Supplemental Digital Content is Available in the Text.
We successfully selected ligamentum flavum stem cells and differentiated ligamentum flavum–derived stem cells demonstrating that coculture and hypoxia play an important part in differentiation. Moreover, we tested genes and protein levels that are considered as special marks of nucleus pulposus (NP). Interestingly, the data suggested that ligamentum flavum–derived stem cells were successfully differentiated into NP-like cells, which produced similar extracellular matrix as NP cells.
Keywords: ligamentum flavum, mesenchymal stromal cells, nucleus pulposus cells, coculture, hypoxia, tissue engineering, intervertebral disc degenerative
Abstract
Study Design.
Human ligamentum flavum (LF)-derived stem cells (LFSCs) and nucleus pulposus cells (NPCs) were cocultured under normoxia or hypoxia.
Objective.
To isolate and identify human LFSCs and determine whether they can differentiate into NPCs when cocultured with NPCs under hypoxia.
Summary of Background Data.
Mesenchymal stem cell (MSC)-based therapies have been proposed as a biological treatment for intervertebral disc degeneration. MSCs derived from various tissues are leading candidates for cell-based therapies, but such cells have not been reported in LF.
Methods.
LF cells were isolated from patient samples and cultured using culture flasks coated with fibronectin, and their identity was confirmed using flow cytometry. The cells were induced to differentiate into osteoblasts, chondrocytes, and adipocytes, and their morphology, immunophenotype, cell proliferation capacity, cell cycle, and expression of stem cell–specific genes were compared with those of bone marrow-MSCs (BM-MSCs) derived from the same patients. NPCs and LFSCs were cocultured in 1-μm-pore-size insert transwell-culture systems under hypoxia (2% O2) or normoxia. CD24 expression was measured by flow cytometry and confocal microscopy assay. On day 14, reverse transcription-polymerase chain reaction was used for comparing the expression of chondrogenic genes (Sox-9, collagen-II, aggrecan) and novel marker genes (KRT19, CA12, FOXF1, HIF-1α) between the 2 groups.
Results.
LFSCs were obtained using the fibronectin differential-adhesion assay. The morphology of LFSCs was altered, and their immunophenotype, multilineage induction, cell proliferation capacity, cell cycle, and stem cell–specific gene expression were closely related—but not identical—to BM-MSCs, CD24 expression was highly significant in the differentiated LFSCs. RT/Real-time polymerase chain reaction revealed that compared with LFSCs grown under normoxia, hypoxia-treated LFSCs expressed higher levels of Sox-9, collagen-II, aggrecan, KRT19, CA12, and HIF-1α genes except FOXF1.
Conclusion.
Stem cells were identified in human LF, and LFSCs cocultured with NPCs were successfully differentiated into NP-like cells under hypoxia. This potentially provides new cell candidates for cell-based regenerative medicine and tissue engineering.
Level of Evidence: N/A
Intervertebral disc (IVD) degeneration is closely related to low back pain,1 which is experienced in around 40% of patients with back pain.2 IVD degeneration involves a reduction in the function and number of viable cells in the disc, mostly through cellular senescence3 and apoptosis,4,5 and this is accompanied by diminished synthesis of extracellular matrix components such as collagens and proteoglycans and a reduction of the water content. Current IVD degeneration treatments mainly involve drug application and surgery, which is limited to relieve symptoms. However, in cell-based tissue engineering, the aim is to restore IVD structure and function. In clinical trials, pain was reduced in patients treated with disc-chondrocyte transplantation,6 and in osteoarthritis treatment, implantation of autologous chondrocytes and matrix-induced autologous chondrocytes yielded favorable results.7 Researchers aim to use a similar strategy in treating IVD degeneration and have previously demonstrated the proliferative capacity and pluripotency of mesenchymal stem cells (MSCs), whose transplantation is considered a promising approach in developing new treatments of IVD degeneration. Several types of multipotent stem cells are present in adult tissues (such as in bone marrow, heart, brain, skeletal muscle, adipose tissues, synovial membrane, and menstrual blood8), and MSCs show similar characteristics such as multigenerational propagation, self-renewal, multilineage differentiation, and specific phenotypes. However, most MSCs are obtained with difficultly or invasively.
Current transforaminal endoscopic surgery and microendoscopic discectomy pass through ligamentum flavum (LF), which has been widely suggested to contain multipotent stem cells.9 However, no study has identified a definitive stem/progenitor-cell population in LF. We tested whether stem cells present in LF (LF-derived stem cells; LFSCs) can be induced into nucleus pulposus cells (NPCs) in vitro. We isolated a rare cell population from human LF and showed that they exhibit several universal stem cell properties. Previously, methods such as hypoxia treatment, use of 3-dimensional scaffolds, and coculturing were employed to induce MSCs' differentiation into NP-like cells.10–13 Here, we used hypoxia and a 3D coculture system to test whether LFSCs can differentiate into NP-like cells. Coculturing with NPCs potently induced chondrogenic differentiation, as shown by the upregulated expression of collagen-II, aggrecan, and Sox-9.14 After differentiation, human NP-specific markers were also detected, such as CD24, cytokeratin-19 (KRT19), carbonic anhydrase XII (CA12), and forkhead box F1 (FOXF1)15; all these genes were upregulated when LFSCs were cocultured with NPCs. The use of autologous stem cells is regarded to be ethically appropriate, and we suggest that LFSCs could emerge as new seed cells for treating IVD degeneration.
MATERIALS AND METHODS
Cell Culture
LF tissue (3–5 g) was obtained from patients who underwent transforaminal endoscopic surgery for lumbar degenerative disease (Table 1) (see Video, Supplemental Digital Content 1.mp4, available at: http://links.lww.com/BRS/A989). Xinqiao Hospital's Institutional Review Board and Ethics Committee approved the study protocol. Written informed consent was obtained from all patients (see Supplemental Digital Content, Figure 1, available at: http://links.lww.com/BRS/A990, which shows the harvesting procedure). Magnetic resonance imaging/computed tomography and histological techniques were used to assess the LF state (see Supplemental Digital Content 2, Figure 2, available at: http://links.lww.com/BRS/A991). The LF-explant samples were cut into 1- to 2-mm3 pieces and digested with 2% type-I collagenase (Sigma, St Louis, MO); counting the nucleated cells revealed that 1 g of tissue provided 2.5 to 5 × 106 cells and plated mononuclear cells at an initial density of 10,000 cells/cm2 for obtaining a high yield. Cells were cultured in α-minimum essential medium (α-MEM) supplemented with 16.6% fetal bovine serum and 100 U/mL penicillin-streptomycin for 12 hours at 37°C in 5% CO2; the medium was changed twice weekly. Passage 3 was transferred to culture flasks coated with fibronectin (5 μg/cm2) as described previously. LFSCs were positive for CD90, CD29, CD44, CD73, and CD105, and negative for STRO-1, CD34, CD45, and CD133, which were examined by an FACScan flow cytometer running CellQuest software (Beckman Coulter, Brea, CA) (see Supplemental Digital Content 3, Figure 3, available at: http://links.lww.com/BRS/A992) to measure the proliferation capacity and cell cycle of the LFSCs and bone marrow mesenchymal stromal cells (BM-MSCs), which was examined by Cell Counting Kit-8 and a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) (see Supplemental Digital Content, Figures 4–5, available at http://links.lww.com/BRS/A993 and http://links.lww.com/BRS/A994). The state of NP was assessed on the basis of magnetic resonance imaging, age, and disc level; the samples were obtained from patients undergoing corrective treatment of scoliosis. NP8 and BM-MSC16 samples were isolated and grown as described previously and used at Passage 3.
TABLE 1. Details of the Patients Enrolled in This Study.
| Case No. | Sex | Surgery | Disc Level | Age, yr | MRI-/CT-Evaluated Hypertrophy | Histological Morphology |
|---|---|---|---|---|---|---|
| 1 | F | TES | L3–L4 | 35 | No | No |
| 2 | M | TES | L4–L5 | 38 | No | No |
| 3 | M | MDS | L4–L5 | 41 | No | No |
| 4 | M | TES | L4–L5 | 45 | No | No |
| 5 | M | TES | L4–L5 | 69 | No | No |
| 6 | M | TES | L5–S1 | 58 | No | No |
| 7 | M | TES | L4–L5 | 54 | No | No |
| 8 | F | MDS | L4–L5 | 28 | No | No |
| 9 | M | TES | L4–L5 | 71 | No | No |
| 10 | M | MDS | L4–L5 | 63 | No | No |
MRI indicates magnetic resonance imaging; CT, computed tomography; F, female; M, male; TES, transforaminal endoscopic surgery; MDS, microendoscopic discectomy surgery.
Figure 2.
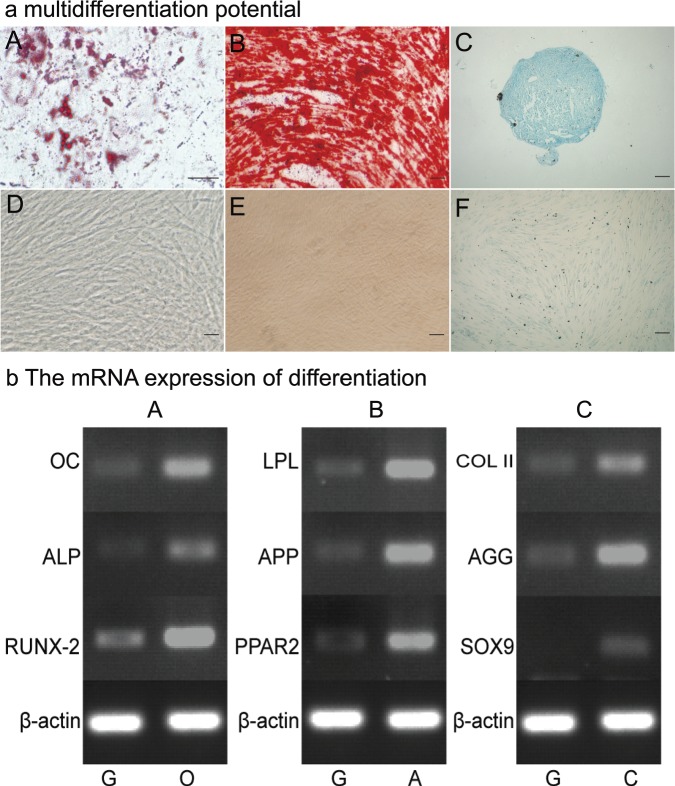
a In vitro osteogenic, adipogenic, and chondrogenic assay of ligamentum flavum–derived progenitor cells from case 10. Cells cultured in adipogenic medium formed intracellular lipid droplets that were stained with Oil Red O (A), and no adipocytes could be found in the noninduction growth medium (D). Cells cultured in osteogenic medium produced an alizarin red positive matrix (B) and no positive staining in the growth medium (E). Pellets cultured in chondrogenic induction medium (C) and the non-induction growth group (F) stained with Alcian blue. Differentiation-related genes were markedly upregulated under induction medium compared with the noninduction growth group. G, O, A, and C indicates the growth, osteogenic, adipogenic, and chondrogenic induction medium, respectively (Figure 2b).
Figure 3.
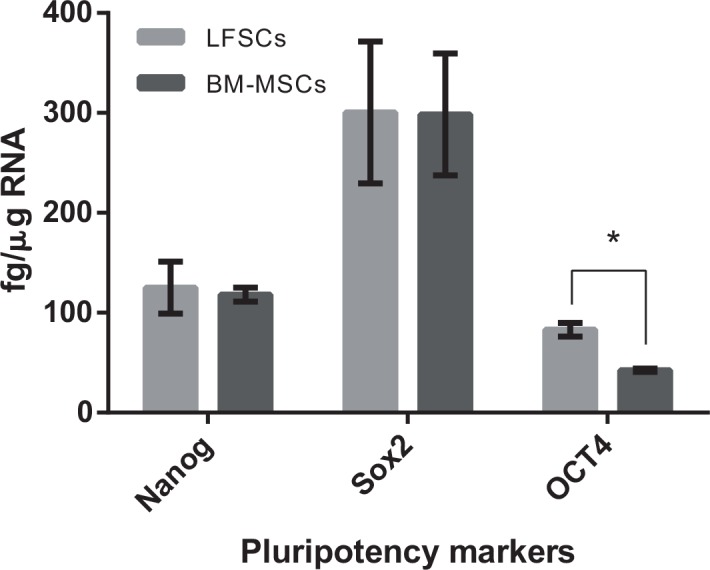
Real-time reverse transcription-polymerase chain reaction for sex-determining region Y-related HMG box 2 (Sox2), Nanog, and POU5F1 stem cell genes (OCT-4, NANOG, and SOX-2), which were expressed both in BM-MSCs and LFSCs from case 10. BM-MSCs indicates bone marrow-mesenchymal stem cells; LFSCs, ligamentum flavum–derived stem cells. *P < 0.05 compared with BM-MSCs.
Testing LFSC Multipotency
Osteogenic Differentiation
Cells were incubated for 21 days in an osteogenic medium containing 10% fetal calf serum (FCS; Hyclone Laboratories, Inc., Logan, UT), 10 nmol/L dexamethasone, 10 mmol/L β-glycerophosphate, and 0.1 mmol/L l-ascorbic acid-2-phosphate; control cells were maintained in α-MEM/10% FCS. Media were changed twice weekly. Mineral deposits were identified using alizarin-red staining.
Adipogenic Differentiation
Cells were incubated for 72 hours in an induction medium containing 10 μg/mL insulin, 1 μmol/L dexamethasone, 500 μmol/L 3-isobutyl-1-methyl xanthine, and 100 μmol/L indomethacin, and then for 24 hours in a maintenance medium (10 μg/mL insulin in α-MEM/10% FCS). This 96-hour treatment cycle was repeated 5 times and then cultures were incubated in the maintenance medium until 30 days. Control cells were maintained in DMEM/F12/10% FCS. Lipid droplets were stained with Oil Red O.
Chondrogenic Differentiation
We used the micromass-culture method; to generate micromass cultures, we placed 20-μL droplets of cell suspensions (in cell-culture medium; 2.5 × 105 cells) in the center of multiwell-plate wells and after culturing for 2 hours under high humidity, we added a chondrogenic-differentiation basal medium (dexamethasone, ascorbate, ITS-Premix (Becton-Deck-inson), sodium pyruvate, proline, and transforming growth factor (TGF)-β3) to the culture vessels and incubated them up to 21 days. The micromass cultures were fixed and stained with 1% Alcian blue.
LFSCs-NPCs Coculture
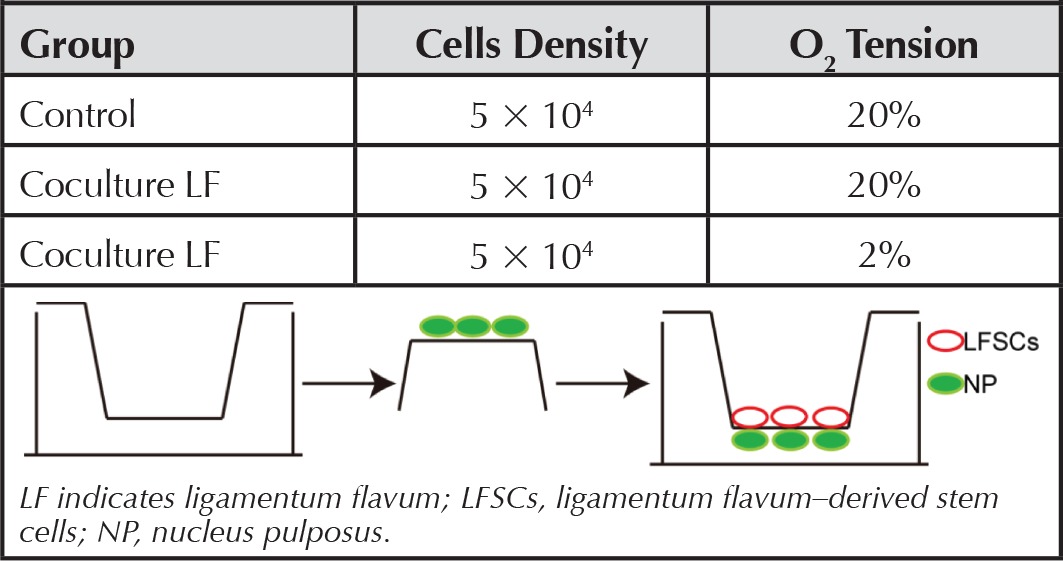
We used 6-well coculture plates consisting of 1-μm-pore-size, high-pore-density, 3D polyethylene terephthalate track-etched tissue-culture inserts. Passage-3 LFSCs and NPCs were cocultured to allow direct contact between the cells, as described.17 Briefly, 5 × 104 NPCs were cultured on the reverse side of the membrane inserts for 12 hours, and after their adhesion, the inserts were turned upright and LFSCs at the same density were cultured on the front side (Table 2), respectively, for 14 days under 2%-O2 or 20%-O2 (normal) tension. We used hypoxia to induce LFSC differentiation because the native NP-tissue environment is hypoxic, and hypoxia represents a critical environmental factor that promotes MSC differentiation.10 The control was non–cocultured LFSCs; Table 2 lists groups used in this study. After coculture, LFSC morphology changes were examined. To denote a nucleus pulposus–like differentiation phenotype, the CD24-FITC (Santa Cruz Biotechnology, Inc., Dallas, TX)–labeled cells were analyzed using an FACScan flow cytometer running CellQuest software (Beckman Coulter, Brea, CA). To detect CD24 were located in differentiated and undifferentiated LFSCs after 14 days and 21 days of coculture, which were methanol-fixed and incubated with CD24-FITC (mouse, 1:100, Santa Cruz Biotechnology, Inc., Dallas, TX) for 3 hours at 4°C, then washed, visualized with a Leica confocal microscope.
TABLE 2. Three Groups in This Research.
Real-Time/Reverse Transcription-Polymerase Chain Reaction Analysis
We used reverse transcription-polymerase chain reaction (RT-PCR) to evaluate the expression of differentiation-related genes (ALP, OC, RUNX-2; PPAR-2, APP, LPL; and AGG, COL II, Sox-9), and real-time PCR for testing stem cell–specific genes (NANOG, Sox-2, and OCT-4) and extracellular matrix or the characteristic NP markers (HIF-1α, Sox-9, collagen-II, aggrecan, KRT19, FOXF1, and CA12). After 2 weeks of coculture, LFSCs were isolated by trypsin from each group. Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Concentration of total RNA was determined with a spectrophotometer (DU-500; Beckman, Fullerton, CA) at 260-nm wavelength. Two micrograms of total RNA were used to synthesize cDNA (MBI Fermantas, Sankt Leon-Rot, Germany). For RT-PCR, 1 mL of cDNA template was used for each reaction and sequences were amplified using Taq DNA polymerase. Differentiation related gene primers were designed and synthesized as shown in Table 3. β-actin was used to normalize the reactions. For Quantitative Reverse Transcription Polymerase Chain, Reaction Analysis Reactions (20 μL) including 10 μL of Sybr Green (BIO-RAD), 2 μL of DNA, 1 μL of each primer, and 6 μL of sterile distilled water were performed in triplicates with C1000 Touch Thermal Cycler (BIO-RAD) for PCR amplification. Target gene expression was calculated by the 2−ΔΔ Ct method and normalized to β-actin and control. Differential expression was examined using 1-factor analysis of variance and was considered significantly different at P value of less than 0.05. The primers used are shown in Tables 4.
TABLE 3. Primer Sequences for Reverse Transcription-Polymerase Chain Reaction Analysis.
| Gene | Primer | Sequences | TA (°C) | Cycles |
|---|---|---|---|---|
| RUNX-2 | Forward | ACGACAACCGCACCATGGT | 59 | 30 |
| Reverse | CTGTAATCTGACTCTGTCCT | |||
| ALP | Forward | TGGAGCTTCAGA AGCTCAACACCA | 59 | 28 |
| Reverse | ATCTCGTTGTCTGAGTACCAGTCC | |||
| OC | Forward | ATGAGAGCCCTCACACTCCTC | 59 | 30 |
| Reverse | GCCGTAGAAGCGCCGATAGGC | |||
| PPAR-2 | Forward | CGAGGGCGATCTTGACAGGAA | 55 | 30 |
| Reverse | CAGGGGGGTGATGTGTTTGAAC | |||
| APP | Forward | CTGTCCAAGTCCAACAGCAA | 55 | 30 |
| Reverse | ACGTTGGCAGCTTTACGTCT | |||
| LPL | Forward | TCCGCGTGATTGCAGAGAGAG | 55 | 30 |
| Reverse | TGCTGCTTCTTTTGGCTCTGACT | |||
| AGG | Forward | TGAGGAGGGCTGGAACAAGTACC | 57 | 28 |
| Reverse | GGAGGTGGTAATTGCAGGGAACA | |||
| COL II | Forward | TTTCCCAGGTCAAGATGGTC | 57 | 30 |
| Reverse | TCACCTGGTTTTCCACCTTC | |||
| SOX-9 | Forward | TGGCCGAGATGATCCTAAAAATAA | 57 | 30 |
| Reverse | GCGCTTGGATAGGTCATGTTTGT | |||
| β-actin | Forward | GTGGGGCGCCCCAGGCACCA | 55 | 45 |
| Reverse | CTTCCTTAATGTCACGCACGATTTC |
TABLE 4. Primer Sequences for Reverse Transcription-Polymerase Chain Reaction Analysis.
| Gene | Primer | Sequences (5′–3′) |
|---|---|---|
| ACAN | Forward | TGAGGAGGGCTGGAACAAGTACC |
| Reverse | GGAGGTGGTAATTGCAGGGAACA | |
| COL2A1 | Forward | TTTCCCAGGTCAAGATGGTC |
| Reverse | TCACCTGGTTTTCCACCTTC | |
| SOX-9 | Forward | TGGCCGAGATGATCCTAAAAATAA |
| Reverse | GCGCTTGGATAGGTCATGTTTGT | |
| KRT19 | Forward | AACGGCGAGCTAGAGGTGA |
| Reverse | GGATGGTCGTGTAGTAGTGGC | |
| CA12 | Forward | CGTGCTCCTGCTGGTGATCT |
| Reverse | AGTCCACTTGGAACCGTTCACT | |
| FOXF1 | Forward | TCCCTGGAGCAGCCGTATC |
| Reverse | TGGGCGACTGCGAGTGATA | |
| HIF-1α | Forward | CCCTCACCCAACGAAAAATTAC |
| Reverse | GGGACTATTAGGTGAAC | |
| SOX-2 | Forward | CCCCCCTGTGGTTACCTCTTC |
| Reverse | GGCCGCTCTGGTAGTGCTG | |
| NANOG | Forward | ACCCCGTTTCACTGTGTTAGC |
| Reverse | GACGGCAGCCAAGGTTATTAAA | |
| OCT4 | Forward | GGCAAGCGATCAAGCAGCGAC |
| Reverse | GGGAAAGGGACCGAGGAGTAC | |
| β-actin | Forward | GTGGGGCGCCCCAGGCACCA |
| Reverse | CTTCCTTAATGTCACGCACGATTTC |
ACAN indicates aggrecan; COL2A1, collagen II α 1; KRT19, cytokeratin-19; CA12, carbonic anhydrase XII; FoxF1, forkhead box F1.
Statistical Analysis
Data are reported as means ± SD. Histological and Immunofluorescence staining data are described qualitatively. Statistical analysis was performed using an analysis of variance, and the Fisher least significant difference test was used as a post hoc test; P value of less than 0.05 was considered statistically significant.
RESULTS
Characteristic of LFSCs
Cell Morphology
After selection using the fibronectin differential-adhesion assay, LFSCs appeared mostly uniform and spread out in culture flasks and remained monoplastic immediately after seeding (Figure 1A, B), and they formed colonies in vitro more efficiently than BM-MSCs did. We also examined the morphology of 7-day–cultured NPCs and BM-MSCs (Figure 1C, D).
Figure 1.
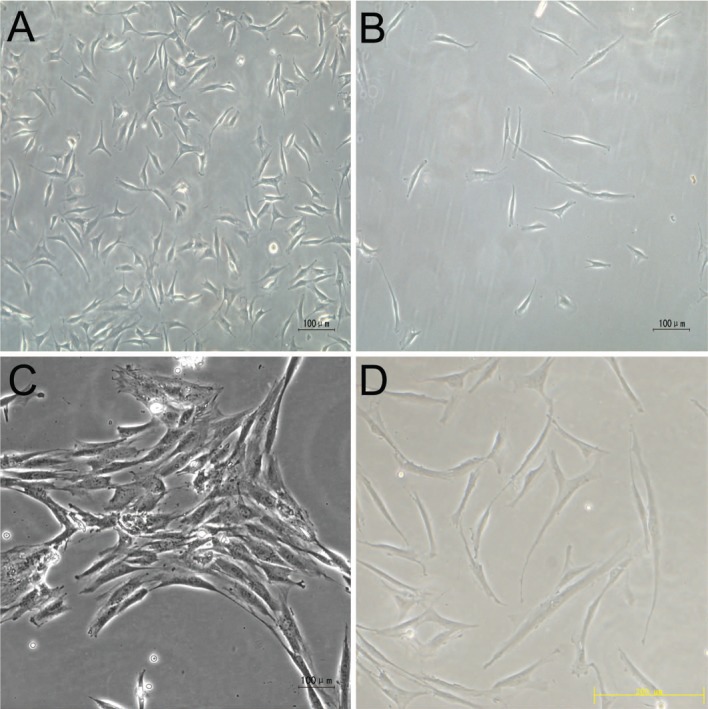
Morphology of cells derived from ligamentum flavum, nucleus pulposus, and bone marrow. Morphology of ligamentum flavum–derived cells was selected by fibronectin differential adhesion assay after immediately seeded (Figure 3B) and before selected (Figure 3A). Morphology of nucleus pulposus (Figure 3C) and bone marrow-mesenchymal stem cells (Figure 3D). A–C Bar = 100 μm, D Bar = 200 μm.
Multipotency of Putative LFSCs
Adipogenic differentiation occurred was confirmed on the basis of the presence of Oil Red O–stained intracellular lipid vacuoles (Figure 2a A); but they were not detected in the growth medium (Figure 2a D). Osteogenic differentiation was verified on the basis of the deposition of an alizarin-red-positive mineralized matrix at 3 weeks after induction (Figure 2a B); this was not observed in control cells (Figure 2a E). Chondrogenic differentiation was confirmed on the basis of Alcian blue staining, which revealed glycosaminoglycan deposition (Figure 2a C), and in the growth culture, no cells stained positively for Alcian blue (Figure 2a F).
PCR for Differentiation- and Stem Cell–Related Gene Expression
RT-PCR results showed that all induction-related genes (ALP, OC, RUNX-2; PPAR-2, APP, LPL; and AGG, COL II, Sox-9) were expressed at higher levels in the 3 induction groups than in the control group (Figure 2b A–C). Compared with BM-MSCs, LFSCs expressed higher levels of NANOG, Sox-2, and OCT-4 (Figure 3). The results were confirmed using real-time RT-PCR. LFSCs and BM-MSCs did not express significantly different levels of NANOG and Sox2 (2-tailed, t = 0.37, P = 0.68, and t = 1.83, P = 0.22, respectively); however, the OCT4 mRNA level was higher in LFSCs than in BM-MSCs (2-tailed, t = 12.8, P = 0.004). In LFSCs, sox2, NANOG, and OCT4 mRNAs were measured to be (mean ± SD) 300.40 ± 71,125 ± 226, and 83 ± 6.9 fg/mg, respectively, and in BM-MSCs, these were 298.45 ± 61,118± 7, and 42.5 ± 1.8 fg/mg, respectively.
Genes Expression After Coculture
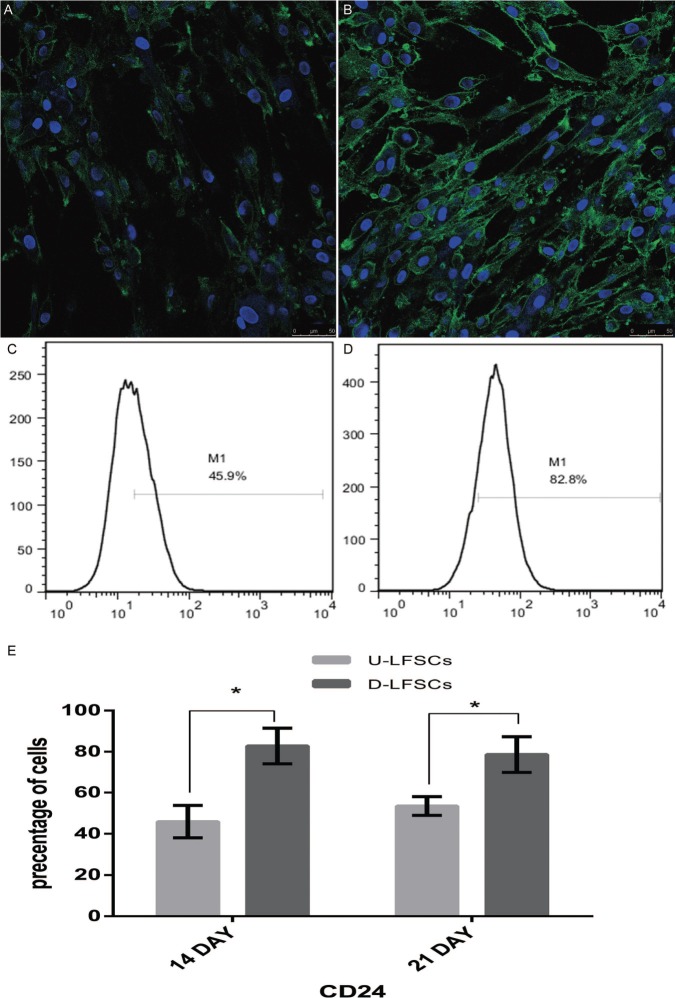
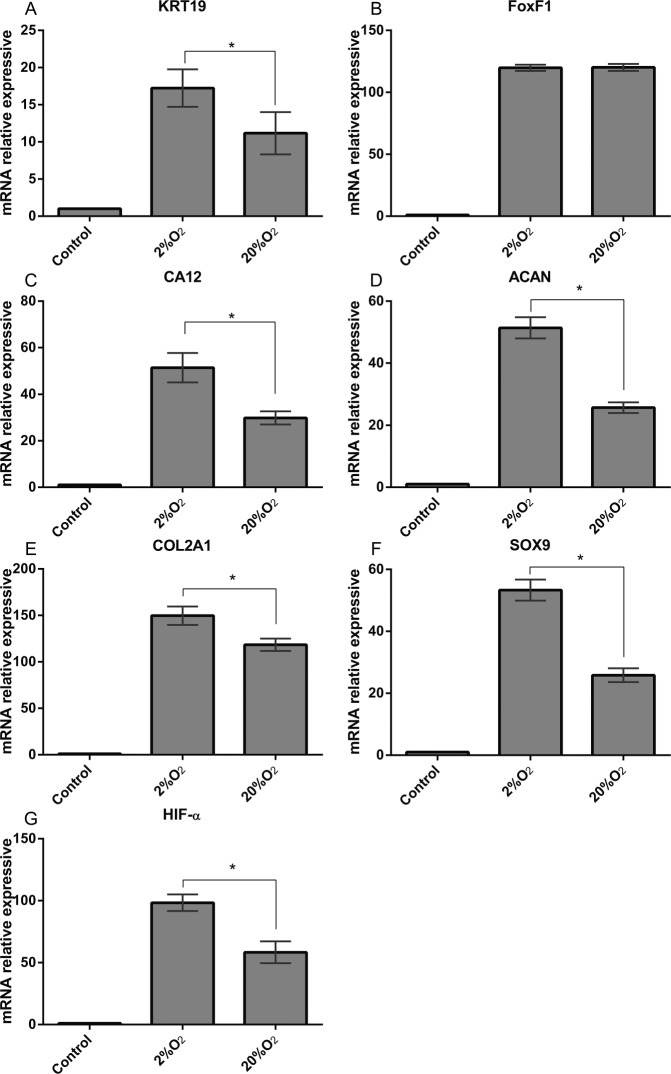
To explore the Expression of CD24 during differentiation of Human LFSCs, we examined its expression pattern by confocal microscopy assay and flow cytometry. Regarding the confocal microscopy assay, the results revealed that there were a large number of cells that expressed CD24 in the differentiated LFSCs. This indicated that LFSCs have some potential to differentiate into cells sharing features with the phenotype of the healthy NP cell. Levels of CD24 expression were consistent with the immunostaining pattern, compared with undifferentiated group (Figure 4A), which was highly significant (P < 0.001) (Figure 4B). The CD24 expression of undifferentiated group (45.9%, Figure 4C) was lower than differentiated (82.8%, Figure 4D). For quantitative analysis in differentiated LFSCs and undifferentiated, the former CD24 level was higher than the latter after 14 days and 21 days of coculture (Figure 4E) (P < 0.05). PCR analysis of NP-marker expression in differentiated LFSCs showed that after 14 days of coculture, the expression of the following genes was significantly increased relative to control (P < 0.05) in both normoxic- and hypoxic-group cells (Figure 5): KRT19 (A), CA12 (C), ACAN (D), COL2A1 (E), Sox-9 (F), and HIF-1α (G). However, under hypoxia, FOXF1 (B) was not upregulated.
Figure 4.
CD24 are expressed in the undifferentiated LFSCs (A). Differentiated LFSCs express high levels of CD24 (B). Scale bars represent 50 μm. Flow cytometric analysis of CD24 in undifferentiated (C) or differentiated LFSCs (D), the former cells indicate that subpopulation is approximately 45% and the latter is up to 82%. Differences in the expression of CD24+ were calculated after 14 days and 21 days of coculture (E). All experiments were performed in triplicate and data are shown as mean ± SD. Data in inset show statistical significance at *P < 0.05. LFSCs indicate ligamentum flavum–derived stems.
Figure 5.
Quantitative reverse transcription-polymerase chain reaction analysis of special mRNA expression in stem cells derived from ligamentum flavum (LFSCs) after coculture. Expression of (A) KRT19, (B) FOXF1, (C) CA12, (D) ACAN, (E) COL2A1, (F) SOX-9, and (G) HIF-1α was normalized to β-actin and control. Values are the mean ± SD. n = 10. *P < 0.05.
DISCUSSION
The tissue-resident adult stem/progenitor cells are unique compared with other embryonic/umbilical cord/fetal/placental-derived stem cell sources in that they are enriched in a readily accessible anatomical location. Bone marrow–derived stromal cells constitute 0.125% of the total marrow cells. However, we identified a large LFSC subpopulation and isolated these cells from LF. LF tissue is an abundant, accessible, and replenishable source of adult stem cells. LFSCs coexpress MSC markers such as CD90 and CD105, which were detected in 2.17% to 3.25% of the total cell mass. We found that 1 to 3 g of tissue is required for expanding the isolated cells. The use of ex vivo –expanded adult stem/progenitor cells and their differentiated progenies offers great promise for cell-replacement therapies with multiple applications in humans. Moreover, the chondrogenesis induction observed highlights the robustness of the described biological phenomenon and suggests that alternative cell sources, which can be harvested under minimally invasive conditions, might be considered for clinical implementation of the described paradigms.
Because LFSCs have been poorly characterized and their specific markers remain unidentified, we sought to identify stem cells in human LF and evaluate their potential to differentiate into NP-like cells in vitro. Using the fibronectin differential-adhesion assay, we obtained homogeneous LFSCs, our results provided evidence that LSCs on FN induced a higher rate of migration than on a glass surface. Migration of cells is facilitated by the adhesive interaction of cells with the substrate, which is determined by specific integrin-ligand binding affinity, level of ligands, generation of forces at binding sites. as demonstrated using flow cytometry, the proliferation rate, cell cycle, and RT-PCR. Recently, primary fibroblast-like cells derived from diverse human tissues were shown to be capable of differentiating into at least 1 mesenchymal lineage.18 Although LFSCs and BM-MSCs expressed many of the same markers, the expression patterns were not identical. LFSCs highly expressed CD90, CD29, CD44, CD73, and CD105 but not the BM-MSC-marker stro-1; although not mentioned in the International Society for Cellular Therapy's (ISCT) criteria as a suitable marker to identify multipotent cells,19 stro-1 might serve a selective negative marker for LFSCs. LFSCs could also differentiate into osteoblasts, adipocytes, and chondrocytes. To further confirmed the unique phenotype of the isolated LFSCs. We also used Immunocytochemistry staining. Specifically, all LFSCs expressed highly expressed a-SMA, type I collagen, and fibronectin, but not expressed type II collagen (Supplemental Digital Content, Figure 6 available at: http://links.lww.com/BRS/A995). LFSCs and BM-MSCs expressed the same stem cell–related genes (OCT-4, NANOG, and Sox-2), and OCT-4 amplifications were significantly detected in LFSCs. These genes are required for efficient self-renewal of embryonic stem cells, Tai et al20 have shown that OCT-4 is expressed in several adult human stem cells. The induction-related genes were markedly upregulated in the respective induction groups. We have also compared the morphology, proliferation potential, and cell cycle of LFSCs with those of BM-MSCs from the same subjects. These data suggest that LFSCs are closely related—but not identical—to BM-MSCs.
We sought to test whether LFSCs can be used to overcome a shortage of seed cells required for disc regeneration. To date, studies have applied methods commonly used to induce chondrogenesis and have reported that 2%-O2 tension promoted the differentiation of human BM-MSCs into NP-like cells.21 Here, we used the transwell system, which facilitates close cell-cell contact and is a widely accepted 3D cultivation system; in this system, hypoxia induced LFSC differentiation into NP-like cells that synthesized an extracellular matrix similar to that synthesized by NPCs. In these transwells, 2 types of cells are adhered to 2 sides of porous membranes, and this coculture method enables direct contact between the 2 types of cells through extended cytoplasmic processes. Recently, the Spine Research Interest Group made the recommendation of the following healthy NP phenotypic markers: stabilized expression of HIF-1a, GLUT-1, aggrecan/collagen II ratio greater than 20, Shh, Brachyury, KRT18/19, CA12, and CD24.22 In our study, We predicted that LFSCs have the potential to differentiate into cells sharing features with populations of NP progenitor cells or immature NP morphology that are CD24 robustly positive. This indicated that LFSCs have some potential to differentiate into cells sharing features with the phenotype of the healthy NP cell. We also examined NP-specific markers HIF-1α, Sox-9, collagen-II, aggrecan, KRT19, FOXF1, and CA12. HIF-1α is a crucial mediator of the adaptive response of cells to hypoxia, which is essential for the growth and survival of growth-plate chondrocytes in vivo,23 but HIF-1α is also robustly expressed under normoxic culture conditions.24 Sox-9 is expressed in all chondrocyte progenitors and chondrocytes,25 and this is required for chondrocyte differentiation, cartilage formation, and expression of chondrocyte-marker genes such as Col2a and aggrecan.26 The collagen-II gene is an early and abundant chondrocyte-differentiation marker. Keratins comprise the largest subfamily of intermediate-filament proteins and provide structural support to the nucleus and tensile strength to the cell.27 Lee et al28 identified KRT19 as an NP-marker gene that is highly differentially expressed, and Sakai et al29 performed comparative microarray analysis on KRT19. FOXF1 has been poorly studied, but recent findings have revealed its nonrandom association with birth defects such as spinal malformations and vertebral fusion.30 Recently, Dahia et al31 reported that hypoxia induced Sonic hedgehog signaling, which is required for IVD differentiation, but we found that FOXF1 expression did not differ between hypoxia and normoxia groups. CA12 is potentially related to NPCs' adaptation to their harsh niche;32 its identification in hypoxic NPCs might reflect the use of a similar homeostatic mechanism under hypoxia.33,34 CA12 also maintains acid-base equilibrium by transporting CO2 from cells residing in acidic environments35; thus, acid-base physiology could play a crucial role in NP-like cell survival and function in the IVD. Although no complete set of markers has yet been established for NP cells, findings of this study suggest that LFSCs may be induced to differentiate into NP-like cells as demonstrated by the presence of multiple NP-associated markers.
In summary, LFSCs are favorable cell-therapy candidates because they can be accessed readily, they proliferate rapidly, and obtaining them causes limited donor-site morbidity. Here, we identified certain LFSC-specific markers and demonstrated that LFSCs were successfully differentiated into NP-like cells, which produced an extracellular matrix similar to that of NPCs and expressed characteristic NP markers. Moreover, hypoxia enhanced the expression of these NP markers in differentiated LFSCs. Furthermore, using an injectable hydrogel to deliver cells to the injured disc might minimize the surgical risk of transplantation. To fully harness LFSCs' potential for IVD regeneration, an appropriate vehicle is required to facilitate effective LFSC delivery into the disc and additionally provide an optimized environment for tissue regeneration once the cells are in situ, and LFSCs can be expanded in vitro in sufficient quantities for tissue engineering. Although expanding LFSCs is less straightforward than originally expected, several approaches have been used for this purpose, including the development of specialized media, coculture with other cell types, identification and use of growth factors that exert proliferative effects on LFSCs, and culture on 3D scaffolds within bioreactors that simulate the in vivo environment. The optimal material used for IVD treatment must be injectable, nonantigenic, nonmigratory, volume-stable, and safe for human use.
Key Points
This study identifies the presence of stem cells in the human ligamentum flavum.
Ligamentum flavum–derived stem cell have some specific markers, such as negative of STRO-1, highly expressed a-SMA, type I collagen, and fibronectin. (Supplemental Digital Content, Figure 6, available at:)
Real-time PCR shows that the LFSCs upregulate the expression of Sox-9, type II collagen, aggrecan, KRT19, CA12, and HIF-1α but not FOXF1.
LFSCs were successfully differentiated into NP-like cells, which could provide new cell candidates for cell-based regenerative medicine and tissue engineering.
Ligamentum flavum is thought to be better candidate for cell therapy because of its ease of access, limited donor-site morbidity, and high proliferation rate.
Acknowledgments
The protocol and the consent form were approved by the institutional review broad and Ethics Committee of Xinqiao Hospital. This work was assisted in part by the Department of Orthopedics, Xinqiao Hospital.
Supplemental digital content is available for this article. Direct URL citations appearing in the printed text are provided in the HTML and PDF version of this article on the journal's Web site (www.spinejournal.com).
Footnotes
Acknowledgment date: November 4, 2014. Revision date: February 11, 2015. Acceptance date: March 2, 2015.
The manuscript submitted does not contain information about medical device(s)/drug(s).
No funds were received in support of this work.
No relevant financial activities outside the submitted work.
References
- 1.Cheung KM, Karppinen J, Chan D. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009;34:934–40. [DOI] [PubMed] [Google Scholar]
- 2.Livshits G, Popham M, Malkin I. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis 2011;70:1740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim KW, Chung HN, Ha KY. Senescence mechanisms of nucleus pulposus chondrocytes in human intervertebral discs. Spine J 2009;9:658–66. [DOI] [PubMed] [Google Scholar]
- 4.Zhao CQ, Wang LM, Jiang LS. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev 2007;6:247–61. [DOI] [PubMed] [Google Scholar]
- 5.Jiang L, Zhang X, Zheng X. Apoptosis, senescence, and autophagy in rat nucleus pulposus cells: implications for diabetic intervertebral disc degeneration. J Orthop Res 2013;31:692–702. [DOI] [PubMed] [Google Scholar]
- 6.Meisel HJ, Siodla V, Ganey T. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng 2007;24:5–21. [DOI] [PubMed] [Google Scholar]
- 7.Marlovits S, Aldrian S, Wondrasch B. Clinical and radiological outcomes 5 years after matrix-induced autologous chondrocyte implantation in patients with symptomatic, traumatic chondral defects. Am J Sports Med 2012;40:2273–80. [DOI] [PubMed] [Google Scholar]
- 8.Hu X, Zhou Y, Zheng X. Differentiation of menstrual blood-derived stem cells toward nucleus pulposus-like cells in a coculture system with nucleus pulposus cells. Spine 2014;39:754–60. [DOI] [PubMed] [Google Scholar]
- 9.Chen YT, Wei JD, Wang JP. Isolation of mesenchymal stem cells from human ligamentum flavum: implicating etiology of ligamentum flavum hypertrophy. Spine 2011;36:E1193–200. [DOI] [PubMed] [Google Scholar]
- 10.McCanless JD, Cole JA, Slack SM, et al. Modeling nucleus pulposus regeneration in vitro: mesenchymal stem cells, alginate beads, hypoxia, bone morphogenetic protein-2, and synthetic peptide B2A. Spine 2011;36:2275–85. [DOI] [PubMed] [Google Scholar]
- 11.Cheng YH, Yang SH, Su WY, et al. Thermosensitive chitosan-gelatin-glycerol phosphate hydrogels as a cell carrier for nucleus pulposus regeneration. Tissue Eng 2011;16:6953–61. [DOI] [PubMed] [Google Scholar]
- 12.Umeda M, Kushida T, Sasai K. Activation of rat nucleus pulposus cells by coculture with whole bone marrow cells collected by the perfusion method. J Orthop Res 2009;27:222–8. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Emery SE, Pei M. Coculture of synovium-derived stem cells and nucleus pulposus cells in serum-free defined medium with supplementation of transforming growth factor-beta1: a potential application of tissue-specific stem cells in disc regeneration. Spine 2009;34:1272–80. [DOI] [PubMed] [Google Scholar]
- 14.Minogue BM, Richardson SM, Zeef LA. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum 2010;62:3695–705. [DOI] [PubMed] [Google Scholar]
- 15.Rutges J, Creemers LB, Dhert W. Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: potential associations with aging and degeneration. Osteoarthritis Cartilage 2010;18:416–23. [DOI] [PubMed] [Google Scholar]
- 16.Bilic G, Zeisberger SM, Mallik AS. Comparative characterization of cultured human term amnion epithelial and mesenchymal stromal cells for application in cell therapy. Cell Transplant 2008;17:955–68. [DOI] [PubMed] [Google Scholar]
- 17.Risbud MV, Albert TJ, Guttapalli A. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine 2004;29:2627–32. [DOI] [PubMed] [Google Scholar]
- 18.Sudo K, Kanno M, Miharada K. Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells 2007;25:1610–7. [DOI] [PubMed] [Google Scholar]
- 19.Dennis JE, Carbillet JP, Caplan AI. The STRO-1+ marrow cell population is multipotential. Cells Tissues Organs 2002;170:73–82. [DOI] [PubMed] [Google Scholar]
- 20.Tai MH, Chang CC, Kiupel M. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis 2005;26:495–502. [DOI] [PubMed] [Google Scholar]
- 21.Stoyanov JV, Gantenbein-Ritter B, Bertolo A. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater 2011;21:533–47. [DOI] [PubMed] [Google Scholar]
- 22.Risbud MV, Schoepflin ZR, Mwale F. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 Annual ORS Meeting. J Orthop Res 2015;33:283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfander D, Cramer T, Schipan E. HIF-1 alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J Cell Sci 2003;116:1819–26. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal A, Guttapalli A, Narayan S. Normoxic stabilization of HIF-1 alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol 2007;293:C621–31. [DOI] [PubMed] [Google Scholar]
- 25.Taipaleenmaki H, Harkness L, Chen L. The crosstalk between transforming growth factor-ss 1 and delta like-1 mediates early chondrogenesis during embryonic endochondral ossification. Stem Cells 2012;30:304–13. [DOI] [PubMed] [Google Scholar]
- 26.Oppenheimer H, Kumar A, Meir H. Set7/9 Impacts COL2A1 expression through binding and repression of SirT1 histone deacetylation. J Bone Mineral Res 2014;29:348–60. [DOI] [PubMed] [Google Scholar]
- 27.Vaidya MM, Kanojia D. Keratins: markers of cell differentiation or regulators of cell differentiation. J Biosci 2007;32:629–34. [DOI] [PubMed] [Google Scholar]
- 28.Lee CR, Sakai D, Nakai T. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J 2007;16:2174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakai D, Nakai T, Mochida J. Differential phenotype of intervertebral disc cells microarray and immunohistochemical analysis of canine nucleus pulposus and anulus fibrosus. Spine 2009;34:1448–56. [DOI] [PubMed] [Google Scholar]
- 30.Stankiewicz P, Sen P, Bhatt SS. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet 2009;84:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahia CL, Mahoney E, Wylie C. Shh signaling from the nucleus pulposus is required for the postnatal growth and differentiation of the mouse intervertebral disc. Plos One 2012;7: e35944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishi H, Inagi R, Kato H. Hemoglobin is expressed by mesangial cells and reduces oxidant stress. J Am Soc Nephrol 2008;19:1500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benneker LM, Heini PF, Alini M. 2004 Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine 2005;30:167–73. [DOI] [PubMed] [Google Scholar]
- 34.Neidlinger-Wilke C, Mietsch A, Rinkler C. Interactions of environmental conditions and mechanical loads have influence on matrix turnover by nucleus pulposus cells. J Orthop Res 2012;30:112–21. [DOI] [PubMed] [Google Scholar]
- 35.Chiche J, Ilc K, Laferriere J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 2009;69:358–68. [DOI] [PubMed] [Google Scholar]