bacteriostatic against the wild-type strain, even at the highest concentration investigated (25x MIC, 7.5 μg/mL) (Fig. 4). The ΔqcrCAB :: hyg mutant showed marginally higher sensitivity for CFZ as compared with the wild-type (Fig. 4). However, the viability of the ΔcydA :: kan mutant was strongly reduced in response to CFZ challenge. CFZ at concentrations >0.3 μg/mL was bacteriostatic for the ΔcydA :: kan mutant and concentrations >1.5 μg/mL were bactericidal. With 7.5 μg/mL CFZ the limit of detection was reached after 72 hours of exposure (Fig. 4). These results indicate that cytochrome bd, but not the cytochrome bc1 complex, can protect the bacteria against the bactericidal effect of clofazimine. We hypothesized that the increased sensitivity of the ΔcydA :: kan strain was due to ROS production by CFZ. To test this hypothesis we investigated the effect of chlorpromazine (CPZ), a phenothiazine-class drug that inhibits type-II NADH dehydrogenase20,23, but does not produce ROS19, on wild-type and the ΔcydA :: kan mutant. As expected, CPZ did not discriminate between wild-type M. smegmatis and the ΔcydA :: kan mutant (Supplementary Figure S1).
Figure 4. Impact of Mycobacterium smegmatis respiratory chain mutations on the susceptibility for clofazimine.
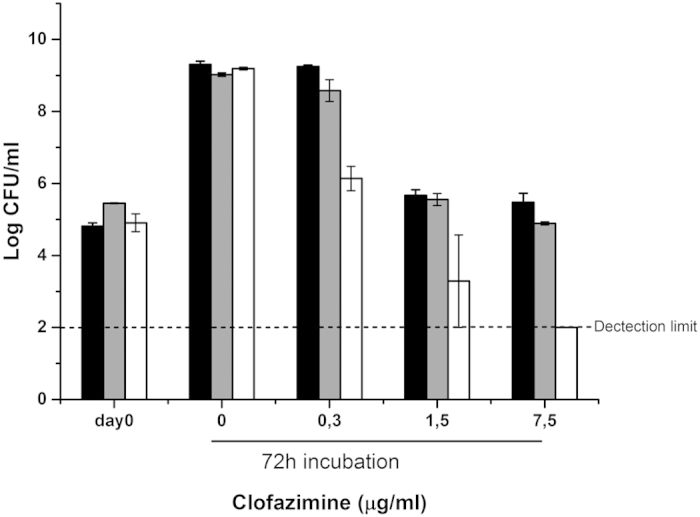
Strains of M. smegmatis were treated with the indicated amounts of clofazimine for 72 hours and CFU/mL were counted on agar plates after three (wild-type, ΔcydA :: kan) or four days (ΔqcrCAB :: hyg) of incubation. Black bars, wild-type; grey bars: ΔqcrCAB :: hyg; white bars: ΔcydA :: kan. Error bars represent means of at least two independent experiments with standard error of the mean (SEM).
Sensitivity for the ATP synthase inhibitor bedaquiline
The results described above demonstrate that genetic inactivation of cytochrome bd, but not of the cytochrome bc1 complex, converts the bacteriostatic effect of hydrogen peroxide and of clofazimine into a bactericidal effect. Next, we expanded our experiments to the ATP synthase inhibitor bedaquiline (BDQ). Whereas BDQ is bactericidal against M. tuberculosis, it is bacteriostatic against M. smegmatis12. A transcriptional and proteomic analysis recently revealed that treatment of M. tuberculosis with BDQ triggers strong upregulation of cytochrome bd38 and deletion of cytochrome bd in M. tuberculosis enhanced the bactericidal activity of BDQ39. We therefore investigated if genetic inactivation of one of the respiratory chain branches would convert the bacteriostatic activity of BDQ on M. smegmatis into bactericidal activity.
BDQ was bacteriostatic against wild-type M. smegmatis, even at the highest concentration used (300x MIC, 5 μg/mL) (Fig. 5). The ΔqcrCAB :: hyg strain was less sensitive to BDQ as compared with the wild-type strain (Fig. 5). However, in case of the ΔcydA :: kan mutant, challenge with BDQ (1 μg/mL) led to a ~1 log10 reduction in colony forming units and 5 μg/mL BDQ caused ~3 log10 kill, approaching the limit of detection after 3 days of treatment (Fig. 5). Cytochrome bd thus protects M. smegmatis against killing by bedaquiline, whereas the cytochrome bc1/aa3 branch does not. We attempted to link the protective function of cytochrome bd to production of ROS in the presence of BDQ, however, inverted membrane vesicles from M. smegmatis did not show increased ROS formation after treatment with BDQ (Supplementary Figure S1).
Figure 5. Impact of Mycobacterium smegmatis respiratory chain mutations on the susceptibility for bedaquiline.
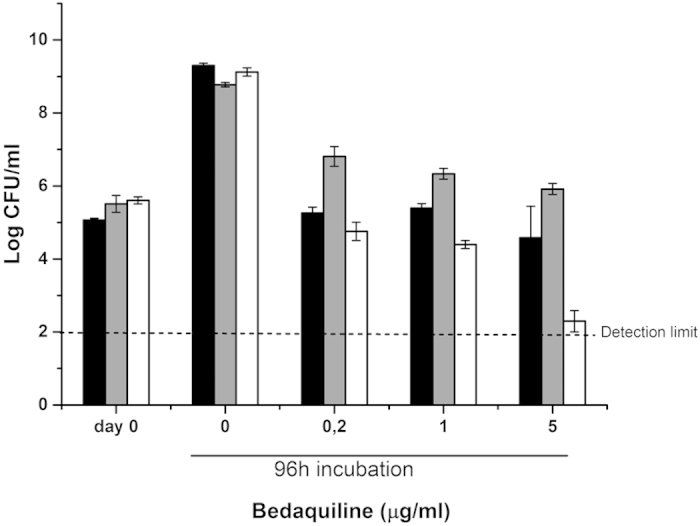
Strains of M. smegmatis were treated with indicated amounts of bedaquiline for 96 hours and CFU/mL were counted on agar plates after three (wild-type, ΔcydA :: kan) or four days (ΔqcrCAB :: hyg) of incubation at 37 °C. Black bars: wild-type; grey bars: ΔqcrCAB :: hyg; white bars: ΔcydA :: kan. Results represent the means of two independent experiments with standard error of the mean (SEM).
The results obtained for CFZ and BDQ demonstrate that inactivation of the cytochrome bd branch, but not of the cytochrome bc1/aa3 branch, can convert bacteriostatic activity of an antibacterial drug into bactericidal activity. Our findings identify cytochrome bd as an important survival factor in mycobacterial metabolism.
Inactivation of mycobacterial cytochrome bd by a small-molecule inhibitor
Genetic inactivation of cytochrome bd can considerably increase the potency of two prominent antibacterial drugs, CFZ and BDQ. Based on these findings we tested if small-molecule inhibitors can block the activity of cytochrome bd in M. smegmatis. The aurachin class of quinone analogs has been reported as inhibitors of a variety of quinone-modifying enzyme40,41,42. Within this class, aurachin D was previously shown to preferentially inhibit E. coli cytochrome bd as compared with other quinone-modifying enzymes42. We investigated the effect of aurachin D on the oxygen consumption activity of inverted membrane vesicles from M. smegmatis. Aurachin D inhibited oxygen consumption in a dose-dependent manner with 50% maximal inhibition for wild-type strain (Fig. 6). Interestingly, this inhibitory effect was clearly stronger in membrane vesicles of the ΔqcrCAB :: hyg strain, where ~90% maximal inhibition was reached (IC50 ~400 nM) (Fig. 6). This suggests that the main target in mycobacterial oxidative phosphorylation was cytochrome bd.
Figure 6. Aurachin D inhibits cytochrome bd activity of Mycobacterium smegmatis membrane vesicles.
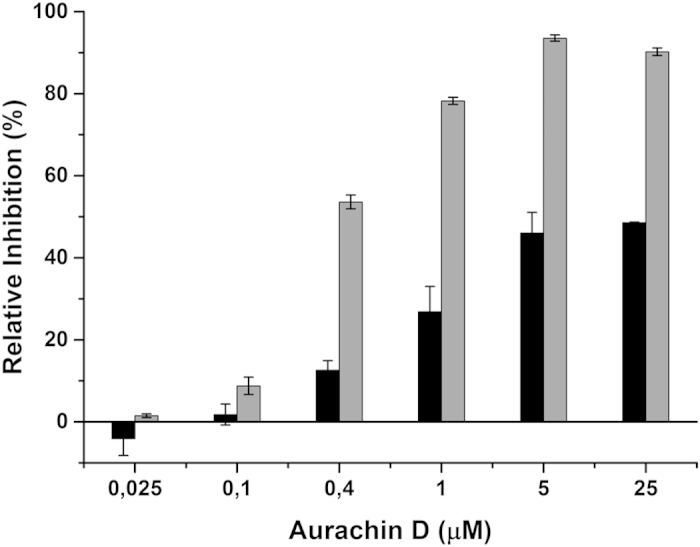
Oxygen consumption activity of inverted membrane vesicles from M. smegmatis was measured with a Clark-type electrode. The reaction was started by addition of NADH (250 μM final conc.) as electron donor and recorded for 90 s. Black bars: wild-type; gray bars: ΔqcrCAB :: hyg. Results represent the means of two independent experiments with standard error of the mean (SEM).
Subsequently, we evaluated the effect of aurachin D on mycobacterial growth. We found that for all three strains tested (wild-type, ΔcydA :: kan, ΔqcrCAB :: hyg) the minimal inhibitory concentrations (MICs) were >85 μM (data not shown). This result suggests that the inhibitor is not capable of effectively crossing the mycobacterial cell envelope.
Discussion
Previously it has been reported that genetic inactivation of cytochrome bd considerably decreased virulence or survival in the host of a variety of pathogenic bacterial strains. In Shigella flexneri, Brucella abortus and Salmonella enterica Serovar Typhymurium, the causative agents of bacterial dysentery, brucellosis and typhoid fever, inactivation of cytochrome bd considerably impaired intracellular survival and virulence43,44,45. In Klebsiella pneumonia cytochrome bd was found crucial for free energy transduction under microaerobic conditions and for protection of anaerobic processes such as nitrogen fixation46. In case of group B streptococci, inactivation of cytochrome bd led to decreased growth in human blood47. Cytochrome bd may also allow strictly anaerobic bacteria such as Bacteriodes fragilis to survive under nanomolar oxygen concentrations, potentially facilitating survival of opportunistic pathogens in the host48.
In this study, we evaluated the function of the two mycobacterial respiratory chain branches in response to stress. The cytochrome bc1 complex is a validated drug target in M. tuberculosis22,24, however, upregulation of cytochrome bd may partially compensate for inhibition of cytochrome bc1 function25,28,29. Therefore, it has been postulated that simultaneously targeting both respiratory chain branches with inhibitors might be required to effectively disrupt mycobacterial respiration29. Whereas the cytochrome bd branch may in part be able to compensate for inactivation of the cytochrome bc1 complex, our results indicate that the cytochrome bc1/aa3 branch is not able to compensate for loss of cytochrome bd functionality. Inactivation of cytochrome bd, although not directly leading to a phenotype, exerts a strong impact on bacterial viability in the presence of antibiotic stress. This highlights the importance of the cytochrome bd branch as a survival factor in M. smegmatis and suggests that targeting this terminal oxidase may be a successful strategy for weakening the mycobacterial stress response.
The hypersensitivity of the cydAB mutants to exogenous hydrogen peroxide is not due to impaired growth of the mutant strain, since growth rate and ATP levels are similar to the wild-type. Giuffre, Borisov and colleagues suggested two molecular mechanisms for peroxide protection by cytochrome bd in E.coli32. First, cytochrome bd as oxygen scavenger may decrease the intracellular oxygen tension, thereby preventing the formation of reactive oxygen species. Second, cytochrome bd displays catalase activity32,34 and might thus directly metabolize peroxides. Both mechanisms may contribute to the protective role of cytochrome bd against hydrogen peroxide stress in M. smegmatis and their respective importance in mycobacteria needs to be further elucidated.
Our experiments revealed that cytochrome bd plays an important role in protection against two prominent anti-tuberculosis drugs, both targeting oxidative phosphorylation. Protection against clofazimine, a ROS-producing drug, is most likely due to the ability of cytochrome bd to metabolize and/or prevent formation of peroxides. Our data do not allow for pinpointing the mechanism of protection against BDQ. Inhibition of ATP synthase may well result in reduction of the electron flow through the respiratory system. As a result, the reduction state of the respiratory complexes increases which in turn leads to increased production of ROS. Higher cellular NADH/NAD+ ratios and enhanced expression of bacterioferritin, indicating BDQ-induced backpressure and ROS formation, have previously been reported for M. tuberculosis treated with BDQ38. However, it is possible that the levels of ROS produced by BDQ are not high enough for detection in case the membrane vesicles used in our study are leaky. Alternatively, protection by cytochrome bd may be due to its lack of proton pump functionality. Cytochrome bd in E. coli has been found electrogenic, but displays a low H+/e- ratio49,50. In this way cytochrome bd may alleviate membrane hyperpolarization.
Inactivation of cytochrome bd converts the bacteriostatic activity of clofazimine and bedaquiline against M. smegmatis into strong bactericidal activity. This finding may be of pharmaceutical and clinical relevance as the bacteriostatic activity of bedaquiline is not restricted to M. smegmatis, but also found for pathogenic non-tuberculous mycobacterial strains, such as the M. avium complex51. These pathogenic strains typically show only low susceptibility towards current antibacterial chemotherapy52. Inactivation of cytochrome bd may assist in improving treatment options for infections caused by these recalcitrant bacteria. It would be important to assess if cytochrome bd deletion mutants in these pathogenic bacteria display increased sensitivity to (ROS-producing) antibacterials as well.
Inhibition of mycobacterial cytochrome bd by aurachin D serves as proof-of-concept for small-molecule inhibition of this important new drug target. Improved aurachin derivatives with better ability to penetrate the mycobacterial cell envelope may constitute a new class of anti-tubercular drugs. Cytochrome bd is of particular interest as potential drug target, as it is only found in prokaryotes. The absence of a human homologue may facilitate selective targeting. However, whole-cell screening on bacteria under aerobic, replicating conditions, which typically are applied for high-throughput discovery procedures53, may not allow for detection of cytochrome bd inhibition. Screening for bacteria under stressed conditions, e.g. in the presence of hydrogen peroxide or bedaquiline, may be applied as an alternative. Additionally, target- or pathway-based screenings, e.g. based on the inverted membrane vesicle system described in this report, against chemical libraries might lead to the discovery of potent cytochrome bd inhibitors.
Materials & Methods
Chemicals
Bedaquiline was obtained from Janssen, Pharmaceutical Companies of Johnson & Johnson. Aurachin D was a kind gift from Dr. Jennifer Herrmann (Helmholtz Centre for Infection Research and Pharmaceutical Biotechnology, Saarbrücken). All other chemicals were bought from Sigma unless indicated otherwise.
Bacterial strains and growth conditions
M. smegmatis mc2 155 was kindly provided by B.J. Appelmelk, Department of Molecular Cell Biology & Immunology, VU University Medical Center Amsterdam, The Netherlands. M. smegmatis mc2155 mutants ΔqcrCAB :: hyg and ΔcydA :: kan were kindly provided by Dr. B. Kana, MRC/NHLS/WITS Molecular Mycobacteriology Research Unit, National Health Laboratory Service, Johannesburg, South Africa. Replicating bacterial cultures were grown in Middlebrook 7H9 broth (Difco) supplied with 0.05% Tween-80 and 10% Middlebrook albumin dextrose catalase enrichment (BBL) at 37 °C with shaking. If applicable, 50 μg/mL kanamycin or 50 μg/mL hygromycin was added to the medium to select for mutant strains.
Growth curves
Growth curves for wild-type and mutant M. smegmatis were determined using a 96-well plate system. Bacteria were diluted to an optical density at 600 nm of 0.01 and optical density was determined at 20 minute intervals for 60 hours. The optical density was measured with a UV-VIS spectrophotometer (Varian Cary50).
Preparation of inverted membrane vesicles
Inverted membrane vesicles (IMVs) of the bacterial strains were prepared as described previously54. Briefly, M. smegmatis was grown for three days in a pre-culture to late-exponential phase. Cells were sedimented by centrifugation at 6000 x g for 20 minutes. The pellet was washed with phosphate buffered saline (PBS, pH 7.4) and centrifuged at 6000 x g for 20 min. Each 5 g of cells (wet weight) was re-suspended in 10 mL of ice-cold lysis buffer (10 mM HEPES, 5 mM MgCl2 and 10% glycerol at pH 7.5) including protease inhibitors (complete, EDTA-free; protease inhibitor cocktail tablets from Roche). Lysozyme (1.2 mg/mL), deoxyribonuclease I (1500 U, Invitrogen) and MgCl2 (12 mM) were added and cells were incubated with shaking for one hour at 37 °C. The lysates were passed three times through a One Shot Cell Disruptor (Thermo Electron, 40 K) at 0.83 kb to break up the cells. Unbroken cells were removed by three centrifugation steps (6000 x g for 20 min at 4 °C). The membranes were pelleted by ultracentrifugation at 222,000 x g for one hour at 4 °C. The pellet was re-suspended in lysis buffer and snap-frozen until use. The protein concentration was measured using the BCA Protein Assay kit (Pierce) as described by the manufacturer.
Oxygen respiration assays
Oxygen respiration and the effect of inhibitors on oxygen respiration were measured by polarography using a Clark-type electrode. The electrode was fully aerated (212 μM O2 at 37 °C) and calibrated with sodium hydrosulfite. The inverted membrane vesicles were pre-incubated for three minutes with the inhibitors in a pre-warmed (37 °C) buffer containing 50 mM MES and 2 mM MgCl2 (pH 6.5). NADH was added as electron donor to a final concentration of 250 μM and oxygen respiration was measured for 90 seconds. Potassium cyanide was used as a control for inhibition. Two independent experiments were performed and average values plus standard errors were calculated.
Cellular ATP levels were determined using the luciferase bioluminescence method described previously55. Briefly, 1.0-mL samples taken from M. smegmatis cultures grown as described above were centrifuged at 8000 * g for 10 min. The pellets were re-suspended in 50 μl water and a 10-fold volume of boiling 100 mM TRIS-HCl, 4 mM EDTA (pH 7.75) was added. After incubation at 100 °C for 2 min the samples were centrifuged (1000 * g, 60 s) and the supernatants transferred to fresh tubes. 100 μl luciferase reagent (ATP Bioluminescence assay, Roche) was added to 100 μl sample and luminescence was measured with a Luminometer (LKB).
Hydrogen peroxide and antibiotic sensitivity assays
Bacterial strains were grown to an optical density at 600 nm of 0.5. For hydrogen peroxide sensitivity assays, hydrogen peroxide (30% (w/v) stock) was added to an Eppendorf tube containing 0.49 mL of bacterial suspension to a final concentration of 20 mM. After the indicated time of incubation at 37 °C with shaking, 15 μl of catalase (10 mg/mL) was added to degrade hydrogen peroxide and thereby stop the reaction. For antibiotic sensitivity assays, 10 mL of bacterial cultures were incubated with the antibiotic for three (clofazimine and chlorpromazine) or four days (bedaquiline) at 37 °C with shaking. All samples were diluted in PBS and 0.1 mL was plated on 7H10 agar plates, containing oleic acid (0.05 g/l) and 10% Middlebrook albumin dextrose catalase enrichment (BBL). Cell viability was measured by counting colony-forming units per mL (CFU/mL) after 72 h (wild-type and ΔcydA :: kan strain) or 96 h (ΔqcrCAB :: hyg strain) incubation at 37 °C. The limit of detection was 100 CFU/mL. Survival was determined as percentage of surviving cells compared to untreated cells at day 0.
ROS detection assays
For detection of reactive oxygen species the Amplex Red® Hydrogen Peroxide/ Peroxidase Assay kit (Invitrogen) was used as described by the manufacturer with minor modifications. To measure ROS production in inverted membrane vesicles, 1 mL samples of 0.05 M sodium phosphate, pH 7.4 containing 20 μg M. smegmatis inverted membrane vesicles, 0.2 mM NADH, 50 μM Amplex Red®, 2 U horseradish peroxidase (HRP), 80 U superoxide dismutase (SOD) and the antibiotic diluted in DMSO in 1x reaction buffer (0.05 M sodium phosphate, pH 7.4) were prepared. Superoxide dismutase was added to allow for detection of superoxide. ROS production was determined by measuring absorbance at 563 nm for 30 minutes with a UV-VIS spectrophotometer (Varian Cary50).
Additional Information
How to cite this article: Lu, P. et al. The cytochrome bd-type quinol oxidase is important for survival of Mycobacterium smegmatis under peroxide and antibiotic-induced stress. Sci. Rep. 5, 10333; doi: 10.1038/srep10333 (2015).
Supplementary Material
Acknowledgments
P.L. is indebted to the Chinese Scholarship Council for a fellowship. The authors wish to thank Henk Hakvoort and Marijke Wagner (VU Amsterdam) for technical assistance, Dr. Bavesh Kana (University of Witwatersrand) for providing the cytochrome bc1 and cytochrome bd mutants and Dr. Jennifer Herrmann (Helmholtz Centre for Infection Research and Pharmaceutical Biotechnology, Saarbrücken) and Dr. Thorsten Friedrich (University of Freiburg) for providing samples of aurachin D.
Footnotes
Author Contributions P.L. and M.H. performed experiments; P.L., M.H., A.K., K.A., G.M.C., H.L., R.v.S. and D.B. designed experiments and analyzed data; D.B. and R.v.S. supervised and coordinated experiments; P.L., M.H. and D.B. wrote the manuscript with contributions from all co-authors, D.B. supervised the overall research.
References
- The World Health Organization, Global tuberculosis report 2014. (2015) available at: http://www.who.int/tb/publications/global_report/en/ (date of access: 01/04/2015).
- Dartois V. The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells. Nat. Rev. Microbiol. 12, 159–167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienhardt C. et al. Global tuberculosis control: lessons learnt and future prospects. Nat. Rev. Microbiol. 10, 407–416 (2012). [DOI] [PubMed] [Google Scholar]
- Barry C. E. III et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 7, 845–855 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C. Latency: A sleeping giant. Nature 502, S14–S15 (2013). [DOI] [PubMed] [Google Scholar]
- Koul A., Arnoult E., Lounis N., Guillemont J. & Andries K. The challenge of new drug discovery for tuberculosis. Nature 469, 483–490 (2011). [DOI] [PubMed] [Google Scholar]
- Hurdle J. G., O’Neill A. J., Chopra I. & Lee R. E. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 9, 62–75 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bald D. & Koul A. Respiratory ATP synthesis: the new generation of mycobacterial drug targets ? FEMS Microbiol. Lett. 308, 1–7 (2010). [DOI] [PubMed] [Google Scholar]
- Black P. A. et al. Energy metabolism and drug efflux in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 58, 2491–2503 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Lill H. & Bald D. ATP synthase in mycobacteria: special features and implications for a function as drug target. Biochim. Biophys. Acta. 1837, 1208–1218 (2014). [DOI] [PubMed] [Google Scholar]
- Cook G. M., Hards K., Vilcheze C., Hartman T. & Berney M. Energetics of Respiration and Oxidative Phosphorylation in Mycobacteria. Microbiol. Spectr. 2, (2014), 10.1128/microbiolspec.MGM2-0015-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andries K. et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307, 223–227 (2005). [DOI] [PubMed] [Google Scholar]
- Haagsma A. C. et al. Selectivity of TMC207 towards mycobacterial ATP synthase compared with that towards the eukaryotic homologue. Antimicrob. Agents Chemother. 53, 1290–1292 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagsma A. C. et al. Probing the interaction of the diarylquinoline TMC207 with its target mycobacterial ATP synthase. PLoS. One 6, e23575 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul A. et al. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat. Chem. Biol. 3, 323–324 (2007). [DOI] [PubMed] [Google Scholar]
- Rao S. P., Alonso S., Rand L., Dick T. & Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 105, 11945–11950 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Infectious disease. Approval of novel TB drug celebrated--with restraint. Science 339, 130 (2013). [DOI] [PubMed] [Google Scholar]
- Jones D. Tuberculosis success. Nat. Rev. Drug Discov. 12, 175–176 (2013). [DOI] [PubMed] [Google Scholar]
- Yano T. et al. Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase: a pathway for the generation of bactericidal levels of reactive oxygen species. J. Biol. Chem. 286, 10276–10287 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein E. A. et al. Inhibitors of type II NADH:menaquinone oxidoreductase represent a class of antitubercular drugs. Proc. Natl. Acad. Sci. USA 102, 4548–4553 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warman A. J. et al. Antitubercular pharmacodynamics of phenothiazines. J. Antimicrob. Chemother. 68, 869–880 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethe K. et al. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat. Med. 19, 1157–1160 (2013). [DOI] [PubMed] [Google Scholar]
- Boshoff H. I. et al. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J. Biol. Chem. 279, 40174–40184 (2004). [DOI] [PubMed] [Google Scholar]
- Abrahams K. A. et al. Identification of novel imidazo[1,2-a]pyridine inhibitors targeting M. tuberculosis QcrB. PLoS. One. 7, e52951 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsoso L. G. et al. Function of the cytochrome bc1-aa3 branch of the respiratory network in mycobacteria and network adaptation occurring in response to its disruption. J. Bacteriol. 187, 6300–6308 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L. et al. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc. Natl. Acad. Sci. USA 102, 15629–15634 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana B. D. et al. Characterization of the cydAB-encoded cytochrome bd oxidase from Mycobacterium smegmatis. J. Bacteriol. 183, 7076–7086 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora K. et al. Respiratory flexibility in response to inhibition of cytochrome C oxidase in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 58, 6962–6965 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. L. et al. Perturbation of cytochrome c maturation reveals adaptability of the respiratory chain in Mycobacterium tuberculosis. MBio. 4, e00475–13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisov V. B., Gennis R. B., Hemp J. & Verkhovsky M. I. The cytochrome bd respiratory oxygen reductases. Biochim. Biophys. Acta. 1807, 1398–1413 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte E. et al. Cytochrome bd oxidase and hydrogen peroxide resistance in Mycobacterium tuberculosis. MBio. 4, e01006–e01013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffre A., Borisov V. B., Arese M., Sarti P. & Forte E. Cytochrome bd oxidase and bacterial tolerance to oxidative and nitrosative stress. Biochim. Biophys. Acta. 1837, 1178–1187 (2014). [DOI] [PubMed] [Google Scholar]
- Borisov V. B. et al. Interaction of the bacterial terminal oxidase cytochrome bd with nitric oxide. FEBS Lett. 576, 201–204 (2004). [DOI] [PubMed] [Google Scholar]
- Borisov V. B. et al. Cytochrome bd oxidase from Escherichia coli displays high catalase activity: an additional defense against oxidative stress. FEBS Lett. 587, 2214–2218 (2013). [DOI] [PubMed] [Google Scholar]
- Lindqvist A., Membrillo-Hernandez J., Poole R. K. & Cook G. M. Roles of respiratory oxidases in protecting Escherichia coli K12 from oxidative stress. Antonie Van Leeuwenhoek 78, 23–31 (2000). [DOI] [PubMed] [Google Scholar]
- Mason M. G. et al. Cytochrome bd confers nitric oxide resistance to Escherichia coli. Nat. Chem. Biol. 5, 94–96 (2009). [DOI] [PubMed] [Google Scholar]
- Cook G. M. et al. A factor produced by Escherichia coli K-12 inhibits the growth of E. coli mutants defective in the cytochrome bd quinol oxidase complex: enterochelin rediscovered. Microbiology 144, 3297–3308 (1998). [DOI] [PubMed] [Google Scholar]
- Koul A. et al. Delayed bactericidal response of Mycobacterium tuberculosis to bedaquiline involves remodelling of bacterial metabolism. Nat. Commun. 5, 3369 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berney M., Hartman T. E. & Jacobs W. R. Jr. A Mycobacterium tuberculosis cytochrome bd oxidase mutant is hypersensitive to bedaquiline. MBio. 5, e01275–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J. et al. Discovery of selective menaquinone biosynthesis inhibitors against Mycobacterium tuberculosis. J. Med. Chem. 55, 3739–3755 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. W. et al. Synthesis and biological activities of the respiratory chain inhibitor aurachin D and new ring versus chain analogues. Beilstein. J. Org. Chem. 9, 1551–1558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier B., Madgwick S. A., Reil E., Oettmeier W. & Rich P. R. New inhibitors of the quinol oxidation sites of bacterial cytochromes bo and bd. Biochemistry 34, 1076–1083 (1995). [DOI] [PubMed] [Google Scholar]
- Way S. S., Sallustio S., Magliozzo R. S. & Goldberg M. B. Impact of either elevated or decreased levels of cytochrome bd expression on Shigella flexneri virulence. J. Bacteriol. 181, 1229–1237 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. K. et al. Contribution of proton-translocating proteins to the virulence of Salmonella enterica serovars Typhimurium, Gallinarum, and Dublin in chickens and mice. Infect. Immun. 71, 3392–3401 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endley S., McMurray D. & Ficht T. A. Interruption of the cydB locus in Brucella abortus attenuates intracellular survival and virulence in the mouse model of infection. J. Bacteriol. 183, 2454–2462 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juty N. S., Moshiri F., Merrick M., Anthony C. & Hill S. The Klebsiella pneumoniae cytochrome bd’ terminal oxidase complex and its role in microaerobic nitrogen fixation. Microbiology 143, 2673–2683 (1997). [DOI] [PubMed] [Google Scholar]
- Yamamoto Y. et al. Respiration metabolism of Group B Streptococcus is activated by environmental haem and quinone and contributes to virulence. Mol. Microbiol. 56, 525–534 (2005). [DOI] [PubMed] [Google Scholar]
- Baughn A. D. & Malamy M. H. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 427, 441–444 (2004). [DOI] [PubMed] [Google Scholar]
- Puustinen A., Finel M., Haltia T., Gennis R. B. & Wikstrom M. Properties of the two terminal oxidases of Escherichia coli. Biochemistry 30, 3936–3942 (1991). [DOI] [PubMed] [Google Scholar]
- Kita K., Konishi K. & Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J. Biol. Chem. 259, 3375–3381 (1984). [PubMed] [Google Scholar]
- Lounis N., Gevers T., Van den Berg J., Vranckx L. & Andries K. ATP synthase inhibition of Mycobacterium avium is not bactericidal. Antimicrob. Agents Chemother. 53, 4927–4929 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ingen. J. & Kuijper E. J. Drug susceptibility testing of nontuberculous mycobacteria. Future Microbiol. 9, 1095–1110 (2014). [DOI] [PubMed] [Google Scholar]
- Bald D. & Koul A. Advances and strategies in discovery of new antibacterials for combating metabolically resting bacteria. Drug Discov. Today 18, 250–255 (2013). [DOI] [PubMed] [Google Scholar]
- Haagsma A. C., Driessen N. N., Hahn M. M., Lill H. & Bald D. ATP synthase in slow- and fast-growing mycobacteria is active in ATP synthesis and blocked in ATP hydrolysis direction. FEMS Microbiol. Lett. 313, 68–74 (2010). [DOI] [PubMed] [Google Scholar]
- Lu P. et al. Pyrazinoic acid decreases the proton motive force, respiratory ATP synthesis activity, and cellular ATP levels. Antimicrob. Agents Chemother. 55, 5354–5357 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.