1. Introduction
Chronic pain has been recognized as pain that persists past normal healing time5 and hence lacks the acute warning function of physiological nociception.35 Usually pain is regarded as chronic when it lasts or recurs for more than 3 to 6 months.29 Chronic pain is a frequent condition, affecting an estimated 20% of people worldwide6,13,14,18 and accounting for 15% to 20% of physician visits.25,28 Chronic pain should receive greater attention as a global health priority because adequate pain treatment is a human right, and it is the duty of any health care system to provide it.4,13
The current version of the International Classification of Diseases (ICD) of the World Health Organization (WHO) includes some diagnostic codes for chronic pain conditions, but these diagnoses do not reflect the actual epidemiology of chronic pain, nor are they categorized in a systematic manner. The ICD is the preeminent tool for coding diagnoses and documenting investigations or therapeutic measures within the health care systems of many countries. In addition, ICD codes are commonly used to report target diseases and comorbidities of participants in clinical research. Consequently, the current lack of adequate coding in the ICD makes the acquisition of accurate epidemiological data related to chronic pain difficult, prevents adequate billing for health care expenses related to pain treatment, and hinders the development and implementation of new therapies.10,11,16,23,27,31,37
Responding to these shortcomings, the International Association for the Study of Pain (IASP) contacted the WHO and established a Task Force for the Classification of Chronic Pain. The IASP Task Force, which comprises pain experts from across the globe,19 has developed a new and pragmatic classification of chronic pain for the upcoming 11th revision of the ICD. The goal is to create a classification system that is applicable in primary care and in clinical settings for specialized pain management.
A major challenge in this process was finding a rational principle of classification that suits the different types of chronic pain and fits into the general ICD-11 framework. Pain categories are variably defined based on the perceived location (headache), etiology (cancer pain), or the primarily affected anatomical system (neuropathic pain). Some diagnoses of pain defy these classification principles (fibromyalgia).
This problem is not unique to the classification of pain, but exists throughout the ICD. The IASP Task Force decided to give first priority to pain etiology, followed by underlying pathophysiological mechanisms, and finally the body site. Developing this multilayered classification was greatly facilitated by a novel principle of assigning diagnostic codes in ICD-11, termed “multiple parenting.” Multiple parenting allows the same diagnosis to be subsumed under more than 1 category (for a glossary of ICD terms refer to Table 1). Each diagnosis retains 1 category as primary parent, but is cross-referenced to other categories that function as secondary parents.
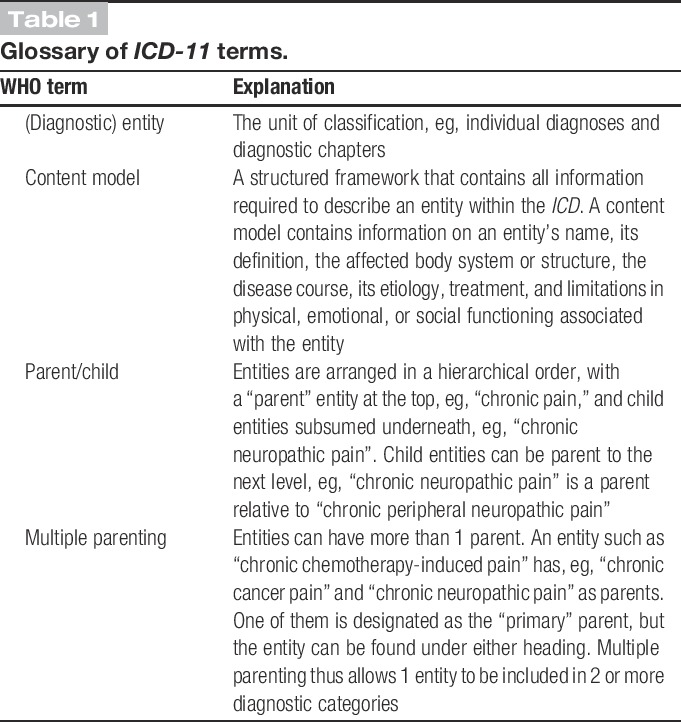
Table 1.
Glossary of ICD-11 terms.
The new ICD category for “Chronic Pain” comprises the most common clinically relevant disorders. These disorders were divided into 7 groups (Fig. 1): (1) chronic primary pain, (2) chronic cancer pain, (3) chronic posttraumatic and postsurgical pain, (4) chronic neuropathic pain, (5) chronic headache and orofacial pain, (6) chronic visceral pain, and (7) chronic musculoskeletal pain. Experts assigned to each group are responsible for the definition of diagnostic criteria and the selection of the diagnoses to be included under these subcategories of chronic pain. Thanks to Bedirhan Üstün and Robert Jakob of the WHO, these pain diagnoses are now integrated in the beta version of ICD-11 (http://id.who.int/icd/entity/1581976053). The Task Force is generating content models for single entities to describe their clinical characteristics. After peer review overseen by the WHO Steering Committee,39 the classification of chronic pain will be voted into action by the World Health Assembly in 2017.
Figure 1.
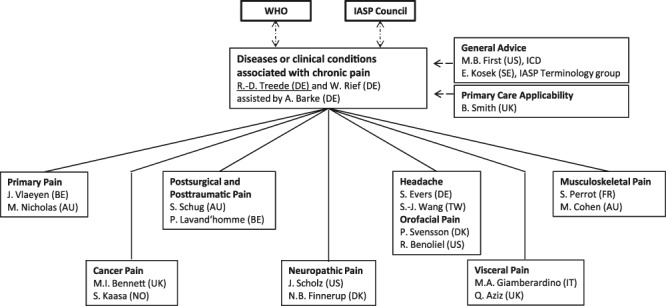
Organizational chart of Task Force, IASP, and WHO interactions. The IASP Task Force was created by the IASP council and its scope defined in direct consultation of the chairs (R.D.T. and W.R.) with WHO representatives in 2012. The Task Force reports to the IASP Council on an annual basis.
2. Classification of chronic pain
Chronic pain was defined as persistent or recurrent pain lasting longer than 3 months. This definition according to pain duration has the advantage that it is clear and operationalized.
Optional specifiers for each diagnosis record evidence of psychosocial factors and the severity of the pain. Pain severity can be graded based on pain intensity, pain-related distress, and functional impairment.
2.1. Chronic primary pain
Chronic primary pain is pain in 1 or more anatomic regions that persists or recurs for longer than 3 months and is associated with significant emotional distress or significant functional disability (interference with activities of daily life and participation in social roles) and that cannot be better explained by another chronic pain condition. This is a new phenomenological definition, created because the etiology is unknown for many forms of chronic pain. Common conditions such as, eg, back pain that is neither identified as musculoskeletal or neuropathic pain, chronic widespread pain, fibromyalgia, and irritable bowel syndrome will be found in this section and biological findings contributing to the pain problem may or may not be present. The term “primary pain” was chosen in close liaison with the ICD-11 revision committee, who felt this was the most widely acceptable term, in particular, from a nonspecialist perspective.
2.2. Chronic cancer pain
Pain is a frequent and debilitating accompaniment of cancer8 that as yet has not been represented in the ICD. The Task Force decided to list it as a separate entity because there are specific treatment guidelines.7,38 Chronic cancer pain includes pain caused by the cancer itself (the primary tumor or metastases) and pain that is caused by the cancer treatment (surgical, chemotherapy, radiotherapy, and others). Cancer-related pain will be subdivided based on location into visceral, bony (or musculoskeletal), and somatosensory (neuropathic). It will be described as either continuous (background pain) or intermittent (episodic pain) if associated with physical movement or clinical procedures. The treatment-related pain will be cross-referenced from the chapters on postsurgical pain and neuropathic pain.
2.3. Chronic postsurgical and posttraumatic pain
Because pain that persists beyond normal healing is frequent after surgery and some types of injuries, the entity of postsurgical and posttraumatic pain was created. This is defined as pain that develops after a surgical procedure or a tissue injury (involving any trauma, including burns) and persists at least 3 months after surgery or tissue trauma26; this is a definition of exclusion, as all other causes of pain (infection, recurring malignancy) as well as pain from a pre-existing pain problem need to be excluded. In view of the different causality, as well as from a medicolegal point of view, a separation between postsurgical pain and pain after all other trauma is regarded as useful. Depending on the type of surgery, chronic postsurgical pain is often neuropathic pain (on average 30% of cases with a range from 6% to 54% and more).15 Pain including such a neuropathic component is usually more severe than nociceptive pain and often affects the quality of life more adversely.21
2.4. Chronic neuropathic pain
Chronic neuropathic pain is caused by a lesion or disease of the somatosensory nervous system.20,22 The somatosensory nervous system provides information about the body including skin, musculoskeletal, and visceral organs. Neuropathic pain may be spontaneous or evoked, as an increased response to a painful stimulus (hyperalgesia) or a painful response to a normally nonpainful stimulus (allodynia). The diagnosis of neuropathic pain requires a history of nervous system injury, for example, by a stroke, nerve trauma, or diabetic neuropathy, and a neuroanatomically plausible distribution of the pain.22 For the identification of definite neuropathic pain, it is necessary to demonstrate the lesion or disease involving the nervous system, for example, by imaging, biopsy, neurophysiological, or laboratory tests. In addition, negative or positive sensory signs compatible with the innervation territory of the lesioned nervous structure must be present.36 Diagnostic entities within this category will be divided into conditions of peripheral or central neuropathic pain.
2.5. Chronic headache and orofacial pain
The International Headache Society (IHS) has created a headache classification17 that is implemented in full in the chapter on neurology. This classification differentiates between primary (idiopathic), secondary (symptomatic) headache, and orofacial pain including cranial neuralgias. In the section on chronic pain, only chronic headache and chronic orofacial pain will be included. Chronic headache and chronic orofacial pain is defined as headaches or orofacial pains that occur on at least 50% of the days during at least 3 months. For most purposes, patients receive a diagnosis according to the headache phenotypes or orofacial pains that they currently present. The section will list the most frequent chronic headache conditions.
The most common chronic orofacial pains are temporomandibular disorders,32 which have been included in this subchapter of chronic pain. Chronic orofacial pain can be a localized presentation of a primary headache.2 This is common in the trigeminal autonomic cephalalgias, less common in migraines, and rare in tension-type headache. Several chronic orofacial pains such as post-traumatic trigeminal neuropathic pain,3 persistent idiopathic orofacial pain, and burning mouth syndrome are cross-referenced to, eg, primary chronic pain and neuropathic pain. The temporal definition of “chronic” has been extrapolated from that of chronic headaches.1
2.6. Chronic visceral pain
Chronic visceral pain is persistent or recurrent pain that originates from the internal organs of the head and neck region and the thoracic, abdominal, and pelvic cavities.24,33,34 The pain is usually perceived in the somatic tissues of the body wall (skin, subcutis, muscle) in areas that receive the same sensory innervation as the internal organ at the origin of the symptom (referred visceral pain).12 In these areas, secondary hyperalgesia (increased sensitivity to painful stimuli in areas other than the primary site of the nociceptive input) often occurs30; the intensity of the symptom may bear no relationship with the extent of the internal damage or noxious visceral stimulation.9 The section on visceral pain will be subdivided according to the major underlying mechanisms, ie, persistent inflammation, vascular mechanisms (ischemia, thrombosis), obstruction and distension, traction and compression, combined mechanisms (eg, obstruction and inflammation concurrently), and referral from other locations. Pain due to cancer will be cross-referenced to the chapter chronic cancer pain and pain due to functional or unexplained mechanisms to chronic primary pain.
2.7. Chronic musculoskeletal pain
Chronic musculoskeletal pain is defined as persistent or recurrent pain that arises as part of a disease process directly affecting bone(s), joint(s), muscle(s), or related soft tissue(s). According to the constraints of the approach as described in the Introduction, this category is therefore limited to nociceptive pain and does not include pain that may be perceived in musculoskeletal tissues but does not arise therefrom, such as the pain of compression neuropathy or somatic referred pain. The entities subsumed in this approach include those characterized by persistent inflammation of infectious, autoimmune or metabolic etiology, such as rheumatoid arthritis, and by structural changes affecting bones, joints, tendons, or muscles, such as symptomatic osteoarthrosis. Musculoskeletal pain of neuropathic origin will be cross-referenced to neuropathic pain. Well-described apparent musculoskeletal conditions for which the causes are incompletely understood, such as nonspecific back pain or chronic widespread pain, will be included in the section on chronic primary pain.
3. Outlook
Irrespective of its etiology, chronic pain is a major source of suffering and requires special treatment and care. Our proposal may not represent a perfect solution for the classification of all manifestations of chronic pain. However, it does represent the first systematic approach to implementing a classification of chronic pain in the ICD. It is based on international expertise and agreement, and consistent with the requirements of the ICD regarding the structure and format of content models. The 7 major categories of chronic pain were identified after considerable research and discussion. They represent a compromise between comprehensiveness and practical applicability of the classification system. Several clinically important conditions that were neglected in former ICD revisions will now be mentioned, eg, chronic cancer pain or chronic neuropathic pain. Etiological factors, pain intensity, and disability related to pain will be reflected. With the introduction of chronic primary pain as a new diagnostic entity, the classification recognizes conditions that affect a broad group of patients with pain and would be neglected in etiologically defined categories. We hope that this classification strengthens the representation of chronic pain conditions in clinical practice and research and welcome comments to improve it further.
Conflict of interest statement
Q. Aziz has attended advisory board meetings for Almirall pharmaceuticals and Grunenthal. He has also received funding for clinical trials from Ono Pharmaceutical and Protexin. M.I. Bennett has received consultancy or speaker fees from Pfizer, Bayer, Astellas, and Grunenthal in the last 5 years. M. Cohen has received honoraria for contributions to educational programs from Mundipharma Pty Limited and Pfizer. S. Evers received honoraria (as speaker and/or member of advisory boards) and research grants within the past 5 years by AGA Medical (now St Jude), Allergan, Almirall, Astra Zeneca, Berlin-Chemie, CoLucid, Desitin, Eisai, GlaxoSmithKline, Ipsen Pharma, Menarini, MSD, Novartis, Pfizer, Reckitt-Benckiser, UCB. N.B. Finnerup has received speaker's honoraria from Pfizer, Grunenthal, and Norpharma, research grant from Grünenthal, and consultancy fee from Astellas and is member of the IMI “Europain” collaboration where industry members of this are: Astra Zeneca, Pfizer, Esteve, UCB-Pharma, Sanofi Aventis, Grünenthal, Eli Lilly, Boehringer Ingelheim, Astellas, Abbott, and Lundbeck. M.B. First on the faculty of the Lundbeck International Neuroscience Foundation. In the past 2 years, M.A. Giamberardino received research funding or honoraria (participation in Advisory Board) from Bayer Healthcare, Helsinn, and Epitech Group. S. Kaasa declares no conflict of interest related to this work. In the past year he received honoraria from Helsinn related to participation in Advisory Board. E. Kosek has received consultancy and speaker fees in the past 24 months from Eli Lilly and Company and Orion and has ongoing research collaborations with Eli Lilly and Company and Abbott and Pierre Fabre. M. Nicholas received honoraria for contributing to educational sessions for Mundipharma and Pfizer in the last 5 years. S. Perrot received honoraria as a speaker and/or member of the advisory board in the past 5 years from Pfizer, BMS, Grunenthal, Elli Lilly, Sanofi, Daichi-Sankyo, Astellas, and Mundipharma. He has received grant support from BMS. W. Rief received honoraria (as speaker and/or member of advisory boards on topics such as adherence, placebo mechanisms) within the past 5 years from Berlin Chemie, Astra Zeneca, Bayer, Heel (research grant). J. Scholz has received speaker fees from Convergence, GlaxoSmithKline, Pfizer, St Jude Medical, and Zalicus. He has served on advisory boards or consulted for Convergence, Pfizer, Sanofi Aventis, and Zalicus Pharmaceuticals. He has received grant support from GlaxoSmithKline and Pfizer. In the last 5 years, the Anaesthesiology Unit of the University of Western Australia, but not S. Schug personally, has received research and travel funding and speaking and consulting honoraria from bioCSL, Bionomics, Eli Lilly, Grunenthal, Janssen, Mundipharma, Pfizer, Phosphagenics and iX Biopharma within the last 2 years. B.H. Smith has received lecture and consultancy fees, on behalf of his institution, from Pfizer, Grunenthal, Eli Lilly, and Napp. He has received unconditional educational grants from Pfizer Ltd; and he has received travel and accommodation support from Napp. P. Svensson served as a paid consultant for Sunstar Suisse SA. R.-D. Treede has received speaker's honoraria, research grants or consultancy fees from AbbVie, Acron, Astellas, Bauerfeind, Boehringer Ingelheim, Grünenthal, Hydra, Mundipharma, and Pfizer and is a member of the IMI “Europain” collaboration where industry members of this are: Astra Zeneca, Pfizer, Esteve, UCB-Pharma, Sanofi Aventis, Grünenthal, Eli Lilly, Boehringer Ingelheim, Astellas, Abbott, and Lundbeck. J.W.S. Vlaeyen is a member of the PHILIPS advisory board on pain management and declares no conflicts of interest with regard to this work. S.-J. Wang has served on the advisory boards of Allergan and Eli Lilly, Taiwan. He has received speaking honoraria from local companies (Taiwan branches) of Pfizer, Elli Lilly, and GSK. He has received research grants from the Novartis Taiwan, Taiwan Ministry of Science and Technology, Taipei-Veterans General Hospital and Taiwan Headache Society. The other authors have no conflicts of interest to declare.
Acknowledgements
The authors are members of the Classification of Pain Diseases Task Force of the International Association for the Study of Pain (IASP), which gave logistical and financial support to perform this work. We acknowledge the contributions of the following IASP Special Interest Groups (SIGs): Abdominal & Pelvic Pain SIG, Acute Pain SIG, Cancer Pain SIG, Neuropathic Pain SIG and the Orofacial Pain SIG, and the Classification Committee of the International Headache Society (IHS).
Author contributions: R.-D. Treede, W. Rief, and A. Barke contributed equally to this topical review.
Appendix. Structure of the chapter on chronic pain
Chronic pain (persistent or recurrent pain lasting longer than 3 months)
-
1. Chronic primary pain
1.1. Widespread chronic primary pain (including fibromyalgia syndrome)
1.2. Localized chronic primary pain (including nonspecific back pain, chronic pelvic pain)
1.x. Other chronic primary pain
1.z. Chronic primary pain not otherwise specified
-
2. Chronic cancer pain
2.1. Chronic pain due to cancer and metastases
2.2. Chronic chemotherapy-induced pain (primary parent: chronic neuropathic pain)
2.3. Chronic pain due to cancer surgery (primary parent: chronic postsurgical and posttraumatic pain)
2.4. Chronic pain due to radiotherapy
2.x. Other chronic pain related to cancer
2.z. Chronic cancer pain not otherwise specified
-
3. Chronic postsurgical and posttraumatic pain
3.1. Chronic postsurgical pain
3.2. Chronic posttraumatic pain
3.x. Other chronic postsurgical and posttraumatic pain
3.z. Chronic postsurgical and posttraumatic pain not otherwise specified
-
4. Chronic neuropathic pain
4.1. Peripheral neuropathic pain
4.2. Central neuropathic pain
4.x. Other neuropathic pain
4.z. Neuropathic pain not otherwise specified
-
5. Chronic headache and orofacial pain
5.1. Chronic primary headaches*
5.2. Chronic secondary headaches*
5.3. Chronic orofacial pains†
5.z. Headache and orofacial pain not otherwise specified*
-
6. Chronic visceral pain
6.1. Chronic visceral pain from persistent inflammation
6.2. Chronic visceral pain from vascular mechanisms
6.3. Chronic visceral pain from obstruction/distension
6.4. Chronic visceral pain from traction/compression
6.5. Chronic visceral pain from combined mechanisms
6.6. Chronic visceral pain referred from other locations
6.7. Chronic visceral pain from cancer (primary parent: chronic cancer pain)
6.8. Functional or unexplained chronic visceral pain (primary parent: chronic primary pain)
6.x. Other chronic visceral pain
6.z. Chronic visceral pain not otherwise specified
-
7. Chronic musculoskeletal pain
7.1. Chronic musculoskeletal pain from persistent inflammation
7.2. Chronic musculoskeletal pain from structural osteoarticular changes
7.3. Chronic musculoskeletal pain due to disease of the nervous system (All neuropathic pain will be classified under 4. Chronic neuropathic pain. Here, other chronic musculoskeletal pain originating from diseases of the nervous system, eg, spastic pain will be listed.)
7.4. Chronic nonspecific musculoskeletal pain (primary parent: chronic primary pain)
7.x. Other chronic musculoskeletal pain syndromes
7.z. Chronic musculoskeletal pain not otherwise specified
*These disorders have in part been renumbered as compared with the ICHD-3. †#5.3 is not part of the ICHD-3.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Benoliel R, Birman N, Eliav E, Sharav Y. The International Classification of Headache Disorders: accurate diagnosis of orofacial pain? Cephalalgia 2008;7:752–62. [DOI] [PubMed] [Google Scholar]
- [2].Benoliel R, Eliav E, Sharav Y. Classification of chronic orofacial pain: applicability of chronic headache criteria. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;110:729–37. [DOI] [PubMed] [Google Scholar]
- [3].Benoliel R, Zadik Y, Eliav E, Sharav Y. Peripheral painful traumatic trigeminal neuropathy: clinical features in 91 cases and proposal of novel diagnostic criteria. J Orofacial Pain 2012;26:49–58. [PubMed] [Google Scholar]
- [4].Bond M, Breivik H, Jensen TS, Scholten W, Soyannwo O, Treede RD. Pain associated with neurological disorders. In: Aarli JA, Dua T, Janca A, Muscetta A, editors. Neurological disorders: public health challenges. Geneva: WHO Press, 2006. p. 127–139. [Google Scholar]
- [5].Bonica JJ. The management of pain. Philadelphia: Lea & Febiger, 1953. [Google Scholar]
- [6].Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287. [DOI] [PubMed] [Google Scholar]
- [7].Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, De Conno F, Fallon M, Hanna M, Haugen DF, Juhl G, King S, Klepstad P, Laugsand EA, Maltoni M, Mercadante S, Nabal M, Pigni A, Radbruch L, Reid C, Sjogren P. Use of opioid analgesics in the treatment of cancer pain: evidence based recommendations from the EAPC. Lancet Oncol 2012;13:e58–68. [DOI] [PubMed] [Google Scholar]
- [8].Caraceni A, Portenoy RK. An international survey of cancer pain characteristics and syndromes. IASP Task Force on Cancer Pain. International Association for the Study of Pain. PAIN 1999;82:263–74. [DOI] [PubMed] [Google Scholar]
- [9].Cervero F. Visceral pain—central sensitization. Gut 2000;47:56–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fillingim RB, Bruehl S, Dworkin RH, Dworkin SF, Loeser JD, Turk DC, Widerstrom-Noga E, Arnold L, Bennett R, Edwards RR, Freeman R, Gewandter J, Hertz S, Hochberg M, Krane E, Mantyh PW, Markman J, Neogi T, Ohrbach R, Paice JA, Porreca F, Rappaport BA, Smith SM, Smith TJ, Sullivan MD, Verne GN, Wasan AD, Wesselmann U. The ACTTION-American Pain Society Pain Taxonomy (AAPT): an evidence-based and multidimensional approach to classifying chronic pain conditions. J Pain 2014;15:241–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Finnerup NB, Scholz J, Attal N, Baron R, Haanpää M, Hansson P, Raja SN, Rice AS, Rief W, Rowbotham MC, Simpson DM, Treede RD. Neuropathic pain needs systematic classification. Eur J Pain 2013;17:953–6. [DOI] [PubMed] [Google Scholar]
- [12].Giamberardino MA, Affaitati G, Costantini R. Referred pain from internal organs. In: Cervero F, Jensen TS, editors. Handbook of clinical neurology. Amsterdam: Elsevier, 2006. p. 343–61. [DOI] [PubMed] [Google Scholar]
- [13].Goldberg DS, Summer JM. Pain as a global public health priority. BMC Public Health 2011;11:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gureje O, von Korff M, Kola L, Demyttenaere K, He Y, Posada-Villa J, Lepine JP, Angermeyer MC, Levinson D, de Girolamo G, Iwata N, Karam A, Borges GLG, de Graaf R, Browne MO, Stein DJ, Haro JM, Bromet EJ, Kessler RC, Alonso J. The relation between multiple pains and mental disorders: results from the World Mental Health Surveys. PAIN 2008;135:82–91. [DOI] [PubMed] [Google Scholar]
- [15].Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. PAIN 2013;154:95–102. [DOI] [PubMed] [Google Scholar]
- [16].Hart OR, Uden RM, McMullan JE, Ritchie MS, Williams TD, Smith BH. A study of National Health Service management of chronic osteoarthritis and low back pain. Prim Health Care Res Dev 2014;27:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- [18].Institute of Medicine (IOM). Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press, 2011. Available at: http://books.nap.edu/openbook.php?record_id=13172. Accessed 10 January 2015. [PubMed] [Google Scholar]
- [19].International Organization for the Study of Pain (IASP). List of Task Force Members. Available at: http://www.iasp-pain.org/AboutIASP/Content.aspx?ItemNumber=1997. Accessed October 01, 2014 and archived: http://www.webcitation.org/6Szl4AxX4.
- [20].International Organization for the Study of Pain (IASP). Available at:(http://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576). Accessed November 11, 2014 and archived: http://www.webcitation.org/6U0KsS4QV.
- [21].Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology 2007;68:1178–82. [DOI] [PubMed] [Google Scholar]
- [22].Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice AS, Treede RD. A new definition of neuropathic pain. PAIN 2011;152:2204–5. [DOI] [PubMed] [Google Scholar]
- [23].Klepstad P, Kaasa S, Cherny N, Hanks G, de Conno F. Pain and pain treatments in European palliative care units. A cross sectional survey from the European Association for Palliative Care Research Network. Palliat Med 2005;19:477–84. [DOI] [PubMed] [Google Scholar]
- [24].Knowles CH, Aziz Q. Basic and clinical aspects of gastrointestinal pain. PAIN 2009;141:191–209. [DOI] [PubMed] [Google Scholar]
- [25].Koleva D. Pain in primary care: an Italian survey. Eur J Public Health 2005;15:475–79. [DOI] [PubMed] [Google Scholar]
- [26].Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008;101:77–86. [DOI] [PubMed] [Google Scholar]
- [27].Manchikanti L, Falco FJE, Kaye AD, Hirsch JA. The disastrous but preventable consequences of ICD-10. Pain Physician 2014;E111–18. [PubMed] [Google Scholar]
- [28].Mäntyselkä P, Kumpusalo E, Ahonen R, Kumpusalo A, Kauhanen J, Viinamäki H, Halonen P, Takala J. Pain as a reason to visit the doctor: a study in Finnish primary health care. PAIN 2001;89:175–80. [DOI] [PubMed] [Google Scholar]
- [29].Merskey H, Bogduk N. Classification of chronic pain. 2nd ed. Seattle: IASP Press, 1994. p. 1. [Google Scholar]
- [30].Procacci P, Zoppi M, Maresca M. Clinical approach to visceral sensation. In: Cervero F, Morrison JFB, editors. Visceral sensation: progress in brain research. Amsterdam: Elsevier, 1986. p. 21–7. [DOI] [PubMed] [Google Scholar]
- [31].Rief W, Kaasa S, Jensen R, Perrot S, Vlaeyen JW, Treede RD, Vissers KC. The need to revise pain diagnoses in ICD-11. PAIN 2010;149:169–70. [DOI] [PubMed] [Google Scholar]
- [32].Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet J-P, List T, Svensson P, Gonzalez Y, Lobbezoo F, Michelotti A, Brooks L, Ceusters W, Drangsholt M, Ettlin D, Gaul C, Goldberg LJ, Haythornthwaite JA, Hollender L, Jensen R, John M, De Laat A, de Leeuw R, Maixner W, van der Meulen M, Murray GM, Nixdorf DR, Palla S, Petersson A, Pionchon P, Smith B, Visscher CM, Zakrzewska J, Dworkin SF. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. J Oral Facial Pain Headache 2014;28:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schwartz ES, Gebhart GF. Visceral Pain. Curr Top Behav Neurosci 2014;20:171–97. [DOI] [PubMed] [Google Scholar]
- [34].Stein SL. Chronic pelvic pain. Gastroenterol Clin North Am 2013;42:785–800. [DOI] [PubMed] [Google Scholar]
- [35].Treede RD. Entstehung der Schmerzchronifizierung. In: Baron R, Koppert W, Strumpf M, Willweber-Strumpf A, editors. Praktische Schmerztherapie. Heidelberg: Springer, 2011. p. 3–13. [Google Scholar]
- [36].Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008;70:1630–5. [DOI] [PubMed] [Google Scholar]
- [37].van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437–49. [DOI] [PubMed] [Google Scholar]
- [38].WHO. Cancer pain relief. Second edition. With a guide to opioid availability. Geneva: WHO, 1996. p. 36–37. [Google Scholar]
- [39].WHO ICD-11 Content Model Guide. Available at: http://www.who.int/classifications/icd/revision/contentmodel/en/. Accessed October 01, 2014 and archived: http://www.webcitation.org/6Szknpm36.


