Abstract
The 22q11.2 deletion syndrome (22q11DS) presents with medical and neuropsychiatric manifestations including neurocognitive deficits. Quantitative neurobehavioral measures linked to brain circuitry can help elucidate genetic mechanisms contributing to deficits. To establish the neurocognitive profile and neurocognitive “growth charts”, we compared cross-sectionally 137 individuals with 22q11DS ages 8–21 to 439 demographically matched non-deleted individuals with developmental delay (DD) and medical comorbidities and 443 typically developing (TD) participants. We administered a computerized neurocognitive battery that measures performance accuracy and speed in executive, episodic memory, complex cognition, social cognition and sensorimotor domains. The accuracy performance profile of 22q11DS showed greater impairment than DD, who were impaired relative to TD. Deficits in 22q11DS were most pronounced for face memory and social cognition, followed by complex cognition. Performance speed was similar for 22q11DS and DD, but 22q11DS individuals were differentially slower in face memory and emotion identification. The growth chart, comparing neurocognitive age based on performance relative to chronological age, indicated that 22q11DS participants lagged behind both groups from the earliest age assessed. The lag ranged from less than a year to over three years depending on chronological age and neurocognitive domain. The greatest developmental lag across the age range was for social cognition and complex cognition, with the smallest for episodic memory and sensorimotor speed, where lags were similar to DD. The results suggest that 22q11.2 microdeletion confers specific vulnerability that may underlie brain circuitry associated with deficits in several neuropsychiatric disorders and thereby help identify potential targets and developmental epochs optimal for intervention.
Keywords: 22q11.2 Deletion Syndrome, developmental delay, neurocognition
INTRODUCTION
The 22q11.2 deletion syndrome (22q11DS) is characterized by heterogeneous medical and neuropsychiatric presentations.1–8 Neuropsychiatric features consist of developmental delay with mild to moderate intellectual disability and multiple psychiatric disorders, including anxiety, attention deficit hyperactivity and autism spectrum in childhood, with depression and schizophrenia emerging in adolescence and early adulthood.2–8 While the frequency of these disorders in 22q11DS is relatively high, the developmental patterns and psychiatric phenotypes are similar to manifestations of major psychiatric disorders in the general population. Therefore, the 22q11.2 genetic variation may provide a unique window for elucidating mechanisms of developmental neuropsychiatric disorders.9–11 Indeed, rare copy number variants (CNVs) like 22q11.2, have been associated with several psychiatric disorders where the diagnosis is based on clinical symptom phenotypes.2–8 Quantitative neurobehavioral measures that are linked to brain circuitry can be useful in evaluating underlying genetic mechanisms of behavioral domains dimensionally, across psychiatric disorders, and thereby advance translational research with animal models.9–13 Thus, 22q11DS provides an inimitable opportunity for dissecting associated neurobehavioral deficits in a way that could eventually lead to a mechanistic account of psychiatric phenomenology.
Reduced intellectual abilities, nonverbal greater than verbal, have been observed in individuals with 22q11DS.9,10,14–16 Neuropsychological reports indicate impaired executive functions, attention, working memory, verbal and nonverbal memory, visuospatial processing and visuo-motor functioning.17–24 Notably, most studies examined relatively small samples and did not include age matched comparison groups. Furthermore, investigations have largely focused on children and on a limited number of cognitive domains. Neuropsychological measures utilize a healthy comparison group to gauge performance and demographic variables, such as age and sex, are considered. Given the phenotypic complexity of 22q11DS, the choice of an appropriate comparison group is important when examining neurocognitive functioning. To date, there have been no studies comparing performance of individuals with 22q11DS, commonly associated with developmental delay and medical comorbidities, to non-deleted youths with developmental delay, medical comorbidities, and no known genetic disorder. Such a comparison is needed to identify neurobehavioral features that can be attributable to the deletion rather than to nonspecific effects of developmental delay or medical sequelae.
To provide quantitative phenotypic measures that can be linked to brain function, we have previously developed a computerized neurocognitive battery (CNB) that consists of tests validated with functional neuroimaging25, 26 and applied in large-scale genetic studies of schizophrenia.27–32 The battery measures accuracy and speed of performance in several domains including executive, episodic memory, complex cognition, social cognition, and sensorimotor speed. The CNB was used, concomitant with a comprehensive medical and psychiatric assessment, in a large population-based sample of genotyped youths age 8–21 years old, who participated in the Philadelphia Neurodevelopmental Cohort. We documented age and sex effects on performance in a subsample of 3,500, establishing the sensitivity and validity of the CNB in a developmental cohort.33
We earlier reported results from a small sample of twenty-one 22q11DS patients assessed for psychotic features. They were compared to non-deleted participants in four groups, varying along the psychosis dimension: low risk, genetic risk, clinical risk and schizophrenia.34 Individuals with 22q11DS were significantly less accurate in nearly all domains, but had similar speed of response compared to the other groups. Their profile resembled that of the psychosis groups in accuracy, except for more pronounced deficits in face memory and social cognition.
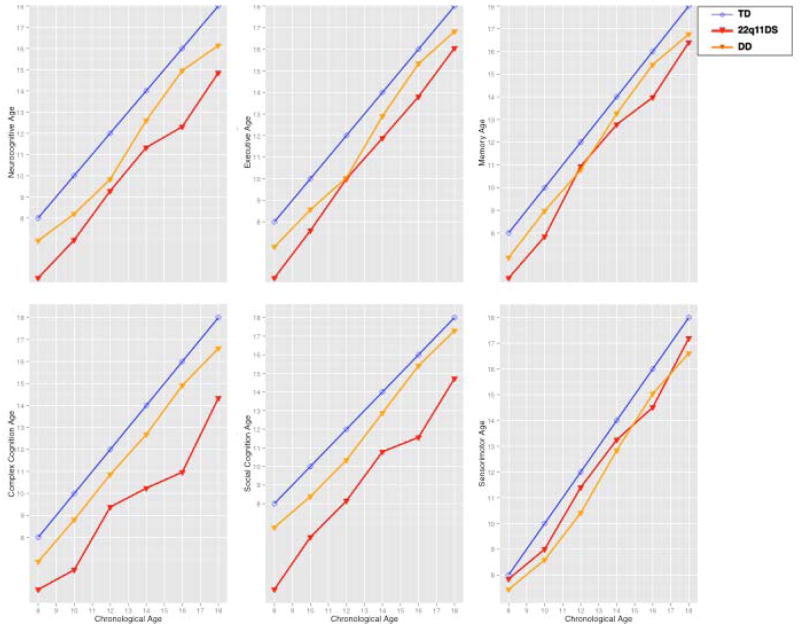
In addition to documenting the profile of deficits, understanding the neurocognitive effects of the 22q11.2 deletion requires elucidation of these deficits in a developmental context. Creating a “growth chart” of cognitive development in 22q11DS relative to non-deleted healthy individuals, as well as individuals with developmental delay and medical comorbidities can help detect lags that relate specifically to the microdeletion. To generate growth charts for integrating brain-behavior phenotypes in a developmental context, “neurocognitive age” indices are calculated reflecting data driven predicted age based on performance. When the neurocognitive age lags behind chronological age, there is a delay or below age-expected performance; conversely, when the neurocognitive age surpasses chronological age there is accelerated or above age-expected performance. We recently reported that non-deleted youths endorsing psychosis spectrum symptoms showed developmental lags compared to healthy participants.35 Determining the neurocognitive age of individuals with 22q11DS can provide efficient tools for staging and intervention as well as integration with other parameters of brain development that are used to determine “brain age”.36,37 The goal of this study is to examine the neurocognitive profile and neurocognitive age in 22q11DS relative to youths with developmental delay and medical comorbidities as well as typically developing participants. We hypothesized that youths with 22q11DS are impaired across neurocognitive domains and have lower neurocognitive age relative to chronological age than the other groups. From our original exploratory analysis,34 we expected that the deficit associated with 22q11DS is larger for performance accuracy than speed and is largest for face memory and social cognition. We also examined whether the developmental lag is comparable across domains or is greater for specific domains.
PATIENTS AND METHODS
Sample
The cross-sectional sample included three groups aged 8–21 years that were balanced demographically (Table 1). The 22q11DS sample was recruited as part of a collaborative RO1 between the University of Pennsylvania (Penn) and Children’s Hospital of Philadelphia (CHOP). The two comparison groups of genotyped youths were evaluated contemporaneously by the Penn team as part of the Philadelphia Neurodevelopmental Cohort (PNC). The PNC was a Grand Opportunity collaborative project between the Brain Behavior Laboratory at Penn and the Center for Applied Genomics at CHOP in which genotyped children were phenotyped as detailed below. The comparison groups were selected from the larger PNC sample of 9,500 participants to match the 22q11DS participants for age, sex and ethnicity.33,38 The PNC sample was recruited from the CHOP pediatric network that did not include psychiatric services.
Table 1.
Sample characteristics
| 22Q | DD | TD | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | 137 | 78M; 59F | 439 | 243M; 196F | 443 | 243M; 200F |
|
| ||||||
| Mean | SD | Mean | SD | Mean | SD | |
|
| ||||||
| Age | 12.1 | 3.5 | 12.2 | 3.6 | 12.2 | 3.6 |
|
| ||||||
| Parental education | 14.6 | 2.2 | 14.8 | 2.2 | 15.1 | 2.3 |
|
| ||||||
| Age bin | 8–10 | 40 | 130 | 128 | ||
|
|
||||||
| 11–12 | 17 | 55 | 54 | |||
| 13–14 | 26 | 76 | 82 | |||
| 15–16 | 17 | 56 | 53 | |||
| 17–18 | 22 | 69 | 71 | |||
| >18 | 15 | 53 | 55 | |||
Abbreviations: 22q11DS = 22q11.2 Deletion Syndrome; DD = Developmental Delay; TD = Typically Developing; M =Male; F = Female; CA = Caucasian; AA = African American; OT = Other
1) 22q11.2 Deletion Syndrome (22q11DS)
137 individuals were recruited through the “22q and You Center” at CHOP and social networks. They had a confirmed deletion of the 22q11.2 region. Three megabase deletions were identified in 124 participants and smaller, nested 1.5–1.7MB deletions were seen in 13 participants [7 A–B: counting non-segmental duplication sequence in Build (GRCh37/hg19) chr22:18,893,541–20,312,01; 4 A–C: sequence of LCR-A or LCR-C in Build chr22:18,893,541–21,045,692; 1 B–D sequence of the start and end LCR in Build chr22: 20,704,868–21,418,457;1 C-D, chr22:21,061,979 - 21,418,457]. Twenty-one participants with 22q11DS were included in a previously published pilot study.34
2) Developmental delay and medical comorbidities (DD)
A sample of 439 non-deleted individuals who presented to CHOP were part of the PNC and had developmental delay and co-morbid medical conditions with no known chrosomal anomalies (e.g., Down’s, Williams Beuren, Turner, Angelman syndromes). This sample was selected by matching each 22q11DS participant with 3 DD participants equivalent for sex, race and age using an optimal matching algorithm written in SAS. Developmental delays included failure to achieve age-appropriate developmental milestones in motor skills, language and speech and cognitive functioning. These participants also had significant medical conditions. Information from electronic medical records (EMR) and parent (ages 8–17) or respondent (ages 18–21) was obtained on 42 conditions that were classified into 14 organ systems/specialties. For conditions with insufficient diagnostic information, manual review of EMR ICD 9 codes was completed by qualified medical staff. Discrepant information occurred in about 5% of the cases was reconciled by physician review. An index of the overall severity of medical conditions was created with the following levels: 1 – None, no ongoing medical conditions requiring sustained intervention or which interfere with overall functioning; 2 – Mild, conditions requiring pediatric visits and occasional medication but mild in severity; 3 – Multiple medical conditions requiring standing medications and monitoring; 4 – Severe medical conditions requiring multiple procedures and monitoring that can be life threatening. To balance medical comorbidities associated with 22q11DS, the DD group included individuals with severity ratings of 3–4. Organ systems affected were similar to those impacted by 22q11DS (cardiac, endocrine, musculoskeletal, immunologic, CNS).
3) Typically developing (TD)
443 healthy youths with no developmental, medical disorders (rating of 1) and no psychiatric disorders, who were part of the PNC were selected from the TD participants by matching each of the 22q11DS patients with 3 TD participants for sex, race and age using the optimal matching SAS algorithm.
Exclusion criteria across the three groups included: 1. Unable to provide signed informed consent. For participants under age 18 assent and parental consent were required. 2. Lack of English proficiency. 3. Physically and cognitively unable to participate in an interview and computerized neurocognitive testing. Thus, individuals with moderate to severe intellectual disability, based on clinical evaluation and IQ testing when available (estimated IQ<70) were excluded. Patients from the 22q11.2 DS group with mild hearing impairment were not excluded. Notably, all the tests have visual instructions that were read out loud. Study procedures were conducted while the participants were medically stable and ambulatory. No changes were made in the participants’ usual medical and behavioral treatment. All procedures were approved by the Institutional Review Boards of the University of Pennsylvania and the Children’s Hospital of Philadelphia. Informed consent/assent was obtained from each participant and accompanying parent.
Procedures
The clinical assessment included a computerized adaptation of the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS)39 and incorporated a timeline of life events, demographics, medical history, and psychopathology evaluation. Following the clinical assessment the computerized neurocognitive evaluation was conducted. The 1-hour CNB includes 14 tests assessing 5 neurobehavioral domains: Executive (Abstraction & Mental Flexibility, Attention, Working Memory), Episodic Memory (Words, Faces, Shapes), Complex Cognition (Verbal Reasoning, Non-Verbal Reasoning, Spatial Processing), Social Cognition (Emotion Identification, Emotion Intensity Differentiation, Age Differentiation), and Sensorimotor Speed (Motor, Sensorimotor).25,33 Except for the tests designed exclusively for measuring speed, each test provides measures of both accuracy and speed. The Reading sub-test of the Wide Range Achievement Test -Fourth Edition (WRAT4),40 was administered prior to the CNB in order to determine participants’ ability to complete the battery and to provide a performance-based estimate of IQ.
Data Analysis
Raw CNB scores were standardized (z-transformed) as previously detailed.33 For consistency of interpretation, higher z-scores always reflect better performance; z-scores where higher numbers reflected poorer performance (i.e. response time) were multiplied by −1. Thus, an individual scoring one standard deviation above the mean would have a score of +1, while an individual scoring one standard deviation below the mean would have a score of −1. These z-scores were available for accuracy and speed on 12 tests and only for speed on 2, yielding a total of 26 performance measures. These z-scores were used as dependent measures in a MANCOVA (SAS PROC GLM), where sex and diagnosis (22q11DS, DD, TD) served as between-group factors, domain as a repeated measures (within) factor, and parental education (average of mother’s and father’s education in years) and age as covariates. To examine whether overall group effects existed for each diagnostic pairing we repeated the MANCOVA contrasting TD with 22q11DS, TD with DD and 22q11DS with DD. The analyses were repeated adding standardized WRAT scores as covariates.
To develop the neurocognitive “growth chart”, we performed a regression analysis with 10-fold cross validation (SAS PROC GLMSELECT), entering age in years: (date of evaluation – date of birth)/365.25 as a dependent measure to be predicted from the 26 performance measures. The regression procedure adds variables to the model until the additional variables do not contribute significantly to the predicted variance (R-squared) in age. Variables selected by the linear model were submitted to further examination of non-linear components using a general additive model (SAS PROC GAM). Variables with significant non-linear trends were entered into the linear model to evaluate whether their squared values added to the ability to predict age. These models were fit separately for males and females because of the well-established sex differences in neurodevelopmental trajectory, which were also evident in the present sample.33 These procedures were applied for the entire set of scores and then separately for each domain (Executive, Episodic memory, Complex cognition, Social cognition and Sensorimotor speed) entering all the scores from that domain. Regressions were run separately for males and females and weights were based on the TD sample. As an outcome of these procedures we calculated the predicted “Neurocognitive Age” across domains and for each domain separately. As the regression line was not as steep as the identity line, the predicted age was adjusted to that of the average for the TD group to facilitate interpretability.
The sample size afforded grouping by six age bins as follows: 8–10, 11–12, 13–14, 15–16, 17–18 and >18 (Table 1). Age and diagnosis (TD, 22q11DS, DD) effects were evaluated as between group factors in a MANCOVA with parental education as a covariate (SAS PROC GLM). The analysis on the overall cognitive age was followed by an analysis adding domain as a repeated measures (within) factor. To examine whether overall group effects existed for each diagnosis pairing we repeated the MANCOVA contrasting TD with 22q11DS, TD with DD and 22q11DS with DD.
RESULTS
The diagnosis x sex x domain MANCOVAs on the accuracy and speed z-scores revealed highly significant diagnosis main effects and diagnosis x domain interactions for all 3-groups and 2-group contrasts, except for an absence of a diagnosis x domain interaction in the contrast between DD and TD (Table 2). These diagnosis effects and interactions were significant after controlling for the significant effects of the covariates (parental education and age), and showed that 22q11DS is associated with greater impairment in accuracy compared to DD, and the domain profile differed between the groups (Figure 1). Specifically, the largest effect sizes for accuracy in the 22q11DS group, exceeding 1.5 SDs, were for face memory, language, nonverbal reasoning, and social cognition. With respect to speed, face memory and emotion identification were differentially slow for the 22q11DS group. Notably, 22q11DS patients were significantly faster for non-verbal reasoning, in which they performed inaccurately. This likely reflects lack of effort and impulsive responding on the challenging items. While sex x domain interactions were significant, sex did not interact with diagnosis on any of the analyses. The MANCOVA adding WRAT4 scores as a covariate did not change the significant findings.
Table 2.
Comparison of diagnostic groups in performance accuracy and speed across neurocognitive domains
| TD vs 22q11DS vs DD | TD vs 22q11DS | TD vs DD | DD vs 22q11DS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy | F | DF | P | F | DF | P | F | DF | P | F | DF | P | |
| Between Covariates |
Diag | 205.36 | 2,949 | <.0001 | 662.32 | 1,575 | <.0001 | 105.98 | 1,862 | <.0001 | 144.68 | 1,512 | <.0001 |
| Sex | 2.48 | 1,949 | 0.1156 | 0.48 | 1,575 | 0.4893 | 4.15 | 1,862 | 0.042 | 2.53 | 1,512 | 0.1126 | |
| Age | 480.48 | 1,949 | <.0001 | 283.53 | 1,575 | <.0001 | 463.63 | 1,862 | <.0001 | 217.45 | 1,512 | <.0001 | |
| Parental education | 53.71 | 1,949 | <.0001 | 24.48 | 1,575 | <.0001 | 49.51 | 1,862 | <.0001 | 24.4 | 1,512 | <.0001 | |
| Within | Domain | 13.14 | 11,939 | <.0001 | 7.8 | 11,565 | <.0001 | 13.75 | 11,852 | <.0001 | 6.86 | 11,502 | <.0001 |
| Interaction | Diag*Domain | 2.79 | 22,1605.6 | <.0001 | 5.3 | 11,565 | <.0001 | 0.79 | 11,852 | 0.6551 | 4.89 | 11,502 | <.0001 |
| Speed | |||||||||||||
| Between Covariates |
Diag | 24.68 | 2,949 | <.0001 | 13.53 | 1,522 | 0.0003 | 49.48 | 1,862 | <.0001 | 0.53 | 1,512 | 0.4675 |
| Sex | 14.35 | 1,949 | 0.0002 | 14.71 | 1,522 | 0.0001 | 6.4 | 1,862 | 0.0116 | 8.94 | 1,512 | 0.0029 | |
| Age | 225.11 | 1,949 | <.0001 | 155.71 | 1,522 | <.0001 | 205.21 | 1,862 | <.0001 | 102.77 | 1,512 | 0.0878 | |
| Parental education | 6.75 | 1,949 | 0.0095 | 2.34 | 1,522 | <.0001 | 7.92 | 1,862 | <.0001 | 3.51 | 1,512 | <.0001 | |
| Within | Domain | 21.19 | 13,937 | <.0001 | 15.46 | 13,510 | <.0001 | 19.32 | 13,850 | <.0001 | 10.6 | 13,500 | <.0001 |
| Interaction | Diag*Domain | 5.17 | 26,1635.4 | <.0001 | 5.76 | 13,510 | <.0001 | 6.23 | 13,850 | <.0001 | 3.21 | 13,500 | 0.0001 |
22q11DS = 22q11.2 Deletion Syndrome; DD = Developmental Delay; TD= Typically Developing
Figure 1.
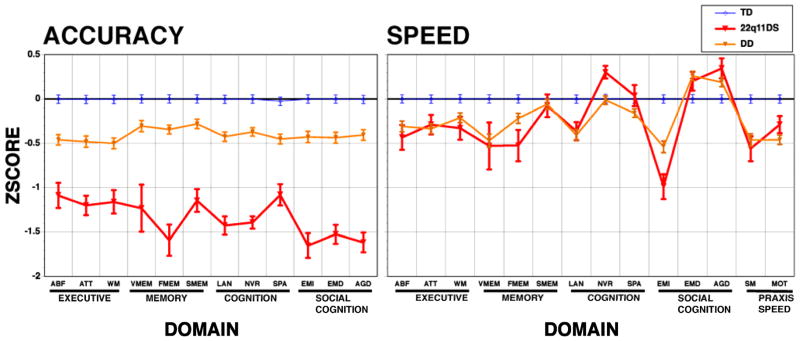
The neurocognitive profile (mean ± SEM Z-scores) for accuracy and speed in Typically Developing (TD, blue line), 22q11.2 Deletion Syndrome (22q11DS, red line) and Developmental Delay with medical comorbidities (DD, orange line). Accuracy scores are presented for Executive (ABF =Abstraction and mental flexibility; ATT = Attention; WM = Working memory), Episodic Memory (VMEM = Verbal Memory, FMEM = Face Memory, SMEM = Spatial Memory), Complex Cognition (LAN = Verbal-Language mediated reasoning, NVR = Nonverbal Reasoning, SPA = Spatial processing), Social Cognition (EMI = Emotion Identification, EMD = Emotion Intensity Differentiation, AGD = Age Differentiation). Speed measures are also available for Praxis (MOT = Motor Speed, SM = Sensorimotor Speed).
The MANCOVAs on cognitive age variables used age-group (cross-sectional) and diagnosis as a between-group factor and domain (Executive, Episodic memory, Complex cognition, Social cognition and Sensorimotor speed) as a within-group factor. Sex was not used as a between-group factor because the cognitive age was calculated separately for males and females and there were no sex x diagnosis interactions in the analysis of the CNB profile. The results indicated highly significant diagnosis x domain x age group interactions for all 3-groups and 2-group contrasts, except for an absence of such an interaction in the contrast between DD and TD (Table 3). These interactions were significant after controlling for the significant effects of the covariates (parental education and age), and showed that 22q11DS is associated with specific abnormalities in the developmental trajectories (Figure 2). The largest developmental delays in the 22q11DS group were for complex cognition and social cognition, whereas their lag was much smaller for executive and memory domains and comparable to that of the developmentally delayed group for sensorimotor speed.
Table 3.
Comparison of diagnostic groups in neurocognitive age across neurocognitive domains
| TD vs 22q11DS vs DD | TD vs 22q11DS | TD vs DD | DD vs 22q11DS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Neurocognitive age | F | DF | P | F | DF | P | F | DF | P | F | DF | P | |
| Between | Diag | 105.86 | 2,938 | <.0001 | 242.04 | 1,515 | <.0001 | 115.05 | 1,855 | <.0001 | 28.15 | 1,505 | <.0001 |
| Age group | 626.86 | 5,938 | <.0001 | 490.60 | 5,515 | <.0001 | 1054.58 | 5,855 | <.0001 | 236.81 | 5,505 | <.0001 | |
| Covariates | Parental education | 35.88 | 1,938 | <.0001 | 23.15 | 1,515 | <.0001 | 36.75 | 1,855 | <.0001 | 15.06 | 1,505 | 0.0001 |
| Within | Domain | 14.09 | 4,935 | <.0001 | 13.38 | 4,512 | <.0001 | 7.49 | 4,852 | <.0001 | 8.2 | 4,502 | <.0001 |
| Interaction | Diag*Domain | 21.14 | 8,1333.4 | <.0001 | 40.42 | 4,512 | <.0001 | 0.58 | 4,852 | 0.678 | 34.95 | 4,502 | <.0001 |
| Diag*Domain*Age group | 3.24 | 20,2049.6 | <.0001 | 2.78 | 20,1119 | <.0001 | 1.18 | 20,1867 | 0.2641 | 3.28 | 20,1097 | <.0001 | |
TD= Typically Developing; 22q11DS= 22q11.2 Deletion Syndrome; DD= Developmental Delay
Figure 2.
Chronological age compared to predicted neurocognitive age in years for Typically Developing participants (TD, blue line), 22q11.2 Deletion Syndrome (22q11DS, red line) and Developmental Delay with medical comorbidities (DD, orange line). Growth charts are provided for a. Predicted age based on all scores (All Domains), b–f. Predicted age based on tests grouped by each of the five domains.
DISCUSSION
In a large well-characterized sample of youths we found that the accuracy performance profile of participants with 22q11DS showed greater impairment than that of non-deleted individuals with concomitant developmental delay and medical comorbidities. The latter group was in turn impaired relative to typically developing healthy controls. In addition to buttressing the sensitivity of the battery to the behavioral effects of developmental delay on brain function, the results indicate that the microdeletion confers neurocognitive deficits beyond those associated with developmental delay and medical conditions that require monitoring, medications and interventions. The diagnosis x domain interactions indicated that against a generally reduced level of accuracy performance, the deficits in 22q11DS are most pronounced for face memory and all social cognition measures, followed by language and nonverbal reasoning. Deficits in face memory and emotion identification of facial expressions have been reported in our initial study of 22q11DS compared to healthy participants34 as well as by others.41,42 The underlying deficits in face processing have been examined in functional magnetic resonance imaging (fMRI) and have been reported in 22q11DS,43 schizophrenia44,45 and autism spectrum disorders.46,47 Complex cognition, specifically language and nonverbal reasoning, was also differentially impaired in 22q11DS. Such measures of complex cognition, which are more akin to IQ scores, have been commonly assessed in 22q11DS and reported to be impaired.14,16–20 Speed of performance, as indicated by response time, was similar overall for 22q11DS and developmentally delayed non-deleted participants. Again, face memory and emotion identification distinguished the two groups, with individuals with 22q11DS being slower. Notably, nonverbal reasoning was associated with faster response despite poorer performance in the deleted group. This enhanced speed of response to complex reasoning items likely reflects “giving up” when the challenge is increased.
The growth charts examine neurocognitive age against chronological age and highlight the extent of developmental lags across the age groups. While the design is cross-sectional, the growth charts of 22q11DS and developmentally delayed individuals diverge for specific domains. Thus, complex cognition and social cognition are most impaired in 22q11DS. There is also evidence of maturation as chronological aging is associated with improved performance for some neurocognitive domains, such as executive functions and memory. This effect is also seen in developmental delay. The significant age group x diagnosis interactions indicate that the neurocognitive lag is not uniform across the age range. It appears that during early adolescence there is a narrowing of the lag, which then widens during late adolescence and early adulthood. Such results in a cross sectional study could reflect cohort effects, but could also suggest periods when an intervention may have a better yield. The age group x diagnosis x domain interactions further indicate that the developmental lag varies by domain. Indeed, it is quite narrow in the sensorimotor and memory domains especially during early adolescence. Domains in which the developmental lag is smaller could be used in educational and rehabilitative efforts in order to employ compensatory strategies to overcome the more marked developmental lag in complex and social cognition. The profile and growth chart analyses complement each other in pointing out that while patients with 22q11DS are most impaired in face memory and social cognition, the greatest developmental lag across the age range is for social cognition and complex cognition and it is minimal for sensorimotor and episodic memory. Combined these findings may point to both appropriate timing and nature of therapeutic efforts.48
The study has several limitations. As this investigation is cross-sectional, we cannot examine developmental trajectories that require longitudinal data. Also, while we assessed participants for psychopathology we do not examine the possible burden of psychiatric disorders that are common in 22q11DS and present in youths with developmental delay. Such an evaluation could be informative but is beyond the scope of this paper and would require an even larger sample of 22q11DS participants. We attempted to tease apart the effects of developmental delay and medical comorbidities on neurocognition, by comparing deleted and non-deleted groups on these potentially important contributors to brain function. However, there are other potential approaches such as examining 22q11DS relative to other neurogenetic disorders. Few studies have compared performance of 22q11DS children to other neurodevelopmental disorders. 22q11DS patients showed differing patterns of intellectual functioning, language processing, mixed handedness and degree of laterality relative to children with other genetic disorders including Down syndrome,49,50 Turner syndrome51 and Williams-Beuren syndrome.50 Finally, the quantitative behavioral tasks were administered to children 8 years of age and older. Deficits are present at earlier ages as documented with traditional testing in 22q11DS49–51 and in children at risk for schizophrenia and other psychoses.52,53
Notwithstanding these limitations, this large-scale study of well phenotyped youths indicates significant neurobehavioral deficits in 22q11DS, with developmental lags in domains that can be pursued in animal and genomic investigations. As multiple psychiatric diagnoses are associated with 22q11DS, a detailed genomic examination in relation to the neurobehavioral domain of social cognition54, 55 and not based predominantly on a specific psychiatric diagnosis, can lead more productively toward a mechanistic account. Neuroimaging, applying fMRI tasks designed to probe the face memory and social cognition circuitry, can help identify the nodes in the circuitry that underlie these deficits.43–45 Such a dimensional approach can be complemented with mouse models that examine affiliative behavior.56–59
Acknowledgments
This study was supported by NIH grants MH087626, MH087636, MH089983 and MH089924. Additional support came from T32 MH019112 (JJY), the Doris Duke Charitable Foundation Clinical Research Fellowship (SXT) and K08 MH 079364 (MEC). We are grateful to the research participants and their parents. We thank Emily Wilkins, Catherine Conroy, Omar Abbas, Amy Cassidy, Allison Mott and Kosha Ruparel of the Neuropsychiatry Section at the University of Pennsylvania for data acquisition and coordination. We also thank Alice Bailey and Jhonna Corson of the “22q and You” Center for clinical coordination of 22q11DS participants and Colleen Franconi and Meghan McNamara of the Human Genetics Division at the Children’s Hospital of Philadelphia for the genetic laboratory assessments of eh 22q11DS participants. We finally thank Dr Hakon Hakonarson and the Center for Applied Genomics at the Children’s Hospital of Philadelphia for their collaboration in establishing the Philadelphia Neurodevelopmental Cohort.
Footnotes
CONFLICT OF INTEREST
The authors report no conflict of interest.
References
- 1.Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, Habel A, et al. Practical guidelines for managing patients with 22q11. 2 deletion syndrome. J Pediatrics. 2011;159:332–339. doi: 10.1016/j.jpeds.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antshel KM, Fremont W, Roizen NJ, Shprintzen R, Higgins AM, Dhamoon A, et al. ADHD, major depressive disorder, and simple phobias are prevalent psychiatric conditions in youth with velo cardio facial syndrome. J Am Acad Child Adolesc Psychiatry. 2006;45:596–603. doi: 10.1097/01.chi.0000205703.25453.5a. [DOI] [PubMed] [Google Scholar]
- 3.Bassett AS, Chow EW, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The schizophrenia phenotype in 22q11 deletion syndrome. Am J Psychiatry. 2003;160:1580–1586. doi: 10.1176/appi.ajp.160.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabbro A, Rizzi E, Schneider M, Debbane M, Eliez S. Depression and anxiety disorders in children and adolescents with velo-cardio-facial syndrome (VCFS) Eur Child Adolesc Psychiatry. 2012;21:379–385. doi: 10.1007/s00787-012-0273-x. [DOI] [PubMed] [Google Scholar]
- 5.Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, et al. Risk factors for the emergence of psychotic disorders in adolescents with 22q11. 2 deletion syndrome. Am J Psychiatry. 2007;164:663–669. doi: 10.1176/ajp.2007.164.4.663. [DOI] [PubMed] [Google Scholar]
- 6.Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, et al. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11. 2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48:1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- 7.Tang SX, Yi JJ, Calkins ME, Whinna DA, Kohler CG, Souders MC, et al. Psychiatric disorders in 22q11. 2 deletion syndrome are prevalent but under-treated. Psycholo Med. 2013;9:1–11. doi: 10.1017/S0033291713001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vorstman JA, Morcus ME, Duijff SN, Klaassen PW, Heineman-de Boer JA, Beemer FA, et al. The 22q11. 2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45:1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- 9.Hiroi N, Takahashi T, Hishimoto A, Izumi T, Boku S, Hiramoto T. Copy number variation at 22q11.2: from rare variants to common mechanisms of developmental neuropsychiatric disorders. Mol Psychiatry. 2013 Aug 6; doi: 10.1038/mp.2013.92. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonas RK, Montojo CA, Bearden CE. The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biol Psychiatry. 2013 Aug 27; doi: 10.1016/j.biopsych.2013.07.019. pii: S0006–3223(13)00672–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meechan DW, Maynard TM, Tucker ES, LaMantia AS. Three phases of DiGeorge/22q11 deletion syndrome pathogenesis during brain development: patterning, proliferation, and mitochondrial functions of 22q11 genes. Int J Dev Neurosci. 2011;29:283–294. doi: 10.1016/j.ijdevneu.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harper KM, Hiramoto T, Tanigaki K, Kang G, Suzuki G, Trimble W, et al. Alterations of social interaction through genetic and environmental manipulation of the 22q11. 2 gene Sept5 in the mouse brain. Hum Mol Genet. 2012;21:3489–3499. doi: 10.1093/hmg/dds180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duijff SN, Klaassen PW, de Veye HF, Beemer FA, Sinnema G, Vorstman JA. Cognitive development in children with 22q11.2 deletion syndrome. Br J Psychiatry. 2012;200:462–468. doi: 10.1192/bjp.bp.111.097139. [DOI] [PubMed] [Google Scholar]
- 15.Bearden CE, Woodin MF, Wang PP, Moss E, McDonald-McGinn D, Zackai E, et al. The neurocognitive phenotype of the 22q11.2 deletion syndrome: selective deficit in visual-spatial memory. J Clin Exper Neuropsychol. 2001;23:447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- 16.De Smedt B, Devriendt K, Fryns J-P, Vogels A, Gewillig M, Swillen A. Intellectual abilities in a large sample of children with Velo--Cardio--Facial Syndrome: an update. J Intellect Disabil Res. 2007;51:666–670. doi: 10.1111/j.1365-2788.2007.00955.x. [DOI] [PubMed] [Google Scholar]
- 17.Bish JP, Ferrante SM, McDonald-McGinn D, Zackai E, Simon TJ. Maladaptive conflict monitoring as evidence for executive dysfunction in children with chromosome 22q11.2 deletion syndrome. Dev Sci. 2005;8:36–43. doi: 10.1111/j.1467-7687.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 18.Campbell LE, Azuma R, Ambery F, Stevens A, Smith A, Morris RG, et al. Executive functions and memory abilities in children with 22q11.2 deletion syndrome. Aust N Z J Psychiatry. 2010;44:364–371. doi: 10.3109/00048670903489882. [DOI] [PubMed] [Google Scholar]
- 19.Henry JC, van Amelsvoort T, Morris RG, Owen MJ, Murphy DG, Murphy KC. An investigation of the neuropsychological profile in adults with velo-cardio-facial syndrome (VCFS) Neuropsychologia. 2002;40:471–478. doi: 10.1016/s0028-3932(01)00136-1. [DOI] [PubMed] [Google Scholar]
- 20.Rockers K, Ousley O, Sutton T, Schoenberg E, Coleman K, Walker E, et al. Performance on the modified card sorting test and its relation to psychopathology in adolescents and young adults with 22q11.2 deletion syndrome. J Intellect Disabil Res. 2009;53:665–676. doi: 10.1111/j.1365-2788.2009.01178.x. [DOI] [PubMed] [Google Scholar]
- 21.Sobin C, Kiley-Brabeck K, Daniels S, Blundell M, Anyane-Yeboa K, Karayiorgou M. Networks of attention in children with the 22q11 deletion syndrome. Dev Neuropsychol. 2004;26:611–626. doi: 10.1207/s15326942dn2602_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabaral MH, Beaton EA, Stoddard J, Simon TJ. Impaired multiple object tracking in children with chromosome 22q11.2 deletion syndrome. J Neurodev Disord. 2012;4:6. doi: 10.1186/1866-1955-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majerus S, Van der Linden M, Braissand V, Eliez S. Verbal short-term memory in individuals with chromosome 22q11.2 deletion: specific deficit in serial order retention capacities? Am J Ment Retard. 2007;112:79–93. doi: 10.1352/0895-8017(2007)112[79:VSMIIW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Woodin M, Wang PP, Aleman D, McDonald-McGinn D, Zackai E, Moss E. Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genet Med. 2001;3:34–39. doi: 10.1097/00125817-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, et al. A cognitive neuroscience based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. J Neurosci Methods. 2010;187:254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roalf DR, Ruparel K, Gur RE, Bilker W, Gerraty R, Elliott MA, et al. Neuroimaging predictors of cognitive performance across a standardized neurocognitive battery. Neuropsychology. doi: 10.1037/neu0000011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calkins ME, Ray A, Gur RC, Freedman R, Green MF, Greenwood TA, et al. Sex differences in familiality effects on neurocognitive performance in schizophrenia. Biol Psychiatry. 2013;73:976–984. doi: 10.1016/j.biopsych.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenwood TA, Swerdlow NR, Gur RE, Cadenhead KS, Calkins ME, Dobie DJ, et al. Genome-wide linkage analyses of 12 endophenotypes for schizophrenia from the consortium on the genetics of schizophrenia. Am J Psychiatry. 2013;170:521–532. doi: 10.1176/appi.ajp.2012.12020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roalf DR, Gur RC, Almasy L, Richard J, Gallagher RS, Prasad K, et al. Neurocognitive performance stability in a multiplex multigenerational study of schizophrenia. Schizophr Bull. 2013;39:1008–1017. doi: 10.1093/schbul/sbs078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calkins ME, Tepper P, Gur RC, Ragland JD, Klei L, Wiener HW, et al. Project among African-Americans to explore risks for schizophrenia (PAARTNERS): evidence for impairment and heritability of neurocognitive functioning in families of schizophrenia patients. Am J Psychiatry. 2010;167:459–472. doi: 10.1176/appi.ajp.2009.08091351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, et al. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry. 2011;168:930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164:813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- 33.Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26:251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldenberg PC, Calkins ME, Richard J, McDonald-McGinn D, Zackai E, Mitra N, et al. Computerized neurocognitive profile in young people with 22q11.2 deletion syndrome compared to youths with schizophrenia and at-risk for psychosis. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:87–93. doi: 10.1002/ajmg.b.32005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gur RC, Calkins ME, Satterthwaite TD, Ruparel K, Bilker WB, Moore TM, et al. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2013.4190. in press. [DOI] [PubMed] [Google Scholar]
- 36.Brown TT, Kuperman JM, Chung Y, Erhart M, McCabe C, Hagler DJ, et al. Neuroanatomical assessment of biological maturity. Curr Biol. 2012;22:1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, et al. Neuroimaging of the Philadelphia Neurodevelopmental Cohort. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.07.064. e-pub ahead of print 3 August 2013; pii: S1053–8119(13)00833–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaufman J, Bismaher B, Brent DA, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Aged Children - Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adol Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson GS, Robertson GJ. Wide Range Achievement Test. 4. Psychological Assessment Resources; Lutz, FL: 2006. (WRAT4) [Google Scholar]
- 41.Jalbrzikowski M, Carter C, Senturk D, Chow C, Hopkins JM, Green MF, et al. Social cognition in 22q11.2 microdeletion syndrome: relevance to psychosis? Schizophr Res. 2012;142:99–107. doi: 10.1016/j.schres.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell L, McCabe K, Leadbeater K, Schall U, Loughland C, Rich D. Visual scanning of faces in 22q11.2 deletion syndrome: attention to the mouth or the eyes? Psychiatry Res. 2010;177:211–215. doi: 10.1016/j.psychres.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Andersson F, Glaser B, Spiridon M, Debbane M, Vuilleumier P, Eliez S. Impaired activation of face processing networks revealed by functional magnetic resonance imaging in 22q11.2 deletion syndrome. Biol Psychiatry. 2008;63:49–57. doi: 10.1016/j.biopsych.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 44.Gur RE, Loughead J, Kohler CG, Elliott MA, Lesko K, Ruparel K, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64:1356–1366. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- 45.Pinkham AE, Loughead J, Ruparel K, Overton E, Gur RE, Gur RC. Abnormal modulation of amygdala activity in schizophrenia in response to direct- and averted-gaze threat-related facial expressions. Am J Psychiatry. 2011;168:293–301. doi: 10.1176/appi.ajp.2010.10060832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tottenham N, Hertzig ME, Gillespie-Lynch K, Gilhooly T, Millner AJ, Casey BJ. Elevated amygdala response to faces and gaze aversion in autism spectrum disorder. Soc Cogn Affect Neurosci. 2013 doi: 10.1093/scan/nst050. e-pub ahead of print 24 May 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shashi V, Veerapandiyan A, Schoch K, Kwapil T, Keshavan M, Ip E, et al. Social skills and associated psychopathology in children with chromosome 22q11.2 deletion syndrome: implications for interventions. J Intellect Disabil Res. 2012;56:865–878. doi: 10.1111/j.1365-2788.2011.01477.x. [DOI] [PubMed] [Google Scholar]
- 49.Scherer NJ, D’Antonio LL, Rodgers JR. Profiles of communication disorder in children with velocardiofacial syndrome: comparison to children with Down syndrome. Genet Med. 2001;3:72–78. doi: 10.1097/00125817-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 50.Carlier M, Desplanches AG, Philip N, Stephanini S, Vicari S, Volterra V, et al. Laterality preference and cognition: Cross-syndrome comparison of patients with Trisomy 21 (Down), del7q11.23 (Williams-Beuren) and del22q11.2 (DiGeorge or Velo-Cardio-Facial) Syndromes. Behavioral Genetics. 2011;41:413–422. doi: 10.1007/s10519-011-9465-2. [DOI] [PubMed] [Google Scholar]
- 51.Simon TJ, Yakarae Y, DeBoer T, McDonald-McGinn DM, Zackai EH, Ross JL. Overlapping numerical impairments in children with chromosome 22q11.2 deletion or Turner syndromes. Neuropsychologia. 2007;46:82–94. doi: 10.1016/j.neuropsychologia.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNeil TF, Cantor-Graae E, Blennow G. Mental correlates of neuromotoric deviation in 6-year olds at heightened risk for schizophrenia. Schizophr Res. 2003;60:219–228. doi: 10.1016/s0920-9964(02)00235-9. [DOI] [PubMed] [Google Scholar]
- 53.Buka SL, Seidman LJ, Tsuang MT, Goldstein JM. The New England family study high-risk project: Neurological impairments among offspring of parents with schizophrenia and other psychoses. Am J Med Genet B Neuropsychiatr Genet. 2013;162:653–660. doi: 10.1002/ajmg.b.32181. [DOI] [PubMed] [Google Scholar]
- 54.Baker K, Vorstman JA. Is there a core neuropsychiatric phenotype in 22q11.2 deletion syndrome? Curr Opin Neurol. 2012;25:131–137. doi: 10.1097/WCO.0b013e328352dd58. [DOI] [PubMed] [Google Scholar]
- 55.Kates WR, Antshel KM, Fremont WP, Shprintzen RJ, Strunge LA, Burnette CP, et al. Comparing phenotypes in patients with idiopathic autism to patients with velocardiofacial syndrome (22q11 DS) with and without autism. Am J Med Genet A. 2007;143A:2642–2650. doi: 10.1002/ajmg.a.32012. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki G, Harper KM, Hiramoto T, Sawamura T, Lee M, Kang G, et al. Sept5 deficiency exerts pleiotropic influence on affective behaviors and cognitive functions in mice. Hum Mol Genet. 2009;18:1652–1660. doi: 10.1093/hmg/ddp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hiroi N, Hiramoto T, Harper KM, Suzuki G, Boku S. Mouse models of 22q11.2-associated autism spectrum disorder. Autism. 2012;S1:1–9. doi: 10.4172/2165-7890.S1-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Babovic D, O’Tuathaigh CM, O’Connor AM, O’Sullivan GJ, Tighe O, Croke DT, et al. Phenotypic characterization of cognition and social behavior in mice with heterozygous versus homozygous deletion of catechol-O-methyltransferase. Neuroscience. 2008;155:1021–1029. doi: 10.1016/j.neuroscience.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Hiramoto T, Kang G, Suzuki G, Satoh Y, Kucherlapati R, Watanabe Y, et al. Tbx1: identification of a 22q11.2 gene as a risk factor for autism spectrum disorder in a mouse model. Hum Mol Genet. 2011;20:4775–4785. doi: 10.1093/hmg/ddr404. [DOI] [PMC free article] [PubMed] [Google Scholar]