Abstract:
Background:
To investigate the factors predicting the onset of major adverse cardiovascular events (MACEs) after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction (STEMI) patients.
Methods:
Two hundred forty-eight STEMI patients (61.4 ± 10.8 years, 186 men) who underwent successful primary percutaneous coronary intervention were enrolled. Patients were followed-up for 1 year. Univariate, multivariate analyses, and receiver operating characteristic curve analysis were performed to determine the factors predicting MACEs.
Results:
There were 36 patients (14.5%) who experienced MACEs in the follow-up period. Multivariate logistic regression analysis demonstrated that hemoglobin (HgB) (odds ratio = 0.972; 95% CI, 0.948–0.998; P = 0.033), neutrophil/lymphocyte ratio (NLR) (odds ratio = 1.511; 95% CI, 1.148–1.987; P = 0.003), Global Registry of Acute Coronary Event score, and postprocedure left ventricular ejection fraction (LVEF) were independent predictors of MACEs. Further subgroup analysis showed higher NLR (>8.61), Global Registry of Acute Coronary Event score (>167) and lower HgB (<131 g/L) all show superior predictive value for patients with relatively higher LVEF (>48%); moreover, the c-statistic of NLR and HgB both exceed 0.7. However, among patients with lower LVEF (≤48%), higher NLR and lower HgB lost the ability for predicting 1 year MACEs independently. In addition, abnormally higher NLR (>8) could predict 1-month MACEs efficiently.
Conclusions:
In summary, among STEMI patients, elevated NLR, decreased HgB level on admission both predicted 1-year MACEs independently, especially for those with relatively preserved LVEF (>48%). Besides, abnormally higher NLR on admission should attract their attention for short-term MACEs.
Key Indexing Terms: STEMI, Major adverse cardiovascular events, Hemoglobin, Neutrophil/lymphocyte ratio
For patients with ST-segment elevation myocardial infarction (STEMI), to achieve full, early and sustained revascularization lies at the core position of the whole therapy and largely affects the subsequent treatment. Primary percutaneous coronary intervention (pPCI) is usually the preferred reperfusion strategy among patients presenting with STEMI without contraindications, in a timely manner.1,2 However, not all the STEMI patients receiving pPCI could benefit equally from the emergency procedure. Thereby, this study was designed to investigate the clinical variables predicting major adverse cardiovascular events (MACEs) after pPCI for STEMI patients, which included cardiovascular mortality, serious ventricular arrhythmia, stroke and reinfarction.
METHODS
Inclusion and Exclusion Criteria
Patients were eligible for this study if they presented with the onset of symptoms of STEMI: persistent chest pain (>30 minutes), prolonged elevtrocardiogram (ECG) changes (including ischemic ST-segment elevation in 2 or more contiguous leads and/or depression) and significantly increased serum myocardial enzyme concentrations.3
Exclusion criteria contains non-STEMI; secondary hypertension or endocrine diseases such as thyroid dysfunction or adrenal cortical dysfunction; severe vascular heart disease; a history of chronic hepatitis or cirrhosis; severe renal insufficiency; known contraindication to statins, heparin, aspirin, clopidogrel, contrast or glycoprotein IIb/IIIa inhibitor; recent serious infections, connective tissue disease, malignancy; active severe bleeding; significant gastrointestinal or genitourinary bleeding, major surgery or trauma (<6 weeks); history of cerebrovascular attack (within 1 year) or cerebrovascular attack with a significant residual neurologic deficit; no unambiguous ST-segment in the ECG before catheterization, balloon angioplasty alone, rescue PCI, conservative treatment without PCI, previous onset of ventricular fibrillation, cardiogenic shock, untreated 3rd or advanced degree of atrioventricular block, estimated life expectancy <12 months and incomplete clinical data.
Therapy Process
To begin with filtering the data using the eligibility and exclusion criteria, a total of 248 patients underwent successful pPCI in the second Hospital of Tianjin Medical University, China were enrolled between January 2011 and October 2013. All patients were admitted to emergency room within 12 hours from onset and were diagnosed definitely with STEMI. All patients received aspirin (300 mg orally) and clopidogrel (600 or 300 mg orally) plus standard heparin (initial 10,000 IU and boost as the operation time prolonged). Postprocedure glycoprotein IIb/IIIa antagonists were administered for at least 1 day. Twelve-lead ECG was recorded on admission and 60 minutes after infarct-related artery recanalization with speed at 25 mm/s and 1.0 mV/10 mm calibration. Ultrasound scans were taken within 5 days after PCI (median 3.6 days) with a commercially available system. Echocardiographic analysis was performed by two operators who were blinded to clinical, electrocardiographic and angiographic data. All the images were obtained at a frame rate of 60 to 90 frames per second. Conventional parasternal short axis views at apical, basal and middle levels were obtained, as well as apical 2-chamber, 3-chamber and 4-chamber views. Three consecutive cardiac cycles were digitally stored for subsequent analysis during breath hold. The parameters of 2D echocardiography and Doppler were measured by standard methods described as follows: using biplane Simpson's method for measuring left ventricular volumes and ejection fraction. Volumes were expressed as indices by normalizing with body surface area.4
All the patients received 1 year follow-up after pPCI until death; in few cases, the occurrence of MACEs after index admission were recorded; besides 1 year, 1 month was another observational time point in the follow-up period. Based on these, the authors stratified the patients into MACEs group and non-MACEs group and tried to identify the independent predictors of poor prognosis. Biochemical indicators were measured: blood routine (including hemoglobin, neutrophil counts and lymphocyte counts), coagulation routine, renal function, myocardial enzyme on admission and hepatic function, lipid level, fast blood glucose on the 2nd day. GRACE score of 6 months was also calculated on admission. The authors further stratified the patients into 2 subgroups according to the postprocedure left ventricular ejection fraction (LVEF) according to the median value of LVEF.
Electrocardiogram Calculation
The sum ST-segment elevation (STE) was measured 40 ms after the J point, elevation in leads I, aVL, V1–V6 plus depression in leads II, III, aVF in case of anterior myocardial infarction (MI), whereas for nonanterior MI, STE equals leads elevation in II, III, aVF plus depression in leads V1–V4. Sum ST-segment resolution was calculated as sum of the STE on the 1st ECG minus the sum of the STE on the 2nd ECG divided by the STE on the 1st ECG and expressed as a percentage.5
Statistical Strategy
Continuous data were presented as mean ± standard deviation or medians with interquartile ranges, whereas categorical data as percentage, unless otherwise denoted. T test, Wilcoxon rank-sum test and χ2 test were used for qualitative and quantitative variables to determine whether there is significant difference between MACEs and non-MACEs. Additionally, the authors performed the receiver operating characteristic (ROC) analysis to identify the appropriate cutoff value of some effective predictive indicators.6 The cutoff point they acquired lied at the maximum sum of sensitivity and specificity. The variables selected to be tested in the multivariate logistic regression analysis were those with P < 0.1 in the univariate models. The adjusted odds ratio and 95% confidence interval were calculated. The ROC curves were conducted with the Medcalc 12.3.0.0. All statistical tests were 2-tailed, performed using SPSS 17.0 (SPSS, Inc, Chicago, IL), and a P-value <0.05 was considered statistically significant.
RESULTS
Baseline Characteristics of Patients From Individual Groups
Of the 248 enrolled patients, 36 (14.5%) suffered MACEs in the 1-year follow-up, whereas 5 of whom died. Among the MACEs group, 14 patients reached end point within 1 month. One hundred twenty- patients presented with an LVEF of exceeding 48% (54.2% ± 3.9%), whereas others with relatively lower LVEF (42.0% ± 4.9%). Basic clinical characteristics, laboratory examinations, electrocardiogram results, angiographic and procedural characteristics are depicted in Table 1.
TABLE 1.
Basic clinical characteristics, laboratory examinations, electrocardiogram results, angiographic and procedural characteristics
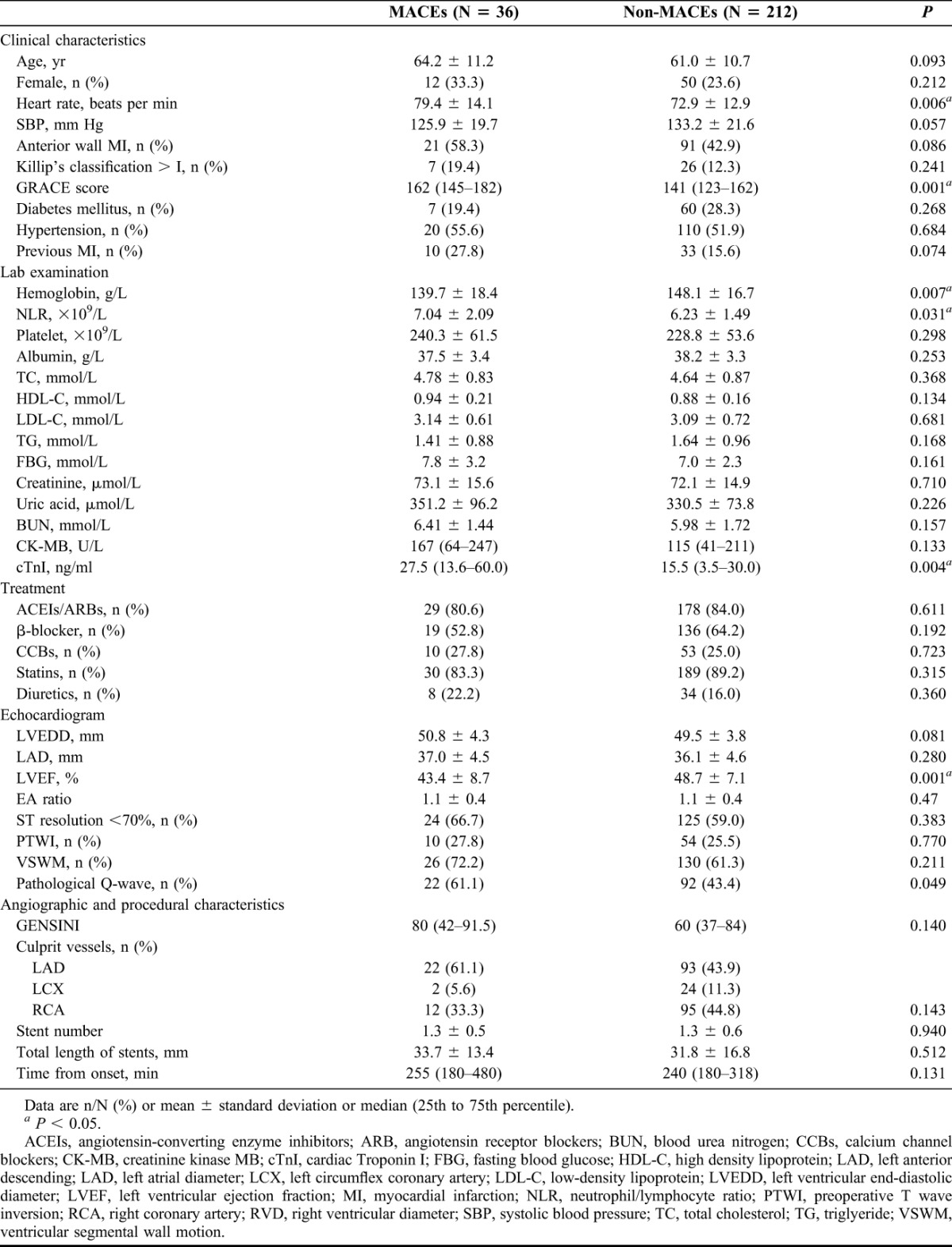
Significant differences could be found between 2 groups in hemoglobin (HgB) (139.7 ± 18.4 versus 148.1 ± 16.7, P = 0.007), neutrophil/lymphocyte ratio (NLR) (7.04 ± 2.09 versus 6.23 ± 1.49, P = 0.031), LVEF (43.4% ± 8.7% versus 48.7% ± 7.1%, P = 0.001) and cardiac Troponin I (cTnI) (P = 0.004). There was an ascending trend of left ventricular end diastolic diameter in MACEs group compared with the non-MACEs group (50.8 ± 4.3 versus 49.5 ± 3.8, P = 0.081). Previous PCI and anterior wall MI was more often present in the MACEs group as compared with the other (P = 0.074 and 0.086, respectively). Although not all the components of Global Registry of Acute Coronary Events (GRACE) score system perform their value in prognostic evaluation, GRACE score was selected to represent the comprehensive stress state, whose efficiency has been validated and compared with thrombolysis in myocardial infarction (TIMI) STEMI scores,7,8 consistent with their expectation, patients who suffered from MACEs usually coincided with higher GRACE score (P = 0.001).
Multivariate Logistic Regression Analysis of Predictor of MACEs
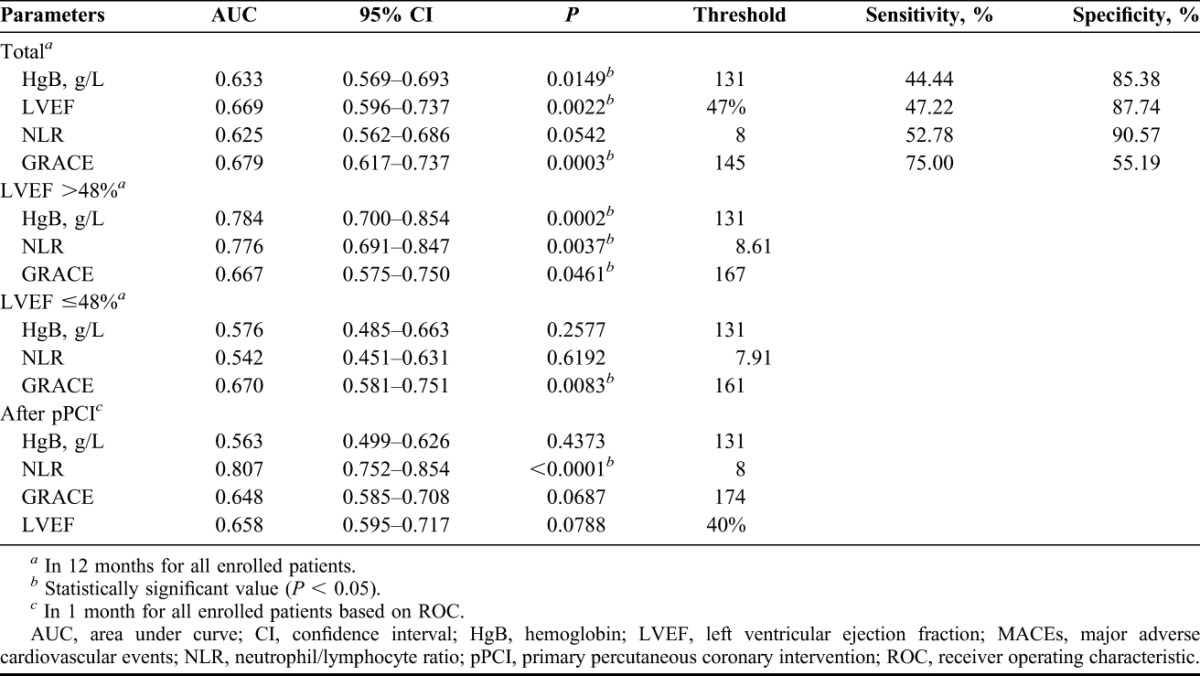
Through the multivariate regression analysis, high GRACE score and NLR were independent predictors of MACEs within 1 year after pPCI, along with low HgB concentration on admission and low postprocedure LVEF (Table 2). The authors conducted the ROC analysis but failed to find any area under curve (AUC) for 1-year MACEs exceeding 0.7 (Figures 1 and 2; Table 3). Further subgroup analysis showed among 121 patients with preserved LVEF (>48%), 13 suffered MACEs within 1 year, HgB, NLR and GRACE score all show their superior predictive value, although only the AUC of HgB and NLR exceed 0.7 (Table 3). However, among patients with lower LVEF (≤48%), neither the forecasting accuracy of HgB nor NLR maintained as the other subgroup presented (c-statistic = 0.576 and 0.542, respectively; Table 3). Finally, when they focused on the 1-month MACEs among total patients, only NLR on admission brought significant predictive value for short-term prognosis after pPCI (AUC = 0.807, P < 0.0001).
TABLE 2.
Multivariate logistic regression analysis of predictor of MACEs
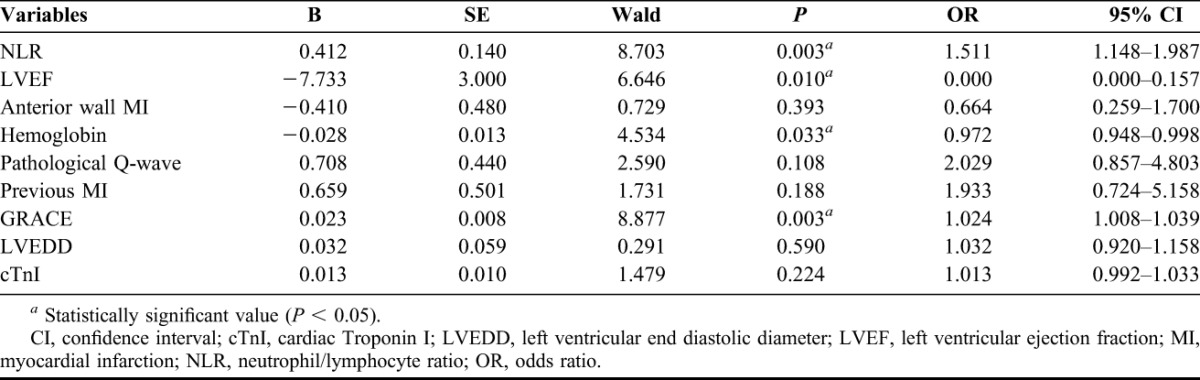
FIGURE 1.
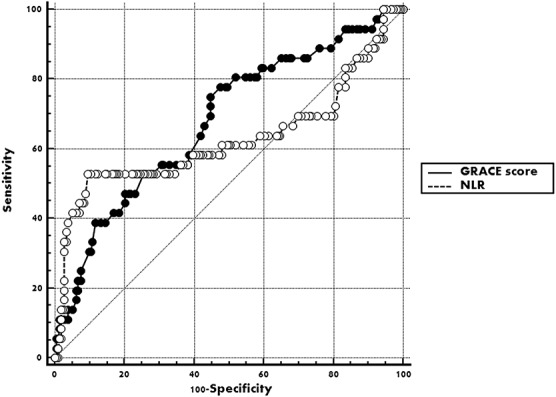
Receiver operating characteristic curve of GRACE score and NLR for predicting 12 months MACEs after pPCI among STEMI patients.
FIGURE 2.
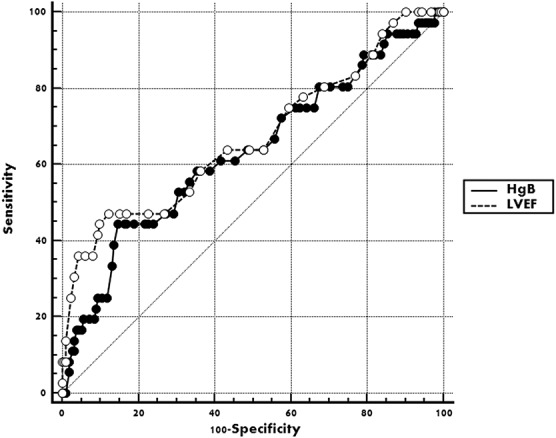
Receiver operating characteristic curve of HgB and LVEF for predicting 12 months MACEs after pPCI among STEMI patients. HgB, hemoglobin; LVEF, left ventricular ejection fraction; MACEs, major adverse cardiovascular events and demonstrated pump failure; NLR, neutrophil/lymphocyte ratio.
TABLE 3.
Univariate predictors of MACEs
DISCUSSION
The results showed relatively anemic state or excessive inflammatory response both predict 1-year MACEs independently, especially for those with relatively preserved LVEF (>48%). In other words, the patients with higher postprocedure LVEF were usually likely to be neglected of poor prognosis, whereas based on the conclusion, HgB and NLR on admission may provide extra risk stratification for the very population. Additionally, higher NLR prompt 1-month MACEs intensively.
Emergency PCI as soon as possible is the most effective therapy in dealing with STEMI patients. As the most straightforward and valid coronary recanalization, pPCI could quickly recover the coronary artery blood flow, save the moribund myocardium, reduce infarction size and improve the prognosis. However, a successful pPCI could not ensure the nonoccurrence of possible adverse cardiovascular events. The necrotic myocardium in corresponding region participate in the progressive expansion and degeneration of left ventricle, along with the left vulnerable endothelium, part of the patients may suffer a serious of severe cardiovascular events during the follow-up period.
The GRACE risk scores have been considered to have higher predictive efficiency of MACEs in patients with cardiovascular disease.9 However, other factors may also simultaneous influence the pronosis, apart from the stress state reflected by GRACE score.
HgB concentration was not only powerful independent determinant of poor prognosis for long-term10 and short-term11 observation but also help risk stratification for acute coronary syndrome patients.12 Although the exact mechanism is not clear, the authors suppose the increased sympathetic release and the activation of renin-angiotension-aldosteron system induced by severe anemia may affect the hemodynamics, aggravate the impaired cardiac function.13 Additionally, as a simple index in blood routine, HgB could be expeditiously measured when the patients were sent to emergency department, which ensure its feasibility in clinical application of prognosis evaluation. The relatively poor heart function in the observation objects may be responsible for the lower cut-point of HgB when compared with other research.14 A recent report indicated, for STEMI patients, the longer delay before revascularization, the lower admission HgB would be, which validate the relationship between admission HgB level and prognosis from another perspective.15 Lower HgB admission was revealed that associated with longer symptom duration and earlier inflammation response, as well, closely correlated with worse prognosis and elevated risk of MACEs during long-term follow-up.16 Another potential explanation may be the relationship of lower HgB concentration and higher likelihood of infection, which was considered the most important inducement of pump failure. Therefore, in patients with severe anemia listed for PCI, this level of Hb should be considered as a precaution.
Abnormally elevated NLR on admission were proved to be correlated with long-term prognosis in STEMI patients who underwent pPCI.17 Results from several previous studies confirmed favorable value of preprocedure NLR in predicting adverse outcomes including pump failure, cardiogenic shock, severe ventricular arrythmias,18 stent thrombosis,19 angiographic thrombus burden20 and long-term mortality21 among STEMI patients, no matter whether pPCI was performed, and even independent of GRACE risk score in patients with STEMI.22 Furthermore, Horne et al23 emphasized the predominant predictive value of NLR in predicting death/MI among a serious of hematological indices containing of neutrophil, lymphocyte and NLR. NLR was also proved significantly correlated with angiographic severity of acute coronary syndrome24 and GRACE risk score in STEMI patients.25 Besides, among coronary artery disease patients, higher NLR usually indicate more vulnerable plaque components, which is closely associated with severe thrombus-related events.26
The conclusion further authenticate that although successful pPCI were proceeded, the prognosis could also be influenced by a serious of factors such as stress state when STEMI onset (GRACE), whether excessive inflammation or severe anemia exists. Patients in different risk stratification should be assigned with respectively reasonable medical resources. In the future daily clinical work, the results above may help them to screen patients with high risk, especially for those who presented relatively considerable LVEF after pPCI. Additionally, differentiating high-risk patients in short term from those with better prognosis may also depend on NLR on admission.
CONCLUSIONS
In summary, regarding STEMI patients, besides GRACE risk score, elevated NLR, decreased HgB level on admission both predicted 1-year MACEs independently, especially for those with relatively preserved LVEF after pPCI (>48%). As a strong inflammatory indicator, NLR could predict 1-month MACEs with high efficacy. In the future, efforts are still warranted to identify these high-risk patients to manage them with target as early as possible to obtain further rehabilitation.
Limitations
Sample size from the single center was not large enough to determine more accurate predicting threshold for both gender respectively, especially for HgB, apart from this, the LVEF decline degree could not be obtained from all the patients. Additionally, NLR on different time point during hospitalization should be analyzed separately and precisely, maybe, in their further prospective cohort study. Finally, the follow-up period could be elongated if possible to explore the predicting of long-term prognosis.
Footnotes
The authors have no financial or other conflicts of interest to disclose.
E.Z. and Z.L. contributed equally to this study.
REFERENCES
- 1.Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC), The European Association for Cardio-Thoracic Surgery (EACTS), European Association for Percutaneous Cardiovascular Interventions (EAPCI), Wijns W, Kolh P, Danchin N, et al. Guidelines on myocardial revascularization. Eur Heart J 2010;31:2501–55. [DOI] [PubMed] [Google Scholar]
- 2.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). Circulation 2004;110:588–636. [DOI] [PubMed] [Google Scholar]
- 3.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J 2007;28:2525–38. [DOI] [PubMed] [Google Scholar]
- 4.Cheitlin MD, Alpert JS, Armstrong WF, et al. ACC/AHA Guidelines for the Clinical Application of Echocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. Circulation 1997;95:1686–744. [DOI] [PubMed] [Google Scholar]
- 5.Sorajja P, Gersh BJ, Costantini C, et al. Combined prognostic utility of ST-segment recovery and myocardial blush after primary percutaneous coronary intervention in acute myocardial infarction. Eur Heart J 2005;26:667–74. [DOI] [PubMed] [Google Scholar]
- 6.Zou KH, O'Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation 2007;115:654–7. [DOI] [PubMed] [Google Scholar]
- 7.Sun YH, Wang GL, Fu YY, et al. Prognostic value of point of care B-type natriuretic peptide testing and GRACE score in patients with acute coronary syndrome. Zhonghua Xin Xue Guan Bing Za Zhi 2009;37:716–20. [PubMed] [Google Scholar]
- 8.Aragam KG, Tamhane UU, Kline-Rogers E, et al. Does simplicity compromise accuracy in ACS risk prediction? A retrospective analysis of the TIMI and GRACE risk scores. PLoS One 2009;4:e7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang EW, Wong CK, Herbison P. Global registry of acute coronary events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J 2007;153:29–35. [DOI] [PubMed] [Google Scholar]
- 10.Tomaszuk-Kazberuk A, Bolińska S, Młodawska E, et al. Does admission anaemia still predict mortality 6 years after myocardial infarction? Kardiol Pol 2014;72:488–93. [DOI] [PubMed] [Google Scholar]
- 11.Dündar C, Oduncu V, Erkol A, et al. In-hospital prognostic value of hemoglobin levels on admission in patients with acute ST segment elevation myocardial infarction undergoing primary angioplasty. Clin Res Cardiol 2012;101:37–44. [DOI] [PubMed] [Google Scholar]
- 12.Archbold RA, Balami D, Al-Hajiri A, et al. Hemoglobin concentration is an independent determinant of heart failure in acute coronary syndromes: cohort analysis of 2310 patients. Am Heart J 2006;152:1091–5. [DOI] [PubMed] [Google Scholar]
- 13.Anand IS, Chandrashekhar Y, Ferrari R, et al. Pathogenesis of oedema in chronic severe anaemia: studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br Heart J 1993;70:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giraldez RR, Sabatine MS, Morrow DA, et al. Baseline hemoglobin concentration and creatinine clearance composite laboratory index improves risk stratification in ST-elevation myocardial infarction. Am Heart J 2009;157:517–24. [DOI] [PubMed] [Google Scholar]
- 15.Shacham Y, Leshem-Rubinow E, Ben-Assa E, et al. Lower admission hemoglobin levels are associated with longer symptom duration in acute ST-elevationmyocardial infarction. Clin Cardiol 2014;37:73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leshem-Rubinow E, Steinvil A, Rogowski O, et al. Hemoglobin nonrecovery following acute myocardial infarction is a biomarker of poor outcome: a retrospective database study. Int J Cardiol 2013;169:349–53. [DOI] [PubMed] [Google Scholar]
- 17.Sen N, Afsar B, Ozcan F, et al. The neutrophil to lymphocyte ratio was associated with impaired myocardial perfusion and long term adverse outcome in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Atherosclerosis 2013;228:203–10. [DOI] [PubMed] [Google Scholar]
- 18.Ghaffari S, Nadiri M, Pourafkari L, et al. The predictive value of total neutrophil count and Neutrophil Lymphocyte Ratio in Predicting In-hospital mortality and complications after STEMI. J Cardiovasc Thorac Res 2014;6:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayca B, Akin F, Celik O, et al. Neutrophil to lymphocyte ratio is related to stent thrombosis and high mortality in patients with acute myocardial infarction. Angiology 2014. pii: 0003319714542997. Epub ahead of print, July 13, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Erkol A, Oduncu V, Turan B, et al. Neutrophil to lymphocyte ratio in acute ST-segment elevation myocardial infarction. Am J Med Sci 2014;348:37–42. [DOI] [PubMed] [Google Scholar]
- 21.Nunez J, Nunez E, Bodi V, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol 2008;101:747–52. [DOI] [PubMed] [Google Scholar]
- 22.Oncel RC, Ucar M, Karakas MS, et al. Relation of neutrophil-to-lymphocyte ratio with GRACE risk score to in-hospital cardiac events in patients with ST-segment elevated myocardial infarction. Clin Appl Thromb Hemost 2015;21:383–8. [DOI] [PubMed] [Google Scholar]
- 23.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol 2005;45:1638–43. [DOI] [PubMed] [Google Scholar]
- 24.Altun B, Turkon H, Tasolar H, et al. The relationship between high-sensitive troponin T, neutrophil lymphocyte ratio and SYNTAX Score. Scand J Clin Lab Invest 2014;74:108–15. [DOI] [PubMed] [Google Scholar]
- 25.Acet H, Ertas F, Akil MA, et al. Relationship between hematologic indices and global registry of acute coronary events risk score in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost 2014. Epub ahead of print, May 8, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Choi YH, Hong YJ, Ahn Y, et al. Relationship between neutrophil to lymphocyte ratio and plaque components in patients with coronary artery disease: virtual histology intravascular ultrasound analysis. J Korean Med Sci 2014;29:950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


