Article first published online 3 April 2015.
Supplemental Digital Content is Available in the Text.
Key Words: critical illness, critical care, population-based, inflammatory bowel disease, Crohn's disease, ulcerative colitis, health care utilization
Abstract
Background:
To determine predictors of intensive care unit (ICU) admission and to assess health care utilization (HCU) post-ICU admission among persons with inflammatory bowel disease (IBD).
Methods:
We matched a population-based database of Manitobans with IBD to a general population cohort on age, sex, and region of residence and linked these cohorts to a population-based ICU database. We compared the incidence rates of ICU admission among prevalent IBD cases according to HCU in the year before admission using generalized linear models adjusting for age, sex, socioeconomic status, region, and comorbidity. Among incident cases of IBD who survived their first ICU admission, we compared HCU with matched controls who survived ICU admission.
Results:
Risk factors for ICU admission from the year before admission included cumulative corticosteroid use (incidence rate ratio, 1.006 per 100 mg of prednisone; 95% confidence interval, 1.004–1.008) and IBD-related surgery (incidence rate ratio, 2.79; 95% confidence interval, 1.99–3.92). Use of immunomodulatory therapies within 1 year, or surgery for IBD beyond 1 year prior, were not associated with ICU admission. In those who used corticosteroids and immunomodulatory medications in the year before ICU admission, the use of immunomodulatory medications conferred a 30% risk reduction in ICU admission (incidence rate ratio, 0.70; 95% confidence interval, 0.50–0.97). Persons with IBD who survived ICU admission had higher HCU in the year following ICU discharge than controls.
Conclusions:
Corticosteroid use and surgery within the year are associated with ICU admission in IBD while immunomodulatory therapy is not. Surviving ICU admission is associated with high HCU in the year post-ICU discharge.
Persons with inflammatory bowel disease (IBD) have high rates of health care utilization (HCU), with an annual frequency of hospitalizations exceeding 20%.1 Admissions for critical illness, as measured by intensive care unit (ICU) admission, occur with increased frequency in the IBD population after adjusting for age, sex, socioeconomic status (SES), and comorbidity.2 However, the existing literature on critical illness among patients with IBD is sparse. We recently published one of the few such studies, exploring the incidence of ICU admission in IBD and mortality among those admitted to the ICU.2 The risk of ICU admission was higher for patients with Crohn's disease (CD) (hazard ratio, 2.31; 1.95–2.75) than ulcerative colitis (UC) (hazard ratio, 1.37; 1.13–1.65). Compared with controls admitted to ICUs, mortality rate 1 year post-ICU admission was increased in both CD (relative risk [RR], 1.42; 95% confidence interval [CI], 1.09–1.77) and UC (RR, 1.38; 95% CI, 1.01–1.78).2
Many relevant and unanswered questions remain about critical illness in IBD. For instance, it is not known what characteristics of these patients, their illness, and their treatments are associated with a higher risk of developing critical illness. Answering this question is relevant because it might illuminate modifiable risk factors. Preventing ICU admission might be associated with preventing other adverse outcomes after discharge from the ICU. Beyond mortality, it is unclear as to what other long-term consequences of critical illness in IBD occur. Although critical care itself is an economic burden, it would be important to determine if surviving an ICU admission presents an additional health care burden postdischarge. This is relevant to health care providers caring for these individuals and for policymakers planning health services.
Therefore, we aimed to determine if HCU, including hospitalizations, surgery, physician visits, and use of disease-specific medications, is associated with ICU admission. We further aimed to determine if persons with IBD had higher HCU in the year post-ICU admission compared with persons from the general population admitted to ICUs.
METHODS
Data Source
This study used anonymized population-based administrative data from the Canadian province of Manitoba for the period April 1, 1984 to March 31, 2010 that were housed in the repository at the Manitoba Centre for Health Policy. Manitoba's 1.2 million residents receive universal health care delivered by Manitoba Health, the provincial health authority.3 All health service claims include a unique personal health identification number that identifies the person who received the service. Hospitalization records include the patient's unique identifier, dates of admission and discharge, and diagnostic codes recorded using International Classification of Disease (ICD)-9 (before 2004) or ICD-10 (from 2004 onward). Physician claims include the patient's unique identifier, date of service, and 3-digit ICD-9-CM code for one diagnosis. The Drug Program Information Network captures all outpatient prescription drug dispensations, including the date, drug name, and drug identification number, for all residents.4 The population registry captures demographic information (sex, date of birth, and date of death) and dates of health care coverage.
Study Population
As previously reported, we identified Manitobans with IBD on the basis of a validated algorithm: ≥5 unique hospital or outpatient physician claims for a diagnosis of either CD (ICD-9 CM code 555) or UC (ICD-9 CM code 556).5 For persons registered with Manitoba Health for less than 2 years, the definition was ≥3 unique hospital or physician claims for a diagnosis of either CD or UC. From 2004 and onward, ICD-10 codes were used for hospital-based claims (K50 for CD and K51 for UC). Prevalent cases were identified as far back as 1984. To identify incident cases, we excluded individuals with CD or UC claims during a 5-year run-in period before the index date. Hence, the first year we chose to include cases as incident was 1989. After identifying the IBD cohort, we selected a general population cohort, matched on sex, exact year of birth, and region of residence based on 6-digit postal code (we used the first 3 digits if a full postal code match was not possible). We excluded anyone with any claims for CD or UC from this cohort. We also excluded individuals with multiple sclerosis or rheumatoid arthritis and related disorders because we also explored critical illness in those populations, separately. We obtained up to 5 controls for each case. General population controls were assigned the same index dates as their matched cases and had to be alive on the index date.
ICU Admission
Admissions to the ICU were identified using codes for special care units6 included in hospital discharge abstracts, as previously described.2 Among prevalent IBD cases, we identified ICU admissions in each year from fiscal years 2000/2001 to 2009/2010, to determine the annual incidence of ICU admission. The findings were age- and sex-standardized to the 2007 Canadian population to aid comparisons with other studies.
Previous HCU
For further analysis, we identified members of the IBD population admitted to the ICU to explore the risk of ICU admission associated with various aspects of previous HCU, including hospitalizations, physician visits, and prescription drug use. First, we compared IBD cases admitted to the ICU for the first time with IBD cases who were not admitted to an ICU. For persons who were not admitted to the ICU, we randomly assigned a pseudo-ICU date corresponding to the date of an actual ICU admission in the IBD population. We report frequency (percent) for categorical variables and mean (SD) or median (interquartile range) for continuous variables. We compared HCU between the groups using chi-square tests, Fisher's exact tests, t tests, and Wilcoxon tests as appropriate.
We then estimated the annual incidence rate of ICU admission in the IBD population according to the amount of HCU using generalized linear models. We used generalized estimating equations with an exchangeable correlation to account for repeated hospitalizations by individuals.7 We included an offset for the duration of Manitoba Health coverage. The independent variables of interest were hospitalizations (any versus none), number of physician visits (tertiles), and total cost of prescription drugs excluding IBD-specific therapy (disease-modifying and immunomodulatory therapy) (quartiles). Immunomodulatory therapy includes azathioprine, 6-mercaptopurine, methotrexate, cyclosporine, tacrolimus, infliximab, and adalimumab. We included separate terms for immunomodulatory therapy (any use versus none), and cumulative corticosteroid use in prednisone equivalents8 in the year before ICU admission.
Covariates for this model included age, sex, comorbidity status, SES, and region of residence. As described previously, we classified comorbidity status using a modified version of the Charlson Comorbidity Index.2 The Charlson Comorbidity Index is a weighted summary index of 17 chronic conditions and predicts HCU following ICU admission.9 SES was divided into quintiles based on average household income in the postal code of residence by linkage to census data. Region of residence was classified as urban (population ≥50,000) or rural.
Subsequent HCU
We also analyzed HCU among survivors of ICU admission. For comparison with our previous work regarding mortality after ICU admission, we used an incident cohort of persons with IBD for this analysis and compared them with persons admitted to the ICU from the matched control group. For these groups, we measured utilization in the year before and in the year after ICU admission using the following measures that focused on the frequency and intensity of use, including (1) proportion with any hospitalizations, (2) proportion with any ICU-containing hospitalizations, (3) number of hospital days, (4) number of outpatient physician visits, and (5) total costs of prescription drugs excluding those specific to IBD.
For multivariable analysis, we constructed general linear models of these post-ICU resource use variables, where the independent variable of interest was population (IBD versus general population matches, as well as separate analyses for persons with CD and UC). To adjust for differing follow-up time between individuals, we used an offset equal to the fraction of the year following incident ICU care that the patient survived and was resident in Manitoba. Other covariates were age, sex, SES, region of residence, comorbidity, and HCU in the year before ICU admission (any previous hospitalization, number of physician visits) as described above. The model was logistic for binary outcomes (proportion hospitalized), and zero-inflated negative binomial for the number of hospital days, and Poisson for the number of physician visits.
The University of Manitoba Health Research Ethics Board and the Manitoba Health Information Privacy Committee approved the study. Statistical analyses were conducted using SAS V9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Characteristics Associated with ICU Admission
From 1984 to 2010, we identified 8224 prevalent persons with IBD, of whom 7098 were alive during the period 2000 to 2010, which was used to evaluate the incidence of ICU admission. Characteristics of these persons are reported in Table 1. Their crude 10-year average annual incidence of ICU admission was 0.74% (95% CI, 0.67%–0.81%).2 Compared with persons with IBD who never got admitted to ICU, those admitted to ICU were more likely to be male, older at IBD diagnosis, urbanites, of the lowest SES, and to have higher comorbidity (Table 2).
TABLE 1.
Demographic Characteristics of Prevalent IBD Cases
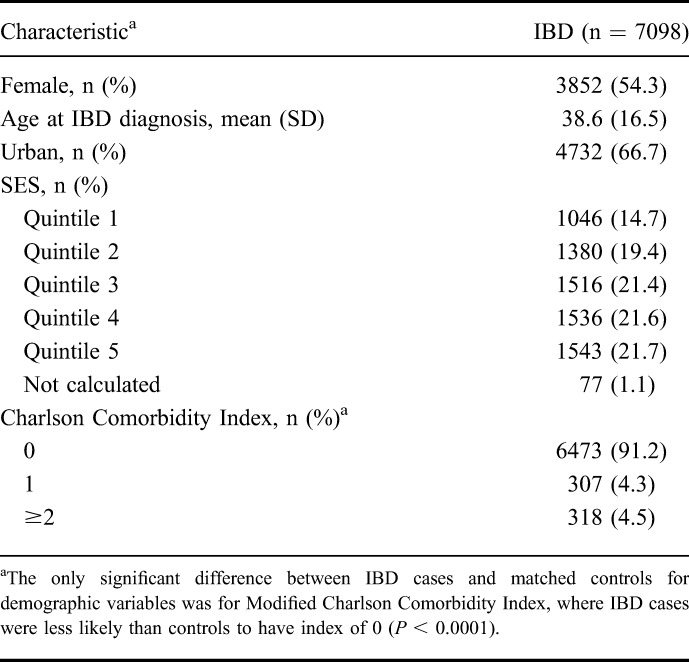
TABLE 2.
Characteristics of Persons with IBD Who were Admitted to an ICU (IBD-ICU) and Persons with IBD not Admitted to an ICU (IBD-Non-ICU) During the Period 2000 to 2010
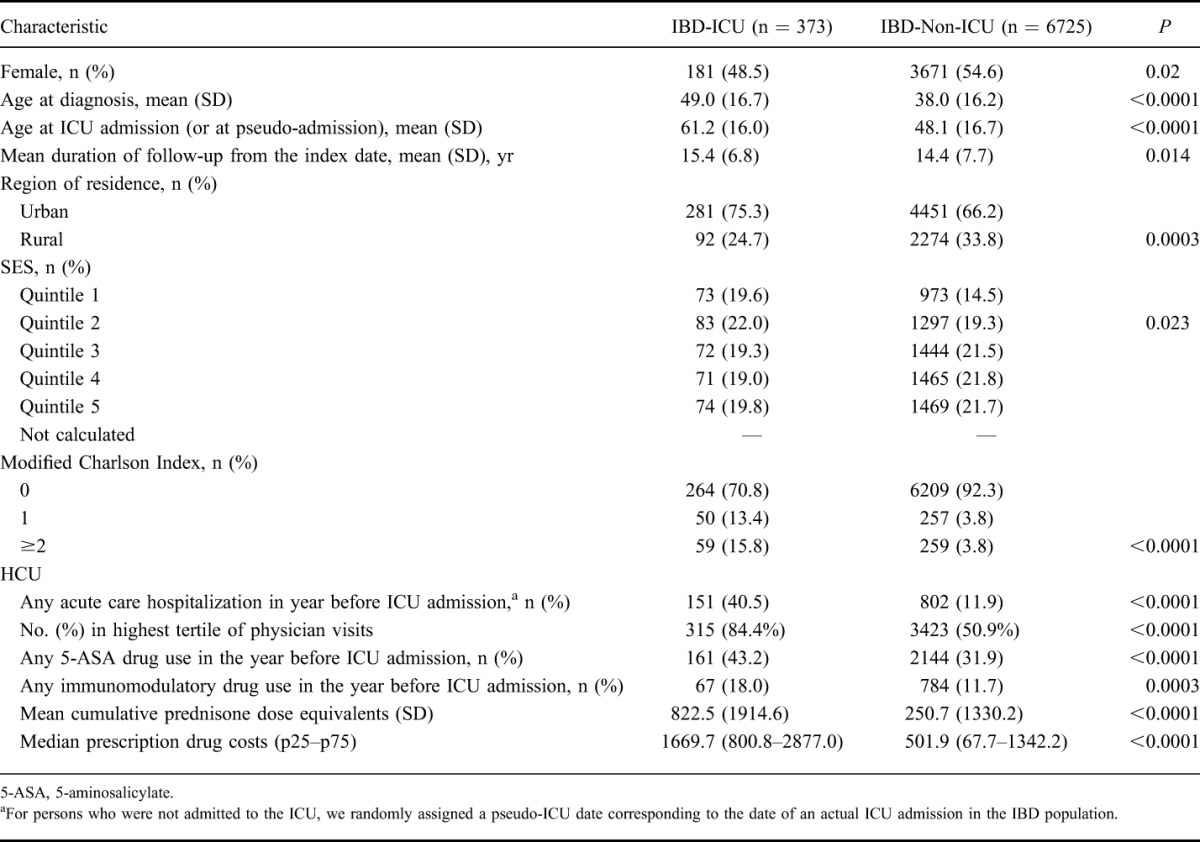
During the 1 year before ICU admission, persons with IBD admitted to an ICU, as compared with persons with IBD who were never admitted to an ICU, had more HCU in every category we assessed. Specifically, they were more likely to have been hospitalized (40.5% versus 11.9%, P < 0.0001), had more physician visits (median, 18 versus 8, P < 0.0001), had higher prescription drug costs for non-IBD drugs ($1670 versus $502, P < 0.0001), had more use of immunomodulatory therapy (18% versus 11.7%, P = 0.0003), and had more use of corticosteroids (823 mg versus 251 mg of prednisone dose equivalents, P < 0.0001) (Table 2). Within the year before admission to ICU, compared with controls, persons with IBD were more likely to have been hospitalized (40.5% versus 26%, P < 0.0001) and to have had a higher median number of physician visits (18 versus 12, P < 0.0001) (Table 2). When we repeated the analysis for CD and UC separately, we found that persons with UC admitted to an ICU compared with persons with UC not admitted to an ICU were more likely to be male, whereas in CD, there was no difference in being admitted to an ICU by sex. All other variables were similar for CD and UC (Table 3).
TABLE 3.
Characteristics of Persons with IBD Who were Admitted to an ICU (IBD-ICU) and Persons with IBD not Admitted to an ICU (IBD-Non-ICU) During the Period 2000 to 2010
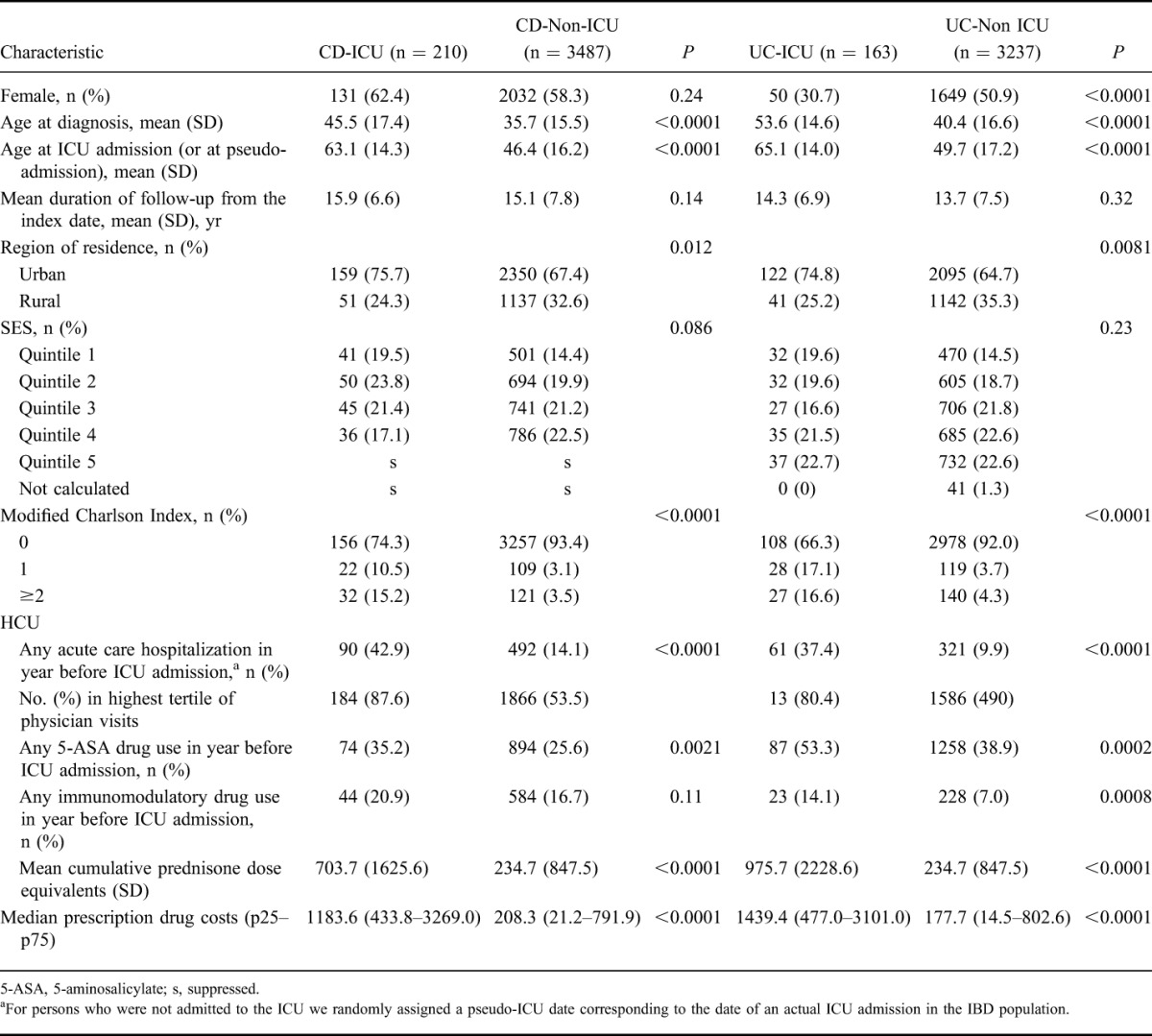
In a generalized linear model, we found several factors to be associated with ICU admission. Consistent with findings in an incident IBD cohort,2 older age, male sex, urban residence, and higher burden of comorbidity were associated with an increased risk of ICU admission. SES was not associated with ICU admission. After accounting for these factors, several measures of HCU during the previous 1 year remained independently associated with risk of ICU admission. These included hospitalization (incidence rate ratio [IRR] 1.78; 95% CI, 1.35–2.35; P < 0.0001), number of physician visits (highest tertile versus lowest IRR 2.17; 95% CI, 1.45–3.23; P = 0.0001), prescription costs for non-IBD–related drugs (highest quartile versus lowest IRR 4.31; 95% CI, 2.08–8.95; P < 0.0001), use of corticosteroids (IRR 1.006 per 100 mg prednisone; 95% CI, 1.004–1.008; P < 0.0001), and surgery for IBD (IRR 2.79; 95% CI, 1.99–3.92; P < 0.0001). Having had a surgery for IBD beyond 1 year from ICU admission was not associated with a risk for ICU admission (P = 0.99). Among those admitted to the ICU who had surgery (69, 18.5%), 31 (44.9%) underwent procedures for the small intestine while 38 (55.1%) underwent procedures for the large intestine. For CD, 36.7% of those admitted to the ICU had previous surgery, with 46.8% (22 of 77) undergoing procedures for the large intestine. For UC, 13.5% of those admitted had previous surgery, with 72.7% involving the large intestine. The use of immunomodulatory medications was not associated with ICU admission (P = 0.62).
To further evaluate the association of IBD therapy with ICU admission, we performed an analysis stratified by the use of corticosteroids in the previous year. In those who used corticosteroids and immunomodulatory medications in the year before ICU admission, the use of immunomodulatory medications conferred a 30% risk reduction in ICU admission (IRR 0.70; 95% CI, 0.50–0.97; P = 0.033). In those not using corticosteroids, immunomodulatory drug use conferred no protection against ICU admission (IRR 0.97; 95% CI, 0.75–1.27; P = 0.84).
Health Care Resource Utilization in the Year Post-ICU Admission
Of the 4580 incident cases of IBD, 293 (6.4%) were admitted to an ICU versus only 794 (3.2%) of matched, non-IBD controls. Of these, 258 IBD cases (88.1%) and 652 controls (82.1%) survived their ICU admissions; these surviving patients with IBD were less likely to be women, were younger at ICU admission, and had a lower burden of comorbidity (see Table, Supplemental Digital Content 1, http://links.lww.com/IBD/A785).
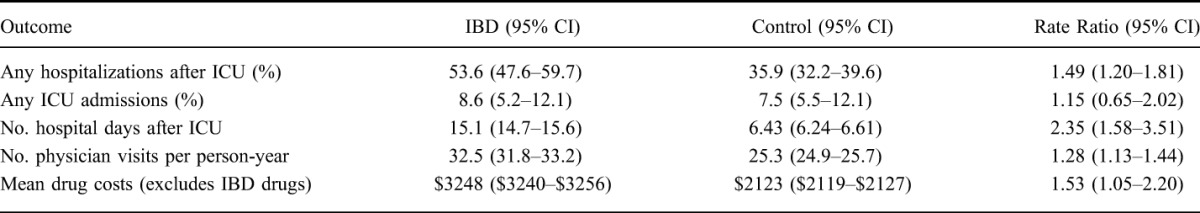
We assessed HCU variables in the 1 year post-ICU admission. After age and sex standardization, the surviving IBD cases were more likely to be hospitalized, had more hospital days and physician visits, and higher non-IBD–related prescription drug costs as compared with matched controls (Table 4). On multivariable analysis, adjusting for age, sex, region of residence, comorbidity, and hospitalizations before ICU admission, IBD cases were still more likely to be hospitalized than matched controls (RR 1.63, 95% CI, 1.19–2.22). Similarly, persons with IBD also had significantly more mean days in hospital once admitted (IRR 1.93; 95% CI, 1.42–2.63). Persons with IBD admitted to an ICU also had more post-hospital physician visits (IRR 1.24; 95% CI, 1.11–1.38).
TABLE 4.
Age and Sex-standardized Outcomes in the 1 Year Post-ICU Admission for IBD Cases and Their Matched Controls
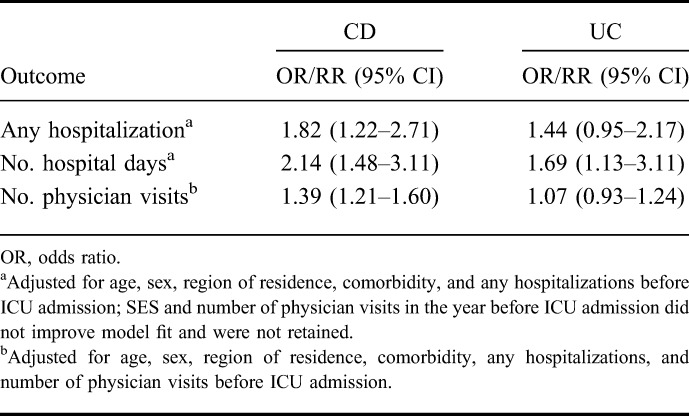
We repeated these analyses identifying CD and UC separately in the model. In the year post-ICU admission, persons with CD had a higher likelihood of being admitted to hospital compared with their matched controls than persons with UC compared with their controls (Table 5). Persons with CD also had relatively more hospital days and physician visits.
TABLE 5.
Multivariable Association of HCU After ICU Admission and CD and UC as Compared with Matched Controls
DISCUSSION
We found that persons with IBD who were admitted to an ICU were more likely to be male, older, live in urban centres, and to have lower SES and higher comorbidity scores than persons with IBD who were not admitted to an ICU. In the year before ICU admission, persons with IBD are also more likely to have used corticosteroids and more likely to have had surgery. Use of immunomodulatory medications did not confer an increased risk for ICU admission and in fact reduced the likelihood of being admitted, among those using corticosteroids. Not surprisingly, persons with IBD admitted to ICU had higher HCU in the year before admission by any parameter (hospitalizations, physician visits, prescription drug costs). We have previously reported that persons with IBD generally have higher HCU than unaffected controls.10–12 Some of this difference reflects IBD-specific utilization, but outpatient utilization for non-IBD–specific reasons is also higher. Post-ICU admission, persons with IBD had higher HCU than controls who were admitted to an ICU; this was especially marked for repeat hospitalizations and physician visits. Hence having a critical illness may have a greater impact from a quality of life, health burden, and economic burden on persons with IBD than other persons in the general population matched for age, sex, SES, and comorbidity.
These results should foster approaches that could reduce the need for critical care in persons with IBD. An obvious consideration would be to ensure that patients with IBD are cared for early, comprehensively, and expeditiously when active disease emerges. Specifically designed intervention studies are needed to determine whether such strategies can reduce ICU admissions. Furthermore, limiting the use of corticosteroids and surgery, although unavoidable in some, should be a consistent goal. This is even more an issue in CD than in UC where HCU post-ICU admission is higher compared with controls. Elsewhere, our group has reported that rates of surgery in both UC and CD have been declining.13,14 This declining rate that has been evident in other jurisdictions is welcome news for the IBD population.15,16 Although well-timed surgeries can provide long-lasting states of remission,17 surgery is associated with critical illness and also is one of the strongest risk factors for death among persons with IBD.18
Conversely, fears regarding immunomodulatory therapies, especially biological therapy, have been mostly allayed as more data on their long-term toxicities have emerged.19 These therapies should continue to supplant corticosteroids where possible. The toxicity of corticosteroids is well known and they are known to be a major contributor to infection in general and postoperatively.20,21 Nonetheless, corticosteroid use has been remarkably consistent over the past 20 years at a rate of approximately 3% to 5% per year in the same Manitoba population-based IBD cohort used in this study.22 Within the first 5 years of disease, 1 in 5 patients with IBD have used at least 3000 mg of prednisone or its equivalent, and by 10 years of disease, nearly two-thirds have been exposed to corticosteroids.22 We have shown although that concurrent use of immunomodulatory therapy with corticosteroids is associated with reduced ICU admissions, such that if corticosteroids are deemed to be necessary therapy clinicians should also be using immunomodulatory therapy. That immunomodulatory therapy use alone did not protect against ICU admission may reflect more long-standing use of these agents. To fully interpret the relationship of long-standing use of immunomodulatory therapy to serious outcomes such as ICU admission, this analysis would need to account for adequacy of drug dosing and disease phenotype, both of which are beyond the scope of this report.
Previously, we reported on the costs of managing persons with IBD and reported that biological therapy consumed a disproportionate percentage of costs.23 Because the use of biological therapy in the early years of their availability was mostly for the more acutely ill of patients, we also observed that their use was typically accompanied by more HCU at least within the first 36 months.24 Although the direct costs of biologic therapies are problematic, especially as their use is liberalized to less ill patients, the answer is not less use of biological therapies but rather reduced costs of these therapies. Our data suggest that therapies that can be less expensive, such as corticosteroids or even surgery (especially if uncomplicated), can also be associated with more expensive care downstream (ICU admission, post-ICU discharge increased HCU). This could undoubtedly impact on quality of life. For those who do require an ICU admission, they will likely have more physician visits and more recurrent hospitalizations and spend more money on medications in the year post-ICU discharge.
There are some limitations to our study. The use of administrative data does not allow for determining disease phenotype or severity. It would be helpful to know what issues related to having surgical procedures within the year before ICU admission factored into this association. However, the use of our population-based administrative dataset limits referral center bias and is comprehensive. Our dataset captures all health system contacts with physicians and for hospitalizations and surgeries, since 1995 for prescription medications, and at least 93% of all ICU admissions within the province of Manitoba. Because the epidemiology of IBD within Manitoba is consistent with that elsewhere in Canada,25 and as universal health care is available in all 10 Canadian provinces and practice approaches do not widely differ across the country, we feel our results are likely representative for all of Canada. There is a higher threshold to admit persons to an ICU in Canada than in the United States,26 so rates of ICU admission in the United States among persons with IBD may differ.
Persons with IBD are more likely to get admitted to ICU than their age, sex, and geographically matched controls within the province of Manitoba. Persons with IBD who get admitted to an ICU compared with persons with IBD who are not admitted to an ICU have higher rates of HCU in the year before ICU admission. In particular, corticosteroid use and surgery within the year before ICU admission were significant risk factors after age, sex, comorbidity, and SES adjustment. Once persons with IBD survive an ICU admission, they are more likely to incur greater HCU over the ensuing year than controls who survived an ICU admission. This includes a higher likelihood of rehospitalization.
ACKNOWLEDGMENTS
The authors acknowledge the Manitoba Centre for Health Policy for use of the Population Health Research Data Repository under project #2010 to 013 (HIPC #2010/11-15b). The results and conclusions presented are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred.
The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
R. A. Marrie, C. N. Bernstein, A. Garland, C. A. Hitchon, and C. A. Peschken designed the study and obtained funding. All authors contributed to the analysis and interpretation of the data. C. A. Bernstein, R. A. Marrie, and A. Garland drafted the manuscript. All authors revised the manuscript and approved of the final version to be published.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.ibdjournal.org).
Supported (in part) by the Canadian Institutes of Health Research and a Don Paty Career Development Award from the MS Society of Canada and the Bingham Chair in Gastroenterology. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
C. N. Bernstein has consulted to Abbvie Canada, Forest Canada, Takeda Canada, Hospira, Bristol Myers Squibb, Pfizer, Shire Canada, and Vertex Pharmaceuticals and has received research grants from Abbvie Canada and unrestricted educational grants from Abbvie Canada, Takeda Canada, and Shire Canada. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Bernstein CN, Nabalamba A. Hospitalization, surgery, and readmission rates of IBD in Canada: a population-based study. Am J Gastroenterol. 2006;101:110–118. [DOI] [PubMed] [Google Scholar]
- 2.Marrie RA, Garland A, Peschken C, et al. Increased incidence of critical illness among patients with inflammatory bowel disease: a population-based study. Clin Gastroenterol Hepatol. 2014;12:2063–2070. [DOI] [PubMed] [Google Scholar]
- 3.Health Information Management Branch. Population Report. Winnipeg, MB: Manitoba Health and Healthy Living; 2008. [Google Scholar]
- 4.Metge CJ, Black C, Peterson S, et al. Manitoba Centre for Health Policy and Evaluation. Analysis of patterns of pharmaceutical use in Manitoba; 1996. Available at: http://mchp-appserv.cpe.umanitoba.ca/reference/dpin.pdf. Accessed December 12, 2014.
- 5.Bernstein CN, Blanchard JF, Rawsthorne P, et al. Epidemiology of Crohn's disease and ulcerative colitis in a central Canadian province: a population-based study. Am J Epidemiol. 1999;149:916–924. [DOI] [PubMed] [Google Scholar]
- 6.Garland A, Yogendran M, Olafson K, et al. The accuracy of administrative data for identifying the presence and timing of admission to intensive care units in a Canadian province. Med Care. 2012;50:e1–e6. [DOI] [PubMed] [Google Scholar]
- 7.Kleinbaum DG, Klein M. Logistic Regression: A Self-Learning Text. New York, NY: Springer-Verlag; 2002:327–375. [Google Scholar]
- 8.Majumdar S, Lix L, Yogendran M, et al. Population-based trends in osteoporosis management after new initiations of long-term systemic glucocorticoids (1998–2008). J Clin Endocrinol Metab. 2012;97:1236–1242. [DOI] [PubMed] [Google Scholar]
- 9.Needham DM, Scales DC, Laupacis A, et al. A systematic review of the Charlson comorbidity index using Canadian administrative databases: a perspective on risk adjustment in critical care research. J Crit Care. 2005;20:12–19. [DOI] [PubMed] [Google Scholar]
- 10.Longobardi T, Bernstein CN. Health care resource utilization in IBD. Clin Gastroenterol Hepatol. 2006;4:731–743. [DOI] [PubMed] [Google Scholar]
- 11.Longobardi T, Bernstein CN. Utilization of health care resources by patients with IBD in Manitoba: a profile of time since diagnosis. Am J Gastroenterol. 2007;102:1683–1691. [DOI] [PubMed] [Google Scholar]
- 12.Longobardi T, Jacobs P, Bernstein CN. Utilization of health care resources by individuals with inflammatory bowel disease in the US: a profile of time since diagnosis. Am J Gastroenterol. 2004;99:650–655. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen GC, Nugent Z, Shaw S, et al. Outcomes of patients with Crohn's disease have improved from 1998 to 2008 and are associated with increased specialist care. Gastroenterology. 2011;141:90–97. [DOI] [PubMed] [Google Scholar]
- 14.Targownik LE, Nugent Z, Singh H, et al. The epidemiology of colectomy in ulcerative colitis: results from a population-based cohort. Am J Gastroenterol. 2012;107:1228–1235. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein CN, Loftus EV, Jr, Ng SC, et al. Hospitalizations and surgery in Crohn's disease. Gut. 2012;61:622–629. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein CN, Ng SC, Lakatos PL, et al. A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis. 2013;19:2001–2010. [DOI] [PubMed] [Google Scholar]
- 17.Silverstein MD, Loftus EV, Sandborn WJ, et al. Clinical course and costs of care for Crohn's disease: Markov model analysis of a population-based cohort. Gastroenterology. 1999;117:49–57. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein CN, Nugent Z, Targownik LE, et al. Predictors and risks for death in a population based study of persons with IBD in Manitoba. Gut. [published online ahead of print September 16, 2014]. doi: 10.1136/gutjnl-2014-307983. [DOI] [PubMed] [Google Scholar]
- 19.Targownik LE, Bernstein CN. Infectious and malignant complications of TNF inhibitor therapy in IBD. Am J Gastroenterol. 2013;108:1835–1842. [DOI] [PubMed] [Google Scholar]
- 20.Toruner M, Loftus EV, Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. [DOI] [PubMed] [Google Scholar]
- 21.Ferrante M, D'Hoore A, Vermeire S, et al. Corticosteroids but not infliximab increase short-term postoperative infectious complications in patients with ulcerative colitis. Inflamm Bowel Dis. 2009;15:1062–1070. [DOI] [PubMed] [Google Scholar]
- 22.Targownik LE, Singh H, Nugent Z, et al. Prevalence of and outcomes associated with corticosteroid prescription in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:622–630. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein CN, Longobardi T, Finlayson G, et al. The direct medical cost of managing IBD patients: a Canadian population based study. Inflamm Bowel Dis. 2012;18:1498–1508. [DOI] [PubMed] [Google Scholar]
- 24.Nugent Z, Blanchard JF, Bernstein CN. A population based study of health care resource use among infliximab users. Am J Gastroenterol. 2010;105:2009–2016. [DOI] [PubMed] [Google Scholar]
- 25.Rocchi A, Benchimol EI, Bernstein CN, et al. Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol. 2012;26:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Targownik LE, Gralnek IM, Dulai GS, et al. Management of acute non-variceal upper gastrointestinal hemorrhage: comparison of a U.S. and a Canadian medical center. Can J Gastroenterol. 2003;17:489–495. [DOI] [PubMed] [Google Scholar]


