Abstract
Background
Studies have examined the development of asthma in children with atopic dermatitis (AD); however, none have looked at the association of asthma or the frequency of wheeze with respect to persistence or difficulty in achieving AD clinical improvement in children.
Objective
To determine whether children with AD who have asthma and increasing frequency of wheezing have more persistent AD.
Methods
This is a cohort study using the Pediatric Eczema Elective Registry (PEER) database, which includes data obtained at enrollment and 3 years later. The AD outcome was the persistence of skin symptoms. Our covariates of interest were asthma diagnosis and wheezing symptoms, which were measured at enrollment and again at year 3 of the study. Multivariate logistic regression models assessed the magnitude of associations among AD symptoms, asthma diagnosis, and the frequency of wheeze. All models were adjusted for sex, age, and ethnicity.
Results
A total of 2104 children were enrolled in the PEER study and had at least 3 years of follow-up at the time of this study. At enrollment, an asthma diagnosis decreased the likelihood of being rash free in the preceding 6 months by 30% (odds ratio, 0.70; 95% confidence interval, 0.59–0.84). At year 3, having asthma decreased the likelihood by 40% (odds ratio, 0.60; 95% confidence interval, 0.49–0.72). Increasing frequency of wheezing also decreased the likelihood that a child was rash free (P < .001).
Conclusion
For children with AD, a history of asthma and an increasing frequency of wheezing correlate strongly with more persistent AD.
Introduction
Atopic dermatitis (AD) is a common chronic relapsing inflammatory skin condition that affects approximately 5% to 20% of children worldwide.1,2 By 3 years of age, many children with AD will have at least one additional form of atopy, such as seasonal allergies, asthma, or food allergies.3 Furthermore, atopic dermatitis is a known risk factor for asthma. Asthma is a chronic inflammatory disorder of the airways, which affects all age groups but most often starts during childhood.4 Studies on the risk of developing asthma indicate that it will develop in approximately 30% of those with AD.5
Historically, studies in the field of AD focused on factors that are associated with its incidence and genetic risk factors.6 In 2010, Peters et al7 evaluated risk factors for the incidence and persistence of AD in an adolescent population. The study found that baseline asthma symptoms were associated with both the incidence and persistence of AD. As noted by Peters et al, adolescent AD and the more common childhood AD may not have the same natural history. As noted by several authors, classic childhood AD, adolescent-onset AD, and adult-onset AD may represent variations of this illness in that cutaneous manifestations, associated atopic illnesses, and prognosis varies by age of onset.8,9
To our knowledge, there have been no studies of children with AD that examine the association between asthma symptoms with respect to the persistence of AD or to the difficulty in achieving clinical improvement of AD. The goals of the study were 2-fold: to determine whether individuals with AD and a diagnosis of asthma have more persistent skin disease and to determine whether the persistence of AD symptoms in those with a diagnosis of asthma are associated with an increasing frequency of wheezing.
Methods
Study Design
This was a secondary analysis of data from an observational cohort study using the Pediatric Eczema Elective Registry (PEER) (www.thepeerprogram.com).10 PEER is part of a manufacturer’s postmarketing commitment to the Food and Drug Administration, as well as the European Drug Agency, to evaluate the long-term safety of any exposure to pimecrolimus cream, 1%. Enrollment was based on a physician-confirmed diagnosis of AD and the use of pimecrolimus cream, 1%, for 6 weeks within 6 months before study entry. The enrolling physicians included dermatologists, pediatric dermatologists, pediatricians, allergists, family practitioners, and other treating physicians. All study participants were children 2 to 18 years of age. Participants were recruited from all regions of the United States.
Information was collected through biannual questionnaires, usually completed by a parent of the enrolled child. We mailed questionnaires to all survey participants who consented to study participation. The questions focused on the activity of the child’s AD and AD medication use. Specifically, the questionnaires captured information on the need to use any topical AD medications, including corticosteroids or calcineurin inhibitors. Many PEER survey questions were modeled after the International Study of Asthma and Allergies in Childhood (ISAAC) questions. Further information on ISAAC can be found online (http://isaac.auckland.ac.nz).
At enrollment and year 3 of participation, the PEER questionnaire captured additional information related to asthma. This information included questions on the diagnosis of asthma and the frequency of wheezing. The questionnaire did not capture information on asthma medication use. To be eligible for our study, PEER participants must have completed both the enrollment and the year 3 questionnaire to gather information on asthma at 2 time points. The study was approved through our institutional review board. All participants provided written consent for enrollment.
Outcomes
The primary outcome of interest was the self-reported persistence of AD symptoms based on mailed queries every 6 months. We defined persistence as the report of a rash during the previous 6 months. We also asked participants to report any use of topical medications (calcineurin inhibitors or corticosteroids).
Covariates
The primary covariate of interest was asthma at baseline, which was based on self-report of a diagnosis of asthma and report on frequency of wheezing. Frequency of wheezing was defined by the participants’ report of the number of episodes of wheezing in the previous 6 months.
Other Covariates
We measured age at enrollment, ethnicity/race, and sex.
Statistical Analyses
Variables were described using percentages or means with SDs. Logistic regression models with both unadjusted single independent variables and adjusted multiple variables were used to ascertain the magnitude of associations between AD participants’ symptoms and related variables. The independent variables were history of asthma diagnosis, the presence of wheezing, and the frequency of wheezing. Multivariate models were adjusted for sex, age, and ethnicity.
Because the data were collected longitudinally and a child could be rash free at any 6-month reporting interval, generalized estimating equations with a logit link function were used. Generalized estimating equations are generalized linear models that account for the within-patient correlation of responses found in longitudinal panel data. The models were expressed as odds ratios (ORs) with 95% confidence interval (CIs). All statistical analyses were conducted using software (STATA, version 12.1; StataCorp, College Station, Texas).
Results
At the time of this study, 2104 children were enrolled in PEER, and all were eligible to complete the year 3 follow-up questionnaire. The year 3 follow-up questionnaire was completed by 2052 of the 2104 participants (97.5%). Table 1 lists the basic demographic information of the entire cohort at enrollment. It also includes data on the subgroup of participants with asthma at enrollment and at the year 3 follow-up. In the overall population at enrollment, 979 participants (46.5%) were male and 1027 (48.8%) were African American. Most of those participants who reported a diagnosis of asthma were male (enrollment, 51.9%; follow-up, 52.3%), African American (enrollment, 52%; follow-up, 53.3%) and between the ages of 5 and 11 years (enrollment, 49.6%; follow-up, 70%). Asthma was only measured at enrollment and at year 3. At enrollment, the mean (SD) age was 7.0 (4.1) years. The median age was 5.9 years, and the interquartile range was 3.8 to 9.7 years. The mean (SD) age at AD onset was 2.1 (3.0) years. The median age was 0.75 years, and the interquartile range was 0.25 to 2 years.
Table 1.
Demographic characteristics of the full study population and asthmatic patients at the time of enrollment and at year 3 follow-up
| Characteristic | No. (%) of participants
|
||
|---|---|---|---|
| Full population at enrollment (n = 2,104) | Asthma at enrollment (n = 1,041) | Asthma at year 3 follow-up (n = 934a) | |
| Sexb | |||
| Male | 979 (46.5) | 540 (51.9) | 488 (52.2) |
| Female | 1,124 (53.4) | 500 (48.0) | 445 (47.7) |
| Ethnicityc | |||
| White | 955 (45.4) | 465 (44.7) | 394 (42.2) |
| African American | 1,027 (48.8) | 541 (52.0) | 493 (52.8) |
| Asian | 88 (4.2) | 24 (2.3) | 27 (2.9) |
| Hawaiian or American Indian or Alaskan Native | 41 (2.0) | 25 (2.4) | 24 (2.6) |
| Hispanic | 173 (8.2) | 76 (7.3) | 75 (8.0) |
| Age, y | |||
| Enrollment age | |||
| 0–4 | 795 (37.8) | 380 (36.5) | 0 |
| 5–11 | 993 (50.7) | 516 (49.6) | 733 (78.5) |
| ≥12 | 316 (17.5) | 145 (13.9) | 308 (33.0) |
A total of 107 participants who completed the questionnaire at enrollment completed some part of the 3-year survey but failed to complete the asthma questions.
One individual did not respond.
Some selected multiple categories.
In participants with AD, those who had asthma on enrollment were very likely to report the diagnosis 3 years later. The agreement of the self-reported asthma diagnosis at enrollment and the year 3 follow-up was substantial (κ = 0.789). However, the frequency of wheezing progressed over time. At enrollment, 794 of the 1041 participants (76.3%) with a diagnosis of asthma noted one or more episodes of wheezing in the previous 6 months. Three years later, 828 participants (88.7%) with a diagnosis of asthma noted one or more episodes of wheezing in the previous 6 months. The categorization of wheezing episodes at both time points is listed in Table 2.
Table 2.
Frequency of wheezing during the previous 6 months for participants with a diagnosis of asthma at enrollment and an asthma diagnosis at year 3 of follow-up
| No. of wheezing episodes | No. (%) of participants
|
|
|---|---|---|
| Asthma at enrollment (n = 1,041) | Asthma at year 3 follow-up (n = 934) | |
| ≤3 | 577 (55.4) | 540 (57.8) |
| 4–12 | 179 (17.2) | 226 (24.2) |
| >12 | 38 (3.7) | 62 (6.6) |
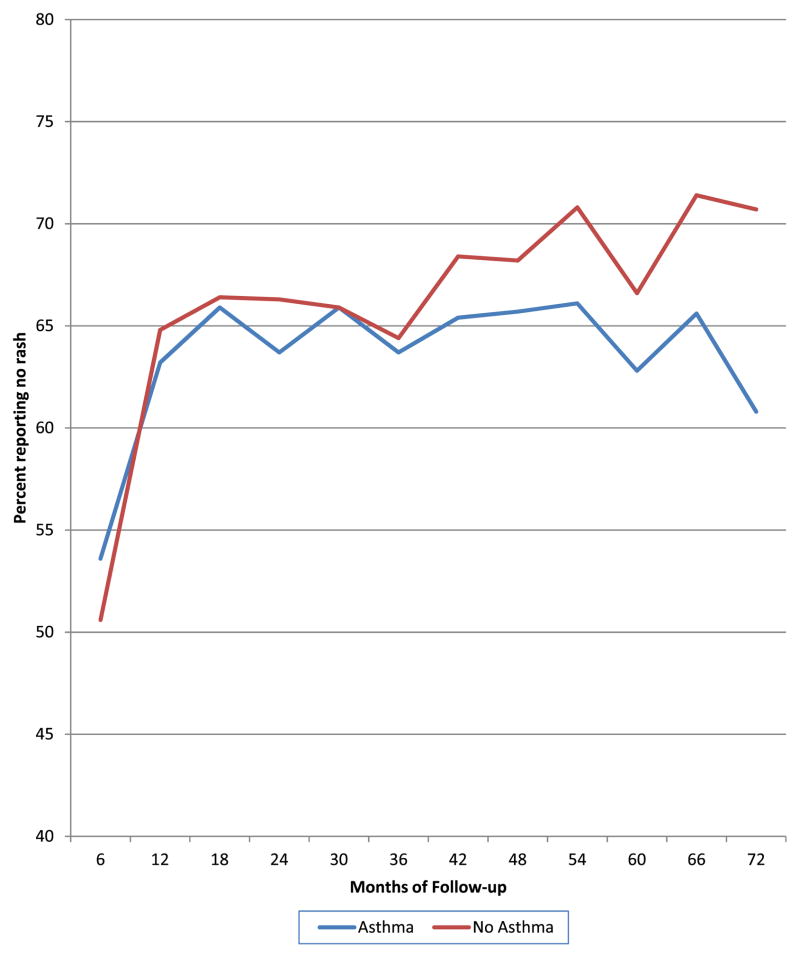
The effect of asthma on AD was substantial. At enrollment, those with a diagnosis of asthma were 30% less likely to be rash free (OR, 0.70; 95% CI, 0.59–0.84; P < .001) compared with those without a history of asthma and wheeze. In fact, at any point during the study having a diagnosis of asthma at enrollment decreased the likelihood of being rash free at any 6-month interval by 40% (OR, 0.60; 95% CI, 0.49–0.72; P <.001) compared with those who did not note a history of asthma on enrollment (Fig 1).
Figure 1.
Percentage of rash-free participants with asthma and without asthma for the first 6 years.
At enrollment, there was an ordinal doselike response relationship between the frequency of wheezing and being rash free (P < .001, test for trend). Compared with those without asthma, those with asthma and 4 to 12 episodes of wheezing were 33% less likely to be rash free (OR, 0.77; 95% CI, 0.72–0.83; P < .001). Those with more than 12 episodes of wheezing were 43% less likely to be rash free (OR, 0.57; 95% CI, 0.49–0.66; P <.001) at any time point in during our study. This effect was not confounded by race (Table 3).
Table 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) comparing episodes of wheezing and being rash free at enrollment for all participants
| No. of wheezing episodes | Full population OR (95% CI) of being rash free |
|---|---|
| No asthma | Reference |
| ≤3 | 0.99 (0.95–1.03) |
| 4–12 | 0.77 (0.72–0.83)a |
| >12 | 0.57 (0.49–0.66)a |
P < .001.
To confirm our findings, we evaluated the likelihood of a participant being rash free without requiring topical AD medications. This serves as a proxy for better AD control. Compared with those without asthma, those with asthma and 3 or fewer episodes of wheezing were 52% less likely to be rash and medication free (OR, 0.48; 95% CI, 0.35–0.67; P < .001). In addition, those with 4 to 12 episodes of wheezing were 50% less likely to be rash and medication free (OR, 0.50; 95% CI, 0.32–0.79; P <.003), and those with more than 12 episodes of wheezing were 79% (OR, 0.21; 95% CI, 0.12–0.35; P < .001) less likely to be rash and medication free (Table 4).
Table 4.
Odds ratios (ORs) and 95% confidence intervals (CIs) comparing episodes of wheezing and absence of atopic dermatitis acute skin symptoms without topical medication use at enrollment and throughout the entire study period for all participants
| No. of wheezing episodes | OR (95% CI)a
|
|
|---|---|---|
| Enrollment | Entire study | |
| No asthma | Reference | Reference |
| ≤3 | 0.48 (0.35–0.67) | 0.69 (0.59–0.81) |
| 4–12 | 0.50 (0.32–0.79) | 0.45 (0.35–0.59) |
| >12 | 0.21 (0.12–0.35) | 0.30 (0.20–0.46) |
P < .001 for all comparisons.
We then evaluated the likelihood of a participant being rash free without requiring topical AD medications throughout the entire study period. Compared with those without asthma, those with asthma and 3 or fewer episodes of wheezing were 31% less likely to be rash and medication free (OR, 0.69; 95% CI, 0.59–0.81; P <.001). Those with asthma and 4 to 12 episodes of wheezing were 55% less likely to be rash and medication free (OR, 0.45; 95% CI, 0.35–0.59; P < .001), and those with asthma and more than 12 episodes of wheezing were 70% less likely to be rash and medication free (OR, 0.30; 95% CI, 0.20–0.46; P <.001) compared with those who did not wheeze (Table 4). In addition, if we limited follow-up to time observed after year 3 and used the report of asthma and wheezing frequency per the year 3 survey, similar effects on the persistence of atopic dermatitis were noted.
Discussion
This study found an association between a history of asthma and the frequency of wheeze with the persistence of rash in children with AD. Our data suggest that the presence of asthma correlates with poorer AD disease control (ie, more likely to have persistent rash). In addition, the frequency of wheeze in asthmatic patients also correlates with the persistence of rash. Our study found that in those with AD, a child with a diagnosis of asthma is 30% less likely to be rash free in any preceding 6-month period compared with those without a history of asthma.
Furthermore, in a dose-dependent fashion, in these asthmatic children, those with more frequent wheezing were less likely to have control of their skin disease. Specifically, as the frequency of wheezing increased, patients were 33% to 43% less likely to be rash free. This clinical finding has not been previously reported in this age group as it has been in the adolescent age group. We also found a similar association between increasing frequency of wheeze and the persistence of cutaneous symptoms requiring use of topical AD medications. Those participants who wheezed were 50% to 79% less likely to be rash free and medication free than those who did not wheeze. This association was found throughout the entire study.
We also found that a diagnosis of asthma in our AD population seemed to be fixed when made at a young age. At enrollment, the mean age at AD diagnosis in our participants was 2.1 years of age. We found that the same children were very likely to still have active asthma and actually more frequent wheezing 3 years later. In other words, in the setting of AD, it is highly likely that the diagnosis of asthma or the presence of wheezing present in the preschool population will persist into school age. This finding is supported by other studies that have reported a greater prevalence of early wheeze in those with early-onset AD.11
Our finding that increasing frequency of wheeze is associated with persistent symptoms in children expands on the findings of Peters et al,7 who reported that asthma symptoms at baseline were a predictor of AD disease course in adolescence. They found that the persistence of AD was 1.48 times (OR, 1.48; 95% CI, 0.70–3.12) more likely in those participants with wheezing or chest symptoms 12 months before the study. Our study differs from the study by Peters et al in that they focused on an adolescent population and measured persistence through surveys administered at 2 time points, 5 years apart only, whereas ours focused on school-aged children. We measured persistence through surveys administered every 6 months for a minimum of 3 years.
Our findings add to the current literature on the natural history of atopic dermatitis and can be used to counsel patients. On the basis of our findings, those with asthma and frequent wheezing will be more likely to have a rash over time and be more likely to require the use of topical medications to alleviate their rash. Furthermore, other studies have used a single 6-month period of the absence of rash as a marker that a child no longer has AD.12 The natural history of AD makes this unlikely. On the basis of our study, the likelihood that a child will no longer have AD is diminished if he/she has a concurrent diagnosis of asthma or more frequent wheezing. This information may be helpful to the clinician in designing a treatment plan for the patient. This study is an epidemiologic study and has certain limitations, including the potential for both recall and selection bias. The former exists because the participants provided information on symptoms during a period of 6 months. It is possible that parents of children with asthma may be more likely to report more skin problems because they may be more mindful of additional symptoms to be reported because of the need to follow up with a physician more often. In addition, a physician diagnosis of AD was required for participation in the PEER study, so it would seem that all parents in our study were likely to engage and report health findings. However, we found a dose response effect related to the frequency of wheeze, and this linear effect would not have been known to the parents or health care professionals. In addition, the population studied represents all regions of the United States and all health care professionals who commonly treat patients with AD.
Our study evaluated the persistence of AD symptoms over time. Since we relied on self-report of AD symptoms and not physician reports, we do not know the severity and extent of these symptoms, as determined by severity scoring systems such as the eczema area and severity score system. However, our questionnaire is modeled on ISAAC-based questionnaires commonly used in clinical research, and because these other studies are the basis for current recommendations, we believe our questionnaire is a validated instrument.
We did not capture information on asthma medication use; hence, we cannot discuss how asthma severity and control affects the control of AD. Because other previously published ISAAC-based studies use a similar template, we believe that this does not lessen the impact of our findings.
In summary, our study suggests that those children with a diagnosis of asthma and more frequent wheezing will have more persistent AD. Future studies are needed in this area. On the basis of our results, it is clinically relevant to consider that those with AD and asthma and more frequent wheezing may have disease that is more persistent and difficult to control using currently available therapies.
Acknowledgments
Funding Sources: This study was funded by National Institutes of Health research grant R01AR056755 and grant funding for PEER from Valeant Pharmaceuticals (Dr Margolis) and National Institute of Health Research grant supplement 5R01AR056755-04 and an American Academy of Allergy, Asthma and Immunology Fellowship of Excellence training grant (Dr Garrett).
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Spergel JM. Epidemiology of atopic dermatitis and atopic march in children. Immunol Allergy Clin North Am. 2010;30:269–280. doi: 10.1016/j.iac.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103:125–138. doi: 10.1016/s0091-6749(99)70536-1. [DOI] [PubMed] [Google Scholar]
- 3.Kapoor R, Menon C, Hoffstad O, Bilker W, Leclerc P, Margolis DJ. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol. 2008;58:68–73. doi: 10.1016/j.jaad.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen SE, Hurd SS, Lemanske RF, Jr, et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46:1–17. doi: 10.1002/ppul.21321. [DOI] [PubMed] [Google Scholar]
- 5.van der Hulst AE, Klip H, Brand PL. Risk of developing asthma in young children with atopic eczema: a systematic review. J Allergy Clin Immunol. 2007;120:565–569. doi: 10.1016/j.jaci.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 6.Girolomoni G, Abeni D, Masini C, et al. The epidemiology of atopic dermatitis in Italian schoolchildren. Allergy. 2003;58:420–425. doi: 10.1034/j.1398-9995.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 7.Peters AS, Kellberger J, Vogelberg C, et al. Prediction of the incidence, recurrence, and persistence of atopic dermatitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. 2010;126:590–595. e1–e3. doi: 10.1016/j.jaci.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 9.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912–917. doi: 10.1016/j.jaci.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Illi S, von Mutius E, Lau S, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004;113:925–931. doi: 10.1016/j.jaci.2004.01.778. [DOI] [PubMed] [Google Scholar]
- 12.Virtanen H, Remitz A, Malmberg P, et al. Topical tacrolimus in the treatment of atopic dermatitis: does it benefit the airways? a 4-year open follow-up. J Allergy Clin Immunol. 2007;120:1464–1466. doi: 10.1016/j.jaci.2007.08.021. [DOI] [PubMed] [Google Scholar]