Abstract
Objectives
Variation in the prevalence of eosinophilic gastrointestinal diseases in different geographical regions has not been extensively studied. The aim of the present study was to define the regional and national prevalence of eosinophilic gastrointestinal diseases, and differences in practice approaches.
Patients and Methods
We administered a survey electronically to members of the American College of Gastroenterology, the American Academy of Allergy, Asthma, and Immunology, and the North American Society Pediatric Gastroenterology, Hepatology, and Nutrition. Questions pertained to the number and proportion of patients seen with eosinophilic gastroenteritis or colitis and eosinophilic esophagitis (EoE), and methods used to diagnose and treat these conditions.
Results
A total of 1836 physicians responded from 10,874 requests (17% response). Extrapolating responses from our US sample, we estimated an overall prevalence of 52 and 28/100,000 for EoE and eosinophilic gastroenteritis or colitis. The patient burden of EoE is higher in urban (0.58) and suburban (0.44) compared with rural settings (0.36, P <0.0065), observations consistent with other allergic disorders. There was also increased prevalence in northeast region when calculated by prevalence per 100,000. There was considerable variability in criteria and initial treatment options used to diagnose EoE. Only one-third of respondents reported using diagnostic criteria proposed in a 2007 consensus document. Seventy-one and 35% of respondents reported treating some patients with EoE with a food elimination or elemental diet, respectively.
Conclusions
EoE is diagnosed more often in northeastern states and urban areas. There is considerable variability in diagnostic criteria and initial treatment approach supporting the need for additional clinical trials and consensus development.
Keywords: eosinophilic esophagitis, eosinophilic gastrointestinal disease, prevalence, treatment options
Eosinophilic gastrointestinal diseases (EGIDs) are increasingly described disorders that include eosinophilic esophagitis (EoE), eosinophilic gastroenteritis (EG), and eosinophilic colitis (EC). Optimal methods to diagnose and care for patients with these conditions remain incompletely defined. As a result, there continues to be variability in clinical practice, the extent of which has not been well studied (1).
The diagnosis of EoE depends in part upon demonstration of esophageal eosinophilia, but diagnostic criteria have varied across different reports, especially as they pertain to the minimal number of eosinophils and the extent to which gastroesophageal reflux disease (GERD) was excluded (1,2). A panel of experts proposed consensus recommendations in 2007, which included the demonstration of ≥15 eosinophils in at least 1 high-power field (HPF) despite treatment with a proton pump inhibitor (PPI) or exclusion of GERD by ambulatory pH monitoring (2). Whether the proposed criteria have been adopted in clinical practice is unknown. In addition, how adjunctive allergy testing, also suggested in the guideline, is used is also uncertain.
Similarly, the guideline suggested management options for these disorders, which included dietary and pharmacological approaches used alone or in combination. How these options are used in clinical practice has not been extensively evaluated. Among the EGIDs, EoE has become increasingly prevalent based on studies in the United States, Switzerland, and Australia (3–6). EoE has been reported throughout the world including most of Europe, Canada, the United States, Japan, Australia, New Zealand, Turkey, Israel, and China (7–16). The increasing prevalence is likely due to increased recognition and an increasing incidence, although there have been limited studies. Several other immune and allergic disorders such as asthma and atopic dermatitis have demonstrated variation in prevalence across geographical regions, but such an association has not been evaluated for EoE (17–22).
To better understand practice variability in diagnosis and management and regional prevalence, we conducted an international survey with a primary focus on the United States, targeting physicians in adult and pediatric gastroenterology and allergy-immunology because these groups are most likely to provide care for patients with EGIDs.
MATERIALS AND METHODS
Survey
An electronic survey (Survey Monkey, Portland, OR) was sent via e-mail invitation (and 2 reminders) to all of the physician members of the American Academy of Allergy, Asthma, and Immunology (N = 3621), the American College of Gastroenterology (n = 5789), and the North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) (n = 1423). These societies represent the large specialty societies for both private practice and academic allergist-immunologists (AIs) and gastroenterologists.
Survey questions pertained to diagnostic criteria, management options, and proportion of patients with EGIDs seen in practice (Supplemental Digital Content Figure 1, http://links.lww.com/MPG/A20). The study was reviewed and approved by the institutional review board of The Children’s Hospital of Philadelphia and by the participating societies. We limited our analysis to responses from the United States because of the relatively small number of respondents in other countries.
Statistical Analysis
Summary and descriptive statistics were generated stratifying responses according to specialty and geographical location. Data were analyzed using SAS 9.13 (SAS Institute Inc, Cary, NC). Chi square and Mantel-Haenszel χ2 tests and logistic regression analyses were used to calculate the P value and odds ratio.
The prevalence of EGIDs in the population was estimated by extrapolating responses from question 3, which pertained to the number of patients seen with EGIDs in the last year. The point estimate for each respondent was estimated to be the midpoint of the response range. For example, a value of 5 was assigned for those who responded in the 0 to 10 range. We assumed that responses were representative of a national distribution of responses for pediatric and adult gastroenterologists and therefore multiplied the mean values for each specialty by the number of specialists. The numbers of pediatric gastrointestinal (GI) and adult GI physicians were obtained from the American Medical Association and American Osteopathic Association as 860 and 13,233, respectively. We restricted the analysis to pediatric and adult gastroenterologists (and excluded allergists) to avoid counting patients managed by both gastroenterologists and allergists twice. We also assumed that patients would not be seen exclusively by allergists.
We then divided the total number of patients by the US population to provide an estimate of overall prevalence. We made no adjustment for the specific diagnostic criteria used by each respondent, and thus the estimates are not based on standardized criteria but rather on the opinions of respondents and/or local criteria. However, for comparison we performed exploratory analysis based on respondents that used 2007 consensus criteria.
To estimate what proportion of patients in gastroenterology or allergy practices have EGIDs, we used questions 2 and 3, which pertained to the number of patients with EGIDs seen in the last year and the total number of patients seen in a typical week (termed patient burden). The point estimate for each respondent was estimated to be the midpoint of the response range. For example, respondents who checked that they see 1 to 25 patients per week were assigned a point estimate of 12.5. The relative proportion of patients with EGIDs was then estimated by dividing the point estimate for each EGID by the point estimate for total patients as the patient burden. The estimate for each respondent was estimated to be the midpoint of the response range. For example, respondents who checked that they see 1 to 25 patients per week were assigned a point estimate of 12.5. The relative proportion of patients with EGIDs was then estimated by dividing the point estimate for each EGID by the point estimate for total patients as the patient burden. We examined state-by-state prevalence by using the midpoint for the number of patients seen per year. This value was summed for each physician per state and averaged. The average value was multiplied by the number of gastroenterologists per state, and prevalence was calculated by dividing by the state population per 2008 US census data (Supplemental Digital Content Table E2, http://links.lww.com/MPG/A20). We also examined whether the prevalence of EGIDs was higher in northern compared with southern states as well as the relative distribution in 4 regions (northeast, south, midwest, and west) or 8 regions (New England, Middle Atlantic, South Atlantic, East North Central, East South Central, West South Central, West North Central, Mountain, and Pacific) as previously described (Supplemental Digital Content, http://links.lww.com/MPG/A20, for state assignments) (22). The definitions of regions were formerly used in the analysis of disease distribution for other allergic disorders (23).
RESULTS
A total of 1836 physicians responded from a total of 10,874 requests (17% response). Respondents represented 57 countries and all 50 states in the United States (1402 GI and AI respondents). Response rates varied from 10% for adult GI to 24% for pediatric GI and AI (Table 1). A total of 35 respondents described their specialty as “other” and were therefore excluded from all of the analysis.
TABLE 1.
Response from US survey
| Group | No. response (% of total) |
|---|---|
| Allergy-immunology | 866/3621 (23%) |
| Pediatric GI | 333/1423 (23%) |
| Adult GI | 602/5789 (10%) |
| Total | 1801/11,033 (16%) |
| Total US respondents | 1836 (35 others*) |
| Academic | 661 (41%) |
| Private practice | 960 (59%) |
| Total | 1621 (180 with no response) |
| Urban | 891 (56%) |
| Suburban | 656 (41%) |
| Rural | 62 (4%) |
| Total | 1616 (185 with no response) |
Were not included in the analysis.
Respondents described their practice settings as private practice (60%) or academic practice (40%). Most respondents practiced in urban (56%) or suburban areas (41%). The range of years in practice varied; 41% had been in practice for >14 years, whereas 26% had been in practice for <5 years, with the remainder in between. The proportion of respondents varied minimally across regions ranging from 8.8% to 16.7% for allergists, 21.9% to 34.5% for pediatric gastroenterologists, and 4.7% to 10.3% for adult gastroenterologists, with 89 of the 106 regional comparisons being not significantly different for the response rate (Supplemental Digital Content Table E1, http://links.lww.com/MPG/A20).
Diagnostic Criteria
Possible choices for a response to the question on diagnostic criteria for EoE were based on criteria most commonly used in the published literature. The “correct” choice was based on criteria proposed in a 2007 consensus statement (2). Overall, only 35% of respondents picked the consensus criteria; responses were similar among AI, pediatric GI, and adult GI (33%, 35%, and 31%, respectively). The youngest physicians (<5 years of experience) were significantly more likely to identify the correct response compared with the most experienced (>15 years) physicians (39 vs 29%, P <0.05).
Treatment Options
We examined the proportion of patients with EoE treated with various options for first-line therapy. Options included PPI, swallowed corticosteroids, swallowed corticosteroids with PPI, oral corticosteroids, leukotriene modifiers, elimination diet, elemental diet, dietary therapy with PPI, dietary therapy with swallowed corticosteroids, or other. The question pertained to the relative use of these approaches as first-line therapy rather than the choice of therapies in specific subgroups of patients (eg, those who failed a previous approach, those who had undergone allergy testing).
There was large variability across treatment options that was not explained by specialty, years of practice, or location of practice. Swallowed corticosteroids plus a PPI (44%) or an elimination diet (39%) were the most common choices for first-line therapy consistent with published data and consensus opinion (Table 2) (2). The use of a PPI alone as first-line therapy was high, with 25% of responders using PPI only most or all of the time, indicating that some physicians are only treating symptomatically and are not treating the underlying inflammation. There was no difference in the use of PPIs alone as initial therapy for EoE between those using >15 or >20 eosinophils/HPF as a diagnostic cutoff (P = 0.50; Cochran-Mantel-Haenszel test)
TABLE 2.
First-line therapy for EoE (add denominators)
| Therapy | All (% of responses) | Most (% of responses) |
|---|---|---|
| PPI only | 221 (13) | 197 (12) |
| Swallowed corticosteroids | 115 (7) | 289 (18) |
| Swallowed corticosteroids + PPI | 178 (11) | 497 (31) |
| Leukotriene inhibitors | 31 (2) | 67 (4) |
| Elimination diet | 181 (11) | 288 (18) |
| Systemic corticosteroids | 11 (1) | 27 (2) |
| Elemental diet | 11 (1) | 50 (3) |
| Dietary therapy + swallowed corticosteroids | 61 (3) | 251 (16) |
| Dietary therapy + PPI | 65 (4) | 133 (8) |
| Other | 7 (0.4) | 16 (1) |
Percentage of response on individual therapeutic options based on 4 choices (all, most, some, or none). EoE = eosinophilic esophagitis; PPI = proton pump inhibitors.
Dietary therapy was not used as first-line therapy in many patients, despite evidence of its effectiveness in children (24). The most common reason that AI and pediatric GI did not use dietary therapy was a concern about compliance whereas among adult GI, it was “insufficient scientific data.” Overall 41% of AI reported using dietary therapy as a first-line approach (data not shown).
Allergy Testing
We examined the preference for allergy testing for EoE and EG-EC among AI. Skin prick test was used for allergen determination in 95% of patients, specific immunoglobulin E 62%, atopy patch test 36%, other 7%, and none 1% for patients with EoE. Allergists performed significantly more tests for EoE compared with EG-EC patients (86% for skin prick test, 57% for specific immunoglobulin E, 26% for atopy patch test, 9% for other, and 7% for none; P <0.01).
Referral to Allergy-Immunology
Forty-six percent of adult GI and pediatric GI refer to AI either most or all of the time for patients with EoE and EG. Academic adult GI or pediatric GI referred to AI significantly more often than those in private practice (55% vs 38%; P <0.0001). There was significantly less referral to AI among rural GI physicians compared with urban and suburban physicians (P <0.004). In addition, pediatric GIs were significantly more likely to refer to allergy than adult GI (74% vs 31%; P <0.0001).
Prevalence
The estimated average number of patients seen by each specialty is reported in Table 3. Pediatric GI saw significantly more patients with EoE and EG-EC compared with adult GI or AI. We estimated that the overall prevalence of EoE in the United States is 52/100,000 population, whereas the estimated prevalence of EG-EC is 28/100,000 population. This prevalence was based on patients seen in adult GI and pediatric GI. If the prevalence was based on patients seen by AI only, then the calculation is 22/100,000 for EoE and 2/100,000 for EG-EC. We did not attempt to make similar estimates in other countries because of the relatively small number of respondents in each country. The prevalence based on the physicians that used the consensus diagnostic criteria is not significantly different at 47/100,000.
TABLE 3.
Estimated prevalence for EGID
| Average no. patients seen per year-EoE (range) | Average no. patients seen per year-EG-EC (range) | |
|---|---|---|
| Pediatric GI | 20.2 (12.4–27.3) | 8.9 (3.2–13.8) |
| Adult GI | 10.7 (4.7–15.7) | 5.9 (0.7–10.1) |
| Estimate of US patients | 158,705 (72,332–231,224) | 85,281 (12,040–145,677) |
| Prevalence/100,000 | 52.2 (23.8–76.1) | 28.1 (4.0–48.0) |
EG-EC = eosinophilic gastroenteritis or colitis; EGID = eosinophilic gastrointestinal disease; EoE = eosinophilic esophagitis.
There were significant differences in the number of patients with EGIDs as examined by patient burden seen in urban, suburban, and rural areas after adjusting for academic, private practice, and geographical region factors. There was no significant difference in the number of patients seen in private versus academic centers (Table 4).
TABLE 4.
Regional differences for patient with EGID burden
| EoE
|
EG-EC
|
|||||
|---|---|---|---|---|---|---|
| Patients/y, mean (range) | Patient burden | Odds ratio for suburban (CI), P* | Patients/y, mean (range) | Patient burden | Odds ratio for suburban (CI), P* | |
| Urban | 13.4 (7.6–20.9) | 0.58 | 0.78 (0.74–0.82), P <0.001 | 7.2 (2.8–12.9) | 0.317 | 0.90 (0.83–0.97), P <0.01 |
| Suburban | 13.7 (7.9–21.1) | 0.44 | 1 | 6.7 (2.3–12.1) | 0.215 | 1 |
| Rural | 10.5 (5.3–16.8) | 0.36 | 1.12 (1.02–1.23), P <0.01 | 5.2 (1.2–10.2) | 0.183 | 1.14 (0.89–1.47) |
CI = confidence interval; EG-EC = eosinophilic gastroenteritis or colitis; EGID = eosinophilic gastrointestinal disease; EoE = eosinophilic esophagitis.
Logistic regression after correcting for academic practices.
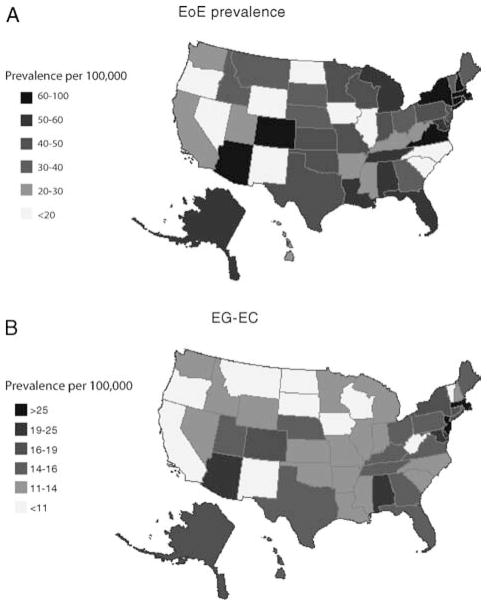
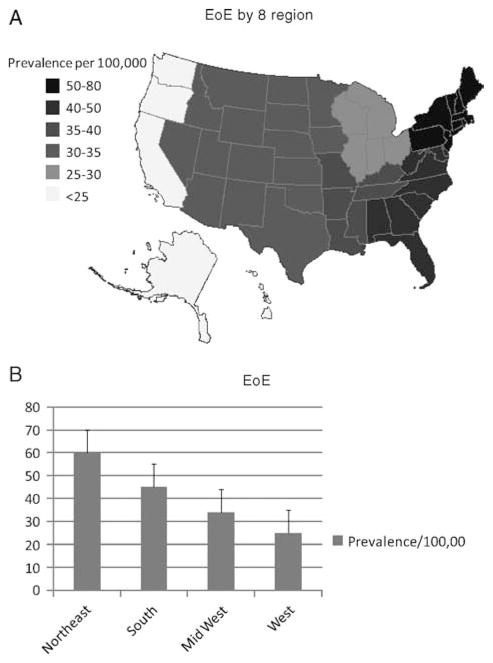
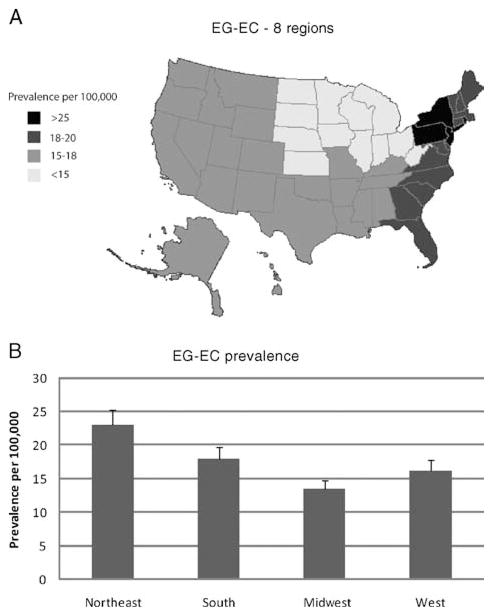
We also calculated prevalence by 100,000 for each individual state based on the assumption that patients were diagnosed by gastroenterologists and not by AI (Fig. 1A and B). Middle Atlantic and New England states have the highest prevalence (P <0.05) for EoE (Fig. 2A). On examination of 4 regions, the northeast has a significantly higher prevalence (P <0.03) (Fig. 2B). The prevalence of EG-EC was significantly higher in the Middle Atlantic (for 8 regions) and northeastern regions (for 4 regions) compared with other regions (Fig. 3A, B).
FIGURE 1.
A, Distribution of EoE in US regions. B, Distribution of EG-EC in the United States. Prevalence based on physician response corrected for the number of physician per state and state population. EG-EC = eosinophilic gastroenteritis or colitis; EoE = eosinophilic esophagitis.
FIGURE 2.
Distribution of EoE in the United States. Prevalence based on physician response corrected for the number of physician per state and state population. States divided into 9 regions (A) or 4 regions (B) based on previous studies (12,13). EoE = eosinophilic esophagitis.
FIGURE 3.
Distribution of EG-EC in the United States. Prevalence based on physician response corrected for the number of physicians per state and state population. States divided into 9 regions (A) or 4 regions (B) based on previous studies (22,23). EG-EC = eosinophilic gastroenteritis or colitis.
DISCUSSION
We have performed the largest US-based survey focusing on EGIDs, which has helped quantify practice variability and provide estimates for the prevalence of these disorders and their regional variation.
We estimated that the prevalence of EoE in the United States is 52/100,000, whereas the prevalence of EG-EC is 28/100,000, suggesting that there are 158,000 people with EoE and 85,000 with EG-EC. The prevalence based on patients seen by AI only is much lower because adult GI and pediatric GI make the initial diagnosis and often do not refer to AI for evaluation. A number of previous studies have also provided population-based prevalence estimates. Ronkainen et al (25) performed endoscopy in a random sample of adults in Sweden. They found a prevalence of EoE of 400/100,000 when the diagnosis was based on >20 eosinophils/HPF, and 1100/100,000 when it was based on >10 eosinophils/HPF; an important limitation was that GERD had not been excluded and most subjects were asymptomatic. Gill et al (26), in review of their cases of EoE from 1995 to 2004, found a prevalence of 7.3/100,000 in their population in West Virginia. Noel et al (3) found a prevalence of 43.6/100,000 in the Cincinnati region. Straumann and Simon (27) found a prevalence of 23/100,000 in study of adults in Switzerland. In a report from Olmsted County Minnesota, the prevalence was estimated to be 55/100,000 in 2006 (28), which is nearly identical to our current estimate.
Multiple factors may account for differences among studies, including methods of data collection and case definitions. However, they may also be related to regional variation in disease burden as suggested by our findings. Nevertheless, the approach we took in deriving our estimates has several limitations. We used the midpoint of the distribution in each response to provide a point estimate, an approach that may not account for potentially relevant variations within the range. However, the approach was used consistently for comparisons across regions, supporting the validity of the differences we observed. Furthermore, we also calculated a range with minimum and maximum values based on the highest and lowest value in each group for EoE and EG-EC (Table 3).
Other potential pitfalls of our approach include response bias and misclassification of cases. One possibility is that only physicians who were interested in EGIDs responded; however, 60% of the responders indicated that they saw only 1 to 9 new EGID patients per year, making this error unlikely. We could not determine whether some responders classified patients as having EGIDs who may have had other disorders. Nevertheless, such misclassification would likely have occurred consistently across the regions and provider types examined, supporting the validity of the comparisons. Another potential pitfall is that some respondents may not have interpreted the prevalence questions as pertaining to unique patients and thereby counted patients more than once. However, such an interpretation of the prevalence questions is unlikely, as underscored by the similarity of our estimates with previously published population-based estimates (3,5,6,26,28).
The overall response rate to the survey was 17%, which is similar to previous electronic surveys in the allergy-immunology literature (29–32). Nevertheless, there remains the possibility that responders differed from the overall pool of eligible providers. It is also possible that patients can be seen by multiple GI physicians and duplicate in our survey. However, we asked responders to identify “unique” patients and second-opinion patients should not have been considered. A post hoc review of the questionnaire with physicians indicated that they would not include a second opinion as a new patient.
We examined adoption of the consensus diagnostic criteria for EoE (2). Only one-third of physicians selected the consensus definition, whereas an additional 39% picked more stringent criteria on 20 eosinophils/HPF. This suggests that they may be missing a small population of EoE patients, which indicates that our estimate prevalence is lower than the actual prevalence. On the contrary, 16% of physicians based the diagnosis on 24 eosinophils/HPF without the need for pretreatment of PPI, potentially leading patients with GERD to be misclassified as EoE. The varying responses underscore the need for additional studies to validate diagnostic criteria and to disseminate the findings.
The treatment options varied for EoE and EG-EC, with no clear preference among physicians. Thus, responses were consistent with the consensus statement, which did not strongly recommend one approach or another (2). For example, dietary therapy has the highest reported success rate of 95% in children (24) with the use of elemental formulas. Elimination diets based on allergy testing (33,34) or removal of 6 common food groups (35) also have a high success rate of 70% to 80%; however, dietary therapy has not been studied on a large scale in adults. Drawbacks of the dietary approach include difficulty with compliance and a decrease in quality of life from the restricted diet.
For the physicians sampled, only 29% reported that they used dietary approaches as first-line therapy for most or all of their patients. AIs were more likely to use dietary approaches as first-line therapy compared with pediatric GI or adult GI. This may be explained by the observation that AI are accustomed to dealing with food allergies and restrictive diets. Among those who did not choose dietary therapy as a first-line approach, the most common reasons were family preference, insufficient data, management issues, and compliance. The most common reason cited for adult GI was lack of sufficient data, whereas the most common reason for pediatric GI was family preference.
Swallowed corticosteroids were most commonly used; 44% of respondents used them in combination with a PPI probably due to the inclusion of AI in the survey. The response rate to swallowed corticosteroids has varied from 50% to 90% depending on the study design, medication, and dose (36–40). The response rate to oral steroids is close to 100% (24). A previous survey of 249 pediatric and adult GI found that 59% of pediatric GI and 73% of adult GI use topical corticosteroids as first-line therapy (41), which is approximately our rate of 66% in the GI community (53% pediatrics and 83% adults).
The rate of referral to AI varied by region and also by type of specialist, adult versus pediatric GI. The low rate of referral in the rural regions may be partly due to decreased access to AI in rural areas. However, the reason for a low rate of referral in adult compared with pediatric GI is unclear because both adults and children have a high rate (>75%) of concomitant allergies (42,43).
We detected a significant increase in EoE and EG-EC in urban populations (EE-13.4, EG-EC-7.2 patients/year) compared with rural populations (EoE-7.2, EG-EC-5.2) (Table 4). The increase in disease prevalence in urban populations is a common feature of atopic diseases (18,19,44,45).
We also observed an increase in EoE and EG-EC in the northern states compared with the southern states, in particular northeastern and Middle Atlantic states. There are several potential reasons for these geographical differences. The recent finding of north versus south gradient for the use of epinephrine emergency devices suggests a role for vitamin D (23) in allergic diseases. Several other epidemiological studies have also suggested a role for low vitamin D levels in the increase in atopic diseases (17,46,47). An alternative albeit less likely explanation may be regional differences in genetic predisposition (48) (Supplemental Digital Content Figure E2, http://links.lww.com/MPG/A20) or differences in response rates.
In summary, this survey of adult and pediatric GI and AI examined the prevalence, distribution, and practice styles for EoE and EG-EC. The variability in diagnostic and treatment approaches underscores the urgent need for additional clinical trials, consensus development, and education. The epidemiological findings suggest geographical variation in disease prevalence in the United States. Additional studies are needed to confirm these observations and clarify the disease burden within and outside the United States.
Supplementary Material
Acknowledgments
Support for the work came from the American Partnership for Eosinophilic Disorders; K01-AI-73729 for SSS.
The authors acknowledge the assistance of the American Academy of Allergy, Asthma, and Immunology, the American College of Gastroenterology, the North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition, and the International Gastrointestinal Eosinophil Researchers.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
The data were originally presented in abstract form at the NASPGHAN annual meeting in October 2009.
The authors report no conflicts of interest.
References
- 1.Dellon ES, Aderoju A, Woosley JT, et al. Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am J Gastroenterol. 2007;102:2300–13. doi: 10.1111/j.1572-0241.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 2.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–1. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 4.Straumann A. The natural history and complications of eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:99–118. ix. doi: 10.1016/j.giec.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Cherian S, Smith NM, Forbes DA. Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Arch Dis Child. 2006;91:1000–4. doi: 10.1136/adc.2006.100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48:30–6. doi: 10.1097/MPG.0b013e3181788282. [DOI] [PubMed] [Google Scholar]
- 7.Yan BM, Shaffer EA. Eosinophilic esophagitis: a newly established cause of dysphagia. World J Gastroenterol. 2006;12:2328–34. doi: 10.3748/wjg.v12.i15.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villalta D, Baragiotta AM. Eosinophilic esophagitis: from the case report to the evidence. Eur Ann Allergy Clin Immunol. 2008;40:53–60. [PubMed] [Google Scholar]
- 9.van der Spek BW, Klemt-Kropp M. A young man with progressive dysphagia. Eosinophilic esophagitis. Neth J Med. 2009;67:202–3. [PubMed] [Google Scholar]
- 10.Straumann A, Spichtin HP, Bernoulli R, et al. Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings. Schweiz Med Wochenschr. 1994;124:1419–29. [PubMed] [Google Scholar]
- 11.Shitrit AB, Reinus C, Zeides S, et al. Eosinophilic esophagitis. Isr Med Assoc J. 2006;8:587. [PubMed] [Google Scholar]
- 12.Sgouros SN, Bergele C, Mantides A. Eosinophilic esophagitis in adults: a systematic review. Eur J Gastroenterol Hepatol. 2006;18:211–7. doi: 10.1097/00042737-200602000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Remedios M, Campbell C, Jones DM, et al. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006;63:3–12. doi: 10.1016/j.gie.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 14.Furuta K, Adachi K, Kowari K, et al. A Japanese case of eosinophilic esophagitis. J Gastroenterol. 2006;41:706–10. doi: 10.1007/s00535-006-1827-9. [DOI] [PubMed] [Google Scholar]
- 15.Molina-Infante J, Hernandez-Alonso M, Perez-Gallardo B, et al. The first Asian case report of eosinophilic esophagitis in an asymptomatic adult: what about a proton pump inhibitor trial? J Chin Med Assoc. 2009;72:166–7. doi: 10.1016/S1726-4901(09)70046-2. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez SA, Ariel Toro M. Eosinophilic esophagitis in adults: diagnosis and treatment. Acta Gastroenterol Latinoam. 2008;38:298–301. [PubMed] [Google Scholar]
- 17.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Von Ehrenstein OS, Von Mutius E, Illi S, et al. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy. 2000;30:187–93. doi: 10.1046/j.1365-2222.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 19.Majkowska-Wojciechowska B, Pelka J, Korzon L, et al. Prevalence of allergy, patterns of allergic sensitization and allergy risk factors in rural and urban children. Allergy. 2007;62:1044–50. doi: 10.1111/j.1398-9995.2007.01457.x. [DOI] [PubMed] [Google Scholar]
- 20.Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225–32. [PubMed] [Google Scholar]
- 21.Lau S, Illi S, Platts-Mills TA, et al. Longitudinal study on the relationship between cat allergen and endotoxin exposure, sensitization, cat-specific IgG and development of asthma in childhood–report of the German Multicentre Allergy Study (MAS 90) Allergy. 2005;60:766–73. doi: 10.1111/j.1398-9995.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- 22.Milner JD. Exogenous vitamin D might contribute to geographic variations in epinephrine prescriptions. J Allergy Clin Immunol. 2008;121:265–6. doi: 10.1016/j.jaci.2007.08.033. author reply 266. [DOI] [PubMed] [Google Scholar]
- 23.Camargo CA, Jr, Clark S, Kaplan MS, et al. Regional differences in EpiPen prescriptions in the United States: the potential role of vitamin D. J Allergy Clin Immunol. 2007;120:131–6. doi: 10.1016/j.jaci.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 24.Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198–206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 25.Ronkainen J, Talley NJ, Aro P, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut. 2007;56:615–20. doi: 10.1136/gut.2006.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill R, Durst P, Rewalt M, et al. Eosinophilic esophagitis disease in children from West Virginia: a review of the last decade (1995–2004) Am J Gastroenterol. 2007;102:2281–5. doi: 10.1111/j.1572-0241.2007.01352.x. [DOI] [PubMed] [Google Scholar]
- 27.Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol. 2005;115:418–9. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Prasad GA, Smryk TC, CS Secular trends in the epidemiology and outcomes of eosinophilic esophagitis in Olmsted County, Minnesota (1976–2007) Gastroenterology. 2008;34:A289. abstract. [Google Scholar]
- 29.Ronmark EP, Ekerljung L, Lotvall J, Toren K, Ronmark E, Lundback B. Large scale questionnaire survey on respiratory health in Sweden: effects of late- and non-response. Respir Med. 2009;103:1807–15. doi: 10.1016/j.rmed.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan K. E mail survey response rates: a review. JCMC. 2001:6. [Google Scholar]
- 31.Larenas Linnemann D, Rodriguez Perez N, Becerril M. Adverse reactions to skin tests and immunotherapy in the practice of Mexican allergologists. Rev Alerg Mex. 2008;55:62–70. [PubMed] [Google Scholar]
- 32.LaBella CR, Sanders DB, Sullivan C. Athletic trainers’ experience and comfort with evaluation and management of asthma: a pilot study. J Asthma. 2009;46:16–20. doi: 10.1080/02770900802460530. [DOI] [PubMed] [Google Scholar]
- 33.Spergel JM, Andrews T, Brown-Whitehorn TF, et al. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol. 2005;95:336–43. doi: 10.1016/S1081-1206(10)61151-9. [DOI] [PubMed] [Google Scholar]
- 34.Spergel JM, Beausoleil JL, Mascarenhas M, et al. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109:363–8. doi: 10.1067/mai.2002.121458. [DOI] [PubMed] [Google Scholar]
- 35.Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097–102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131:1381–91. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol. 2008;6:165–73. doi: 10.1016/j.cgh.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Teitelbaum JE, Fox VL, Twarog FJ, et al. Eosinophilic esophagitis in children: immunopathological analysis and response to fluticasone propionate. Gastroenterology. 2002;122:1216–25. doi: 10.1053/gast.2002.32998. [DOI] [PubMed] [Google Scholar]
- 39.Aceves SS, Bastian JF, Newbury RO, et al. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102:2271–9. doi: 10.1111/j.1572-0241.2007.01379.x. [DOI] [PubMed] [Google Scholar]
- 40.Aceves SS, Dohil R, Newbury RO, et al. Topical viscous budesonide suspension for treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2005;116:705–6. doi: 10.1016/j.jaci.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 41.King J, Khan S. Eosinophilic Esophagitis: perspectives of adult and pediatric gastroenterologists. Dig Dis Sci. 2009;55:973–82. doi: 10.1007/s10620-009-0801-9. [DOI] [PubMed] [Google Scholar]
- 42.Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2008;6:531–5. doi: 10.1016/j.cgh.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 43.Jyonouchi S, Brown-Whitehorn TA, Spergel JM. Association of eosinophilic gastrointestinal disorders with other atopic disorders. Immunol Allergy Clin North Am. 2009;29:85–97. doi: 10.1016/j.iac.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Aamodt G, Jahnsen J, Bengtson MB, et al. Geographic distribution and ecological studies of inflammatory bowel disease in southeastern Norway in 1990–1993. Inflamm Bowel Dis. 2008;14:984–91. doi: 10.1002/ibd.20417. [DOI] [PubMed] [Google Scholar]
- 45.Eder W, von Mutius E. Hygiene hypothesis and endotoxin: what is the evidence? Curr Opin Allergy Clin Immunol. 2004;4:113–7. doi: 10.1097/00130832-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Oren E, Banerji A, Camargo CA., Jr Vitamin D and atopic disorders in an obese population screened for vitamin D deficiency. J Allergy Clin Immunol. 2008;121:533–4. doi: 10.1016/j.jaci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Weiss ST, Litonjua AA. Maternal diet vs lack of exposure to sunlight as the cause of the epidemic of asthma, allergies and other autoimmune diseases. Thorax. 2007;62:746–8. doi: 10.1136/thx.2007.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ethnic distribution of top reported ancestories by county in the United States based on Census 2000. Wikipedia; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.