Article first published online 3 April 2015.
Key Words: infliximab, trough level, fecal calprotectin
Abstract
Background:
Infliximab is an effective therapy for inflammatory bowel disease (IBD). However, more than 50% of patients lose response. Empiric dose intensification is not effective for all patients because not all patients have objective disease activity or subtherapeutic drug level. The aim was to determine how an objective marker of disease activity or therapeutic drug monitoring affects clinical decisions regarding maintenance infliximab therapy in outpatients with IBD.
Methods:
Consecutive patients with IBD on maintenance infliximab therapy were invited to participate by providing preinfusion stool and blood samples. Fecal calprotectin (FCP) and infliximab trough levels (ITLs) were measured by enzyme linked immunosorbent assay. Three decisions were compared: (1) actual clinical decision, (2) algorithmic FCP or ITL decisions, and (3) expert panel decision based on (a) clinical data, (b) clinical data plus FCP, and (c) clinical data plus FCP plus ITL. In secondary analysis, Receiver-operating curves were used to assess the ability of FCP and ITL in predicting clinical disease activity or remission.
Results:
A total of 36 sets of blood and stool were available for analysis; median FCP 191.5 μg/g, median ITLs 7.3 μg/mL. The actual clinical decision differed from the hypothetical decision in 47.2% (FCP algorithm); 69.4% (ITL algorithm); 25.0% (expert panel clinical decision); 44.4% (expert panel clinical plus FCP); 58.3% (expert panel clinical plus FCP plus ITL) cases. FCP predicted clinical relapse (area under the curve [AUC] = 0.417; 95% confidence interval [CI], 0.197–0.641) and subtherapeutic ITL (AUC = 0.774; 95% CI, 0.536–1.000). ITL predicted clinical remission (AUC = 0.498; 95% CI, 0.254–0.742) and objective remission (AUC = 0.773; 95% CI, 0.622–0.924).
Conclusions:
Using FCP and ITLs in addition to clinical data results in an increased number of decisions to optimize management in outpatients with IBD on stable maintenance infliximab therapy.
Anti-tumor necrosis factor–α monoclonal antibodies such as infliximab are effective in inducing and maintaining remission in patients with Crohn's disease (CD)1,2 and ulcerative colitis (UC).3 However, an estimated 40% of patients on infliximab therapy eventually lose response at a rate of 13% per patient-year of treatment.4 Empiric infliximab dose intensification is often the initial management strategy after loss of response, with secondary switch to another anti-tumor necrosis factor medication, and if these strategies fail, a change in the class of medication or proceeding to surgery.5 Although loss of response is frequently manifested with a return of clinical symptoms, the reliability of symptoms to predict active disease is not robust. In contrast, the biomarker fecal calprotectin (FCP) has been shown to accurately reflect the presence of intestinal inflammation and thus active IBD.6–9
Serum infliximab trough levels (ITLs) have been found to be associated with clinical response,10 and algorithms using serum ITLs have been shown to be clinically efficacious and cost effective.11–13 Nevertheless, a subset of patients demonstrate clinical symptoms while ITLs are therapeutic and require further investigation to assess the presence or absence of active disease. The addition of objective markers of disease activity (such as FCP) to therapeutic drug monitoring, and using this combination during maintenance infliximab therapy, may add to the clinical utility of therapeutic drug monitoring. However, FCP and ITL are not easily available to many clinicians who manage patients with IBD, and it is currently unclear if these measurements would best be used in algorithms or incorporated into clinical assessments and whether they would even impact clinical decision-making.
In this study, we prospectively evaluated how FCP and ITL used in algorithms or used in addition to clinical data could affect clinical decision-making in outpatients with IBD receiving maintenance infliximab therapy. We also assessed the ability of FCP in predicting clinical disease activity or subtherapeutic ITL and of ITL in predicting clinical versus objective disease remission.
METHODS
Study Design and Setting
This study was conducted from the University of Alberta IBD infliximab infusion clinic, Edmonton, Canada, in 2013. This study was designed to include prospective recruitment of patients and collection of samples, as well as retrospective chart review.
Participants
Outpatients with IBD receiving maintenance infliximab therapy were prospectively and randomly invited to participate. Patients provided (1) a morning fecal sample on the day of infliximab infusion for FCP determination and (2) a blood sample drawn immediately before the infliximab infusion for ITL assessment. A chart review determined clinical response to infliximab.
Inclusion criteria included: (1) an endoscopic and/or radiologically confirmed diagnosis of CD or UC, (2) a primary response to the induction regimen of infliximab (5 mg/kg at wk 0, 2, and 6) defined by a decrease in the modified Harvey–Bradshaw Score (mHBI; Harvey–Bradshaw index less abdominal examination) by >3 points14 or a decrease in the partial Mayo (pMayo; Mayo score less endoscopic subscore) score by >2 points,15 (3) receiving stable maintenance infliximab infusions, defined as infusions subsequent to the third induction dose or subsequent to the first infusion after any dose or interval change, (4) both a stool sample and an ITL from the same infusion, and (5) complete records on infliximab infusions since initial induction.
Data Sources and Definitions
Baseline Demographics
Demographic data recorded included gender, age, disease type, infusion premedication with methylprednisolone, and use of concomitant medications (azathioprine, 6-mercaptopurine, and methotrexate). Infliximab treatment characteristics included (1) infliximab dose in milligrams per kilograms, and (2) infliximab dosing interval in weeks.
FCP Level
FCP (MRP8/14) was measured by enzyme linked immunosorbent assay using a monoclonal antihuman calprotectin-coated plate (Immunodiagnostik, Bensheim, Germany). Stool samples were stored at −20°C before analysis. The thawed stool was mixed and 15 g weighed, diluted with buffer, and analyzed in duplicate. The lower limit of detection of this method is 5 μg/g stool. The manufacturer suggested >250 μg/g as positive test, a cutoff shown to predict endoscopic disease activity.16 The variability of the assay was 9.5% at 20 μg/g and 7.2% at 100 μg/g.
Infliximab Trough Level
ITLs were determined on serum samples collected within 30 minutes before the infusion. Serum was separated and frozen within 4 hours of collection. An enzyme linked immunosorbent assay method (Immunodiagnostik) was used to quantitate free infliximab. Results are reported down to 0.4 μg/mL with an interassay precision of 8% at 1.8 μg/mL, 9% at 8.6 μg/mL, and 20% at 12.9 μg/mL.
Disease Activity
Clinical remission was defined as a mHBI <5 for patients with CD or a pMayo <2 for patients with UC. Clinical disease activity was defined as a mHBI ≥5 for patients with CD or a pMayo ≥2 for patients with UC. Objective remission was defined as FCP <250 μg/g, and objective disease activity was defined as FCP ≥250 μg/g.16
Actual Clinical Decision
Patients were seen at each infliximab infusion by the attending physician (one of several gastroenterologists with at least 1 yr of subspecialty training in IBD) and an actual clinical management decision made. The actual clinical decision was made with the physician blinded to calprotectin and ITLs and was based on clinical symptoms, mHBI or pMayo score, and any available laboratory and imaging investigations up to the time of the infusion visit. Possible decisions included (1) “no action” (i.e., no change in management) or (2) “action”–“investigation (i.e., endoscopy),” “dose escalation,” “dose de-escalation,” or “switch drugs.”
Hypothetical Decisions
FCP algorithmic decision
An FCP <250 μg/g led to “no action” while an FCP ≥250 μg/g led to “an action.”
ITLs algorithmic decision
ITLs <3.0 μg/mL led to “dose escalation,” ITLs between 3.0 μg/mL and 7.0 μg/mL led to “no action,” ITLs >7.0 μg/mL led to “dose de-escalation.”
Expert Panel Decisions
Three expert IBD clinicians (R.F., K.K., H.W.) constituted the panel. Expert panel decisions were determined based on the majority decision (i.e., 2 of the 3 clinicians had to agree).
Expert panel decision based on clinical data only
Based only on the available clinical data (disease type and location, mHBI or pMayo score, and any available laboratory and imaging investigations up to the time of the infusion visit). Possible decisions included (1) “no action” or (2) “action”–“investigation (i.e., endoscopy),” “dose escalation,” “dose de-escalation.”
Expert panel decision based on clinical data plus FCP
The panel was provided with the FCP level for each patient case in addition to the available clinical data. Possible decisions included (1) “no action” or (2) “action”–“investigation (i.e., endoscopy),” “dose escalation.” “dose de-escalation.”
Expert panel decision based on clinical plus FCP plus ITL
The panel was provided the FCP and the ITL for each patient case in addition to the available clinical data. Possible decisions included (1) “no action” or (2) “action”–“investigation (i.e., endoscopy),” “dose escalation,” “dose de-escalation,” or “switch drugs.”
Study Objectives
The primary objectives of this study were to evaluate how (1) decisions based on FCP and ITL used in algorithms and (2) decisions based on the addition of FCP and ITL to clinical data differ from the actual clinical decision made based on clinical assessment, in outpatients with IBD receiving maintenance infliximab therapy.
The secondary objectives of this study were to (1) assess the relationship between FCP, ITL, and clinical disease activity, (2) determine the ability of FCP to predict clinical disease activity or subtherapeutic ITL, and (3) determine the ability of ITL to predict clinical versus objective remission in outpatients with IBD on stable maintenance infliximab therapy
Bias
This study involved a random cross-sectional sampling of patients with IBD receiving stable maintenance infliximab therapy. A retrospective chart review for patient demographics was conducted subsequent to patient recruitment and therefore minimizes selection bias. The FCP and ITL algorithmic decisions were based purely on the measured FCP and ITL. The expert panel decisions were determined by majority agreement and were independent of the algorithmic decisions.
Statistical Methods
Continuous variables are presented as median (interquartile range [IQR]) because of the small sample size and nonparametric distributions. Nonparametric Mann–Whitney and Kruskall–Wallis tests were used to determine significant differences between medians. For categorical variables, proportions were calculated and comparison between subgroups was performed using the Fisher's Exact test. A P < 0.05 was considered significant. Each category of decision made was compared with the actual clinical decision made and presented in table format as number of cases (in percentages).
Receiver-operating curves (ROC) were used to assess the discriminate ability of (1) FCP to predict clinical disease activity and subtherapeutic ITL and (2) ITL to predict clinical remission and objective remission. The area under the ROC was calculated with 95% confidence intervals (CI), and the optimum ITL was determined using the Youden's method.
RESULTS
Patient Characteristics
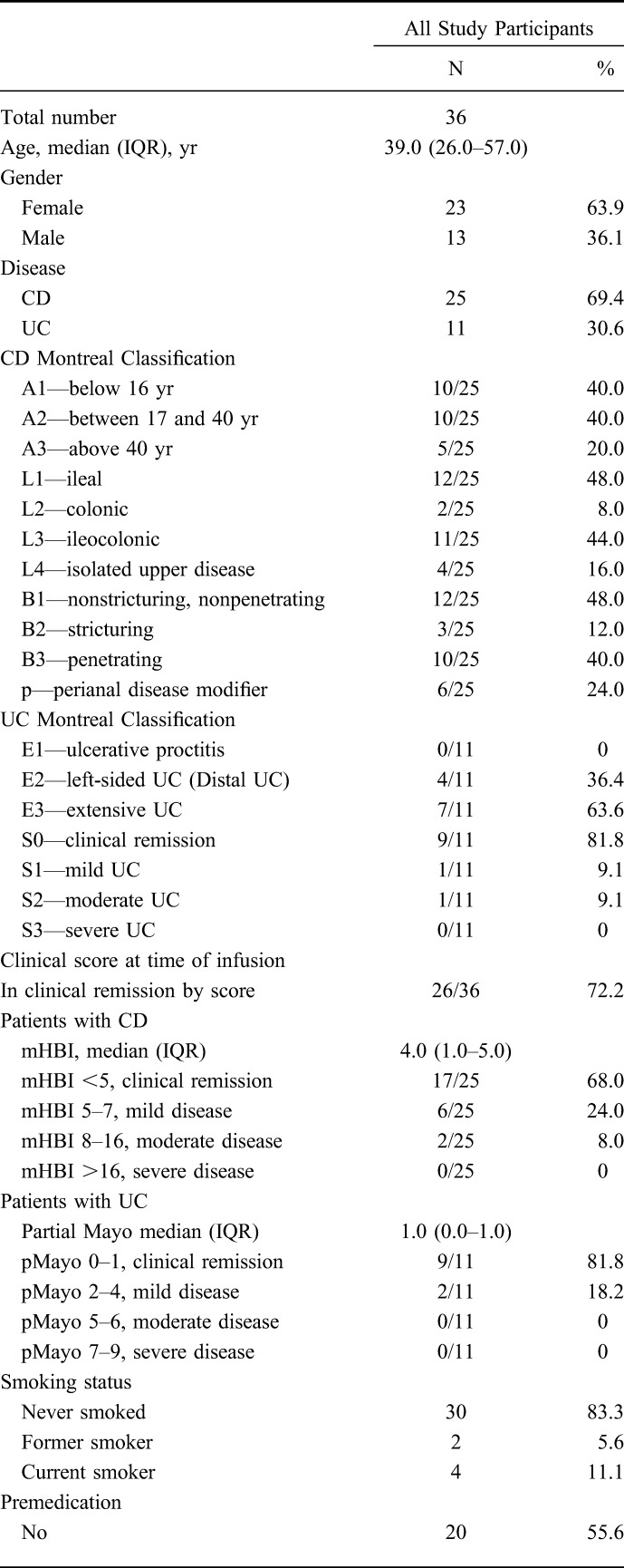
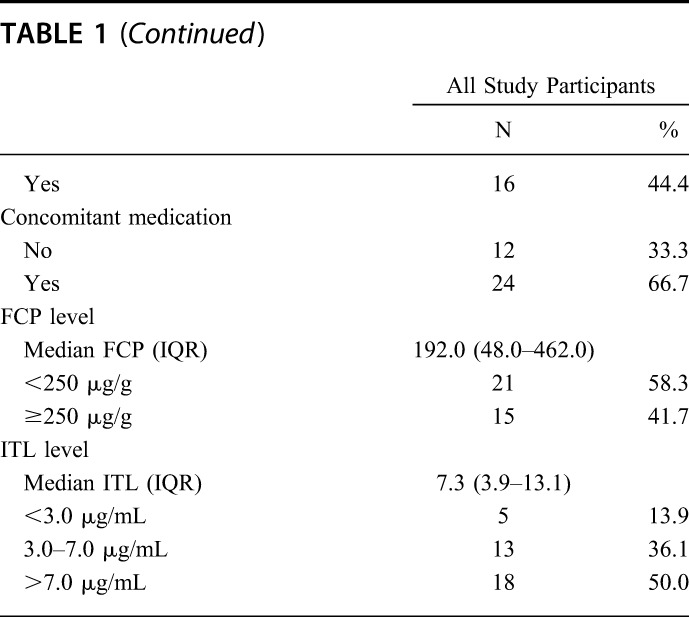
Table 1 shows the demographic and infliximab treatment characteristics of the 36 included patients. There were 23 (63.9%) females and 13 (36.1%) males. The majority had CD (25/36, 69.4%), with ileal (12/25, 48.0%) or ileocolonic (11/25, 44.0%) involvement. Two-thirds (24/36, 66.7%) of patients were on concomitant medication. Only 72.2% (26/36) of the patients were in clinical remission at the time of sample collection. The median FCP was 192.0 (IQR: 48.0–462.0) μg/g, with 21/36 (58.3%) patients having an FCP< 250 μg/g. The median ITL was 7.3 (IQR: 3.9–13.1) μg/mL, with 5/36 (14%) having subtherapeutic levels <3.0 μg/mL with 18/36 (50.0%) having supratherapeutic levels >7.0 μg/mL. Of the 15 patients who had FCP ≥250 μg/g, 4 (26.7%) had supratherapeutic ITLs >7.0 μg/mL, 7 (46.7%) had therapeutic ITLs in the 3.0 to 7.0 μg/mL range, and 4 (26.7%) had subtherapeutic ITLs <3.0 μg/mL. Conversely, of the 13 patients who had therapeutic ITLs in the 3.0 to 7.0 μg/mL range, only 6 (46.2%) had FCP <250 μg/g.
TABLE 1.
Demographics of 36 Outpatients with IBD on Stable Maintenance Infliximab Therapy, Infliximab Infusion Clinic, University of Alberta
Primary Objective: Comparison of Actual Clinical Decision and Hypothetical Decisions
Actual Clinical Decision Compared with FCP and ITLs Algorithmic Decisions
As shown in Table 2, the actual clinical decision and the FCP algorithmic decision differed in 17 of 36 (47.2%) cases (bolded text); 13 (36.1%) from “no action” to “action,” 3 (8.3%) from “action/investigation” to “no action,” and 1 (2.8%) from “dose de-escalation” to “no action,” respectively.
TABLE 2.
Contingency Table Comparing Algorithmic Decisions Based on FCP and ITLs with the Actual Clinical Decisions Made
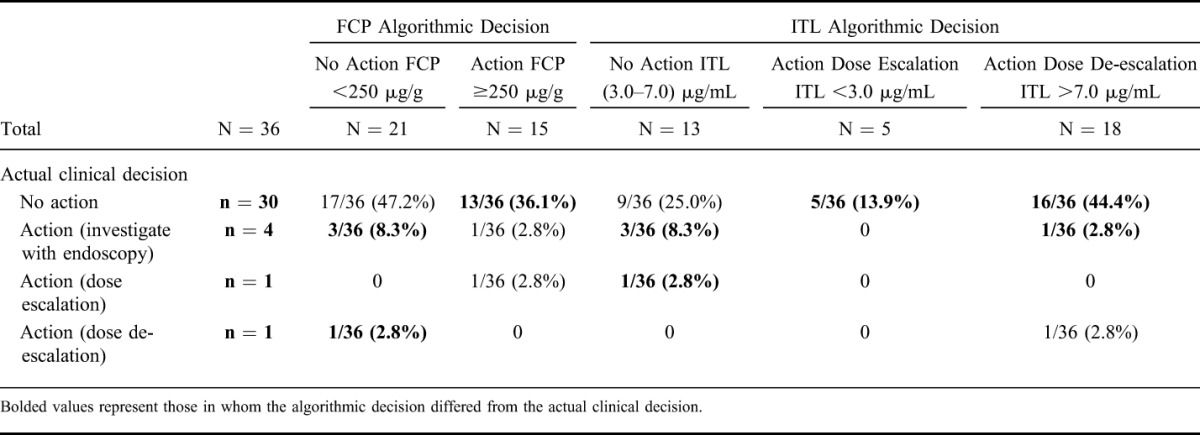
As shown in Table 2, the actual clinical decision and the ITL algorithmic decision differed in 25 of 36 (69.4%) cases (bolded text); 5 (13.9%) from “no action” to “dose escalation,” 16 (44.4%) from “no action” to “dose de-escalation,” 3 (8.3%) from “investigation” to “no action,” and 1 (2.8%) from “dose escalation” to “no action,” respectively.
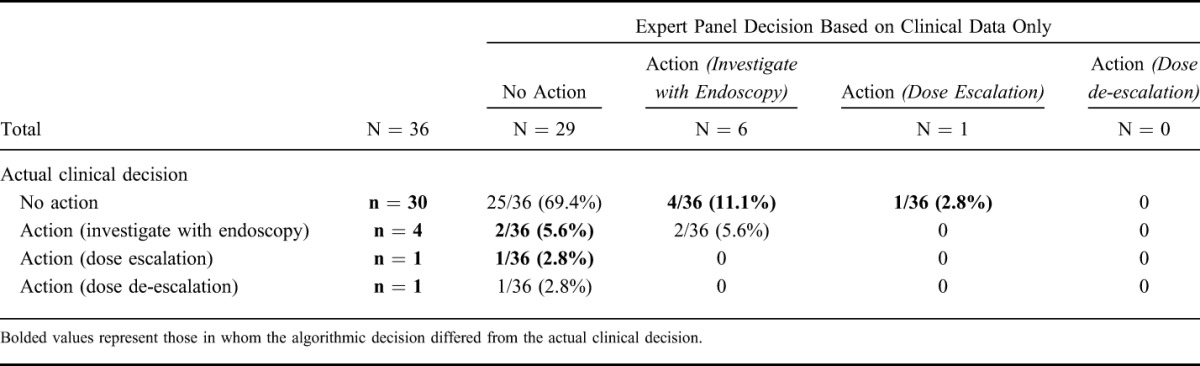
Actual Clinical Decision Compared with Expert Panel Decisions
As shown in Table 3, the actual clinical decision and the expert panel clinical decision differed in 8 of 36 (22.2%) cases (bolded text); 4 (11.1%) from “no action” to “investigation,” 1 (2.8%) from “no action” to “dose escalation,” 2 (5.6%) from “investigation” to “no action,” and 1 (2.8%) from “dose escalation” to “no action,” respectively.
TABLE 3.
Contingency Table Comparing Expert Panel Decisions Based on Clinical Data with the Actual Clinical Decision Made
As shown in Table 4, the actual clinical decision differed from the expert panel clinical plus FCP decision in 16 of 36 (44.4%) cases; 12 (33.3%) from “no action” to “investigation,” 1 (2.8%) from “no action” to “dose escalation,” 1 (2.8%) from “investigation” to “no action,” and 1 (2.8%) from “dose de-escalation” to “no action,” respectively.
TABLE 4.
Contingency Table Comparing Expert Panel Decisions Based on Clinical Plus FCP or FCP and ITL with the Actual Clinical Decisions Made
As shown in Table 4, the actual clinical decision differed from the expert panel clinical plus FCP plus ITL decision in 21 of 36 (58.3%) cases; 9 (25.0%) from “no action” to “investigation,” 3 (8.3%) from “no action” to “dose escalation,” 5 (13.9%) from “no action” to “dose de-escalation,” 1 (2.8%) from “no action” to “switch drugs,” 1 (2.8%) from “investigation” to “no action,” 1 (2.8%) from “dose escalation” to “investigation,” and 1 (2.8%) from “dose de-escalation” to “switch drugs,” respectively.
Secondary Analysis: Relationship of FCP and ITL and Clinical Disease Activity
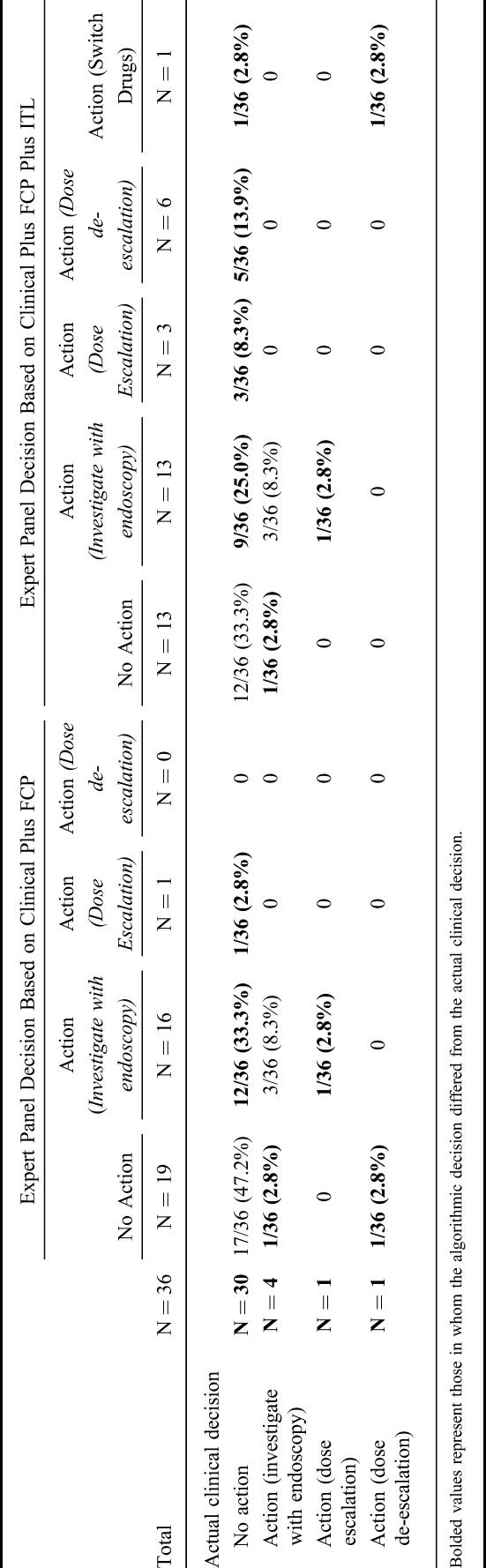
The ROCs showing the relationship of FCP in discriminating clinical disease activity and subtherapeutic ITL are shown in Figure 1A, B. The area under the ROC for FCP to predict clinical disease activity was 0.417 (95% CI, 0.197–0.641). The area under the ROC for FCP to predict subtherapeutic ITL was 0.774 (95% CI, 0.536–1.000). Table 5 presents the sensitivity and specificity for various FCP cutoffs in predicting clinical disease activity or subtherapeutic ITL.
FIGURE 1.
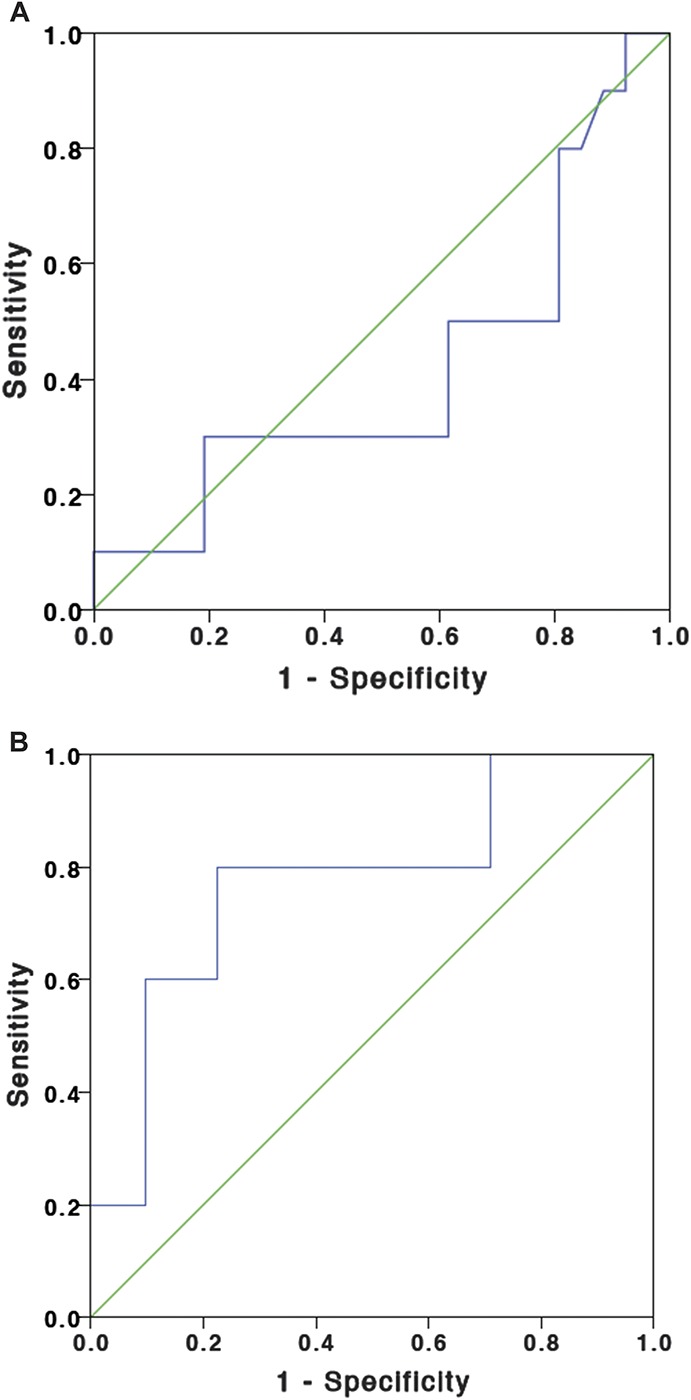
A, ROC for FCP for the prediction of clinical relapse (mHBI ≥5 or pMayo ≥2). B, ROC for FCP for the prediction of subtherapeutic ITL (<3 μg/mL).
TABLE 5.
FCP Levels for Predicting Clinical Disease Activity and Subtherapeutic ITL
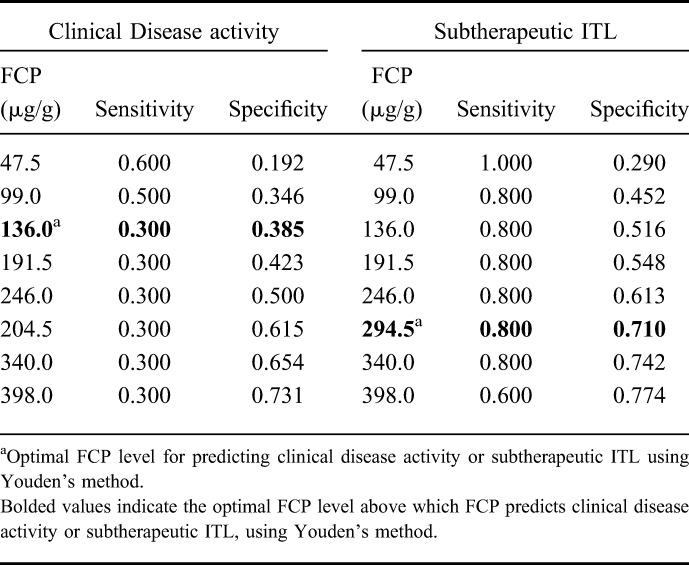
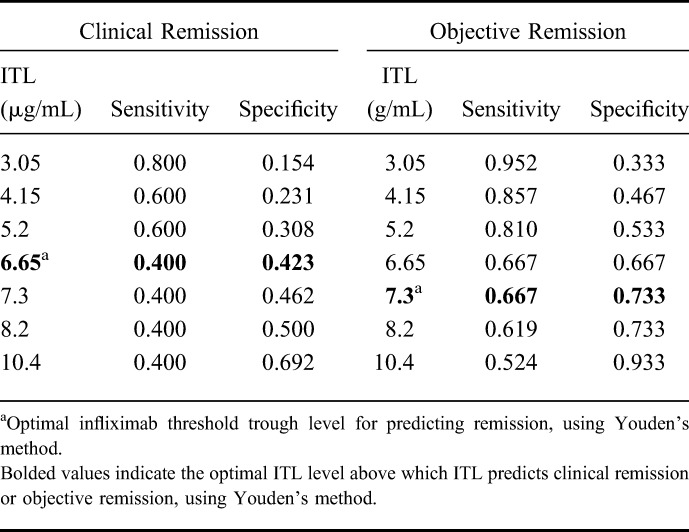
The ROCs showing the relationship of ITL in discriminating clinical remission and objective remission are shown in Figures 2A, B. The area under the ROC for ITL to predict clinical remission was 0.498 (95% CI, 0.254–0.742) and to predict objective remission was 0.773 (95% CI, 0.622–0.924). Table 6 presents the sensitivity and specificity of various ITL cutoffs for predicting clinical or objective remission.
FIGURE 2.
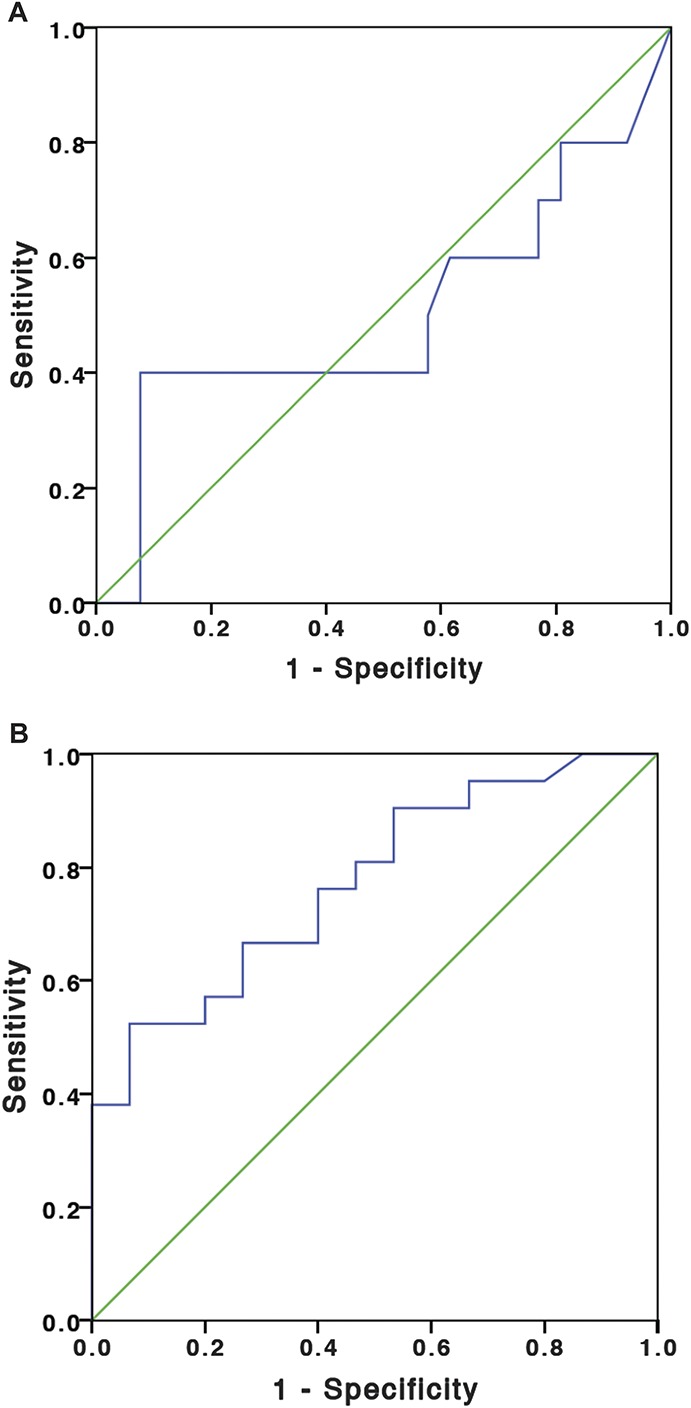
A, ROC for ITL for the prediction of clinical remission. B, ROC for ITL for the prediction of objective remission.
TABLE 6.
ITLs for Predicting Clinical and Objective Remission
DISCUSSION
Infliximab maintenance therapy is often continued indefinitely in patients with IBD when they appear to respond and do not have adverse events. However, when patients have symptoms suggestive of loss of response, their infliximab dose is often empirically escalated. In this study, an analysis of different decisions based on FCP and ITL algorithms or on the addition of FCP and ITL to clinical data, regarding maintenance infliximab therapy was conducted. The addition of FCP and ITLs in all decision scenarios resulted in different decisions in a significant proportion of cases suggesting that these objective markers of disease activity (FCP) and of therapeutic drug level (ITLs) are important in clinical decision-making regarding the management of patients with IBD on infliximab maintenance therapy. The secondary analysis of the study showed that the ability of these objective measurements to predict clinical remission or relapse varied depending on cutoffs used, suggesting that algorithms based on specific cutoffs may not be useful.
In this study, using only the clinical scores, 72.2% of patients with IBD receiving infliximab maintenance therapy were in clinical remission. However, using an FCP cutoff of 250 μg/g, only 58.3% of the patients with IBD had objective remission. Thus, 41.7% of patients actually had objective disease activity and were at risk of disease flares if they remain on their current infliximab maintenance therapy. The similarity in the proportion of cases for which the actual clinical decision differed from the FCP algorithm decision (44.4%) or the expert panel clinical data plus FCP decision (44.4%) suggests that clinicians generally agree on the FCP cutoff of 250 μg/g as an indicator of disease activity or predictor of disease flare and would make decisions to investigate or adjust therapy accordingly.
Previous studies have suggested that therapeutic ITLs range from 3.0 to 7.0 μg/mL and have proposed that therapeutic drug monitoring can be used to manage loss of response in infliximab treated patients with IBD or in optimizing maintenance infliximab therapy. In this study, only 36.1% of the patients with IBD receiving infliximab maintenance therapy had a therapeutic ITL between 3.0 to 7.0 μg/mL. However, only 46.2% of the patients who had therapeutic ITLs were in objective remission (normal FCP <250 μg/g). The actual clinical decision differed from the ITL algorithm decision (72.2%) in more cases than it differed from the expert panel clinical plus FCP plus ITL decision (58.3%). Although there were 18 cases that had supratherapeutic ITLs and therefore should be de-escalated by the ITL algorithm, the expert panel decided to de-escalate only 5 patients because the other patients had elevated FCP suggesting that clinicians are wary of basing their clinical decisions on therapeutic drug monitoring alone.
FCP was a modest biomarker to predict clinical disease activity (area under the curve [AUC] = 0.417; 95% CI, 0.197–0.641) but was a better biomarker to predict subtherapeutic ITL (AUC = 0.774; 95% CI, 0.536–1.000). ITL was a modest predictor of clinical remission (AUC = 0.498; 95% CI, 0.254–0.742) but had better ability to predict objective remission (AUC = 0.773; 95% CI, 0.622–0.924). The limitation of our secondary analysis was that we used clinical scores to define clinical remission, and FCP was used to define objective remission; we did not have endoscopic evaluation or inflammatory markers, such as C-reactive protein, for all patients. However, this strengthens the translatability of our study findings to clinical practice, as in many centers across the world, patients may not have endoscopic evaluation or C-reactive protein measured routinely with each infliximab infusion.
Another limitation of this study was that it was that the algorithmic decisions were based on an FCP cutoff 250 μg/g and a therapeutic ITL range of 3.0 to 7.0 mg/mL, based on the available current literature. We illustrate with our secondary analysis that the test characteristics for FCP or ITL in predicting clinical or objective disease activity varies with cutoff levels. Therefore, using FCP or ITL in algorithms based on cutoff levels may have limited use in clinical practice.
Steenholdt et al13 demonstrated the clinical utility and cost effectiveness of a test-based algorithm to manage secondary loss of response to infliximab therapy. However, their algorithm was based on first measuring the ITLs and anti-infliximab antibodies and then categorizing patients into 1 of 4 subgroups of loss of response based on these levels. However, an estimated 57% of patients with CD and 33% of patients with UC may have concomitant irritable bowel syndrome-like symptoms.17 Patients with IBD who have clinical loss of response may actually have symptoms that are not due to active IBD.18,19 Initial investigations to confirm active IBD before the measurement of infliximab and anti-infliximab antibodies could be recommended based on clinical suspicion.19,20 However, pursuing investigations such as endoscopy or imaging tests on each patient with IBD who has symptoms while on infliximab maintenance therapy may be costly. However, it is currently unclear whether it would be more cost effective to investigate symptoms to confirm active IBD before measuring infliximab and anti-infliximab abs levels or to measure levels first; either strategy would be appropriate.21
Another limitation of our study is that the hypothetical decisions (algorithmic and expert panel) were based on single FCP and ITLs. Consecutively elevated FCP has been shown to predict relapse in patients with UC,22 so it may also be a better strategy to measure consecutive FCP as well as ITLs in addition to monitoring clinical symptoms in patients with IBD receiving infliximab maintenance therapy and adjust infliximab therapy to prevent predicted relapse.
CONCLUSIONS
In conclusion, using FCP and ITL in addition to clinical data results in an increased number of decisions to optimize management in outpatients with IBD on stable maintenance infliximab therapy. The costs and benefits of monitoring FCP and ITLs during maintenance infliximab therapy in outpatients with IBD require additional study.
ACKNOWLEDGMENTS
Author contributions: R. N. Fedorak is acting as the article guarantor and contributed to study design, data analysis, and manuscript editing. V. W. Huang contributed to study design, data collection, data analysis, and manuscript drafting and editing. N. Dhami, D. K. Fedorak, C. Prosser, and C. Shalapay contributed to data collection, and manuscript editing. K. I. Kroeker and H. Wang contributed to data collection and manuscript editing. L. A. Dieleman, K. J. Goodman, and B. Halloran contributed to study concept and manuscript editing. All authors have approved the final version of the manuscript.
Footnotes
V. W. Huang was supported by the Alberta Innovates Health Solutions Clinician Research Fellowship Award. D. K. Fedorak was supported by the Cecile Mactaggart Summer Student Research Fund. This study was supported by the Alberta IBD Consortium and by the Center of Excellence for Gastrointestinal Inflammation and Immunity Research (CEGIIR).
Presented at Canadian Digestive Diseases Week 2014; European Crohn's and Colitis Organization Congress 2014; Digestive Diseases Week 2014; United European Gastroenterology Week 2014.
R. N. Fedorak, K. I. Kroeker, L. A. Dieleman, and B. Halloran have served as speakers and consultants for Abbvie Canada Inc., and Janssen Canada Inc. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hanauer S, Feagan B, Lichtenstein G, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. [DOI] [PubMed] [Google Scholar]
- 2.Sands B, Anderson F, Bernstein C, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–885. [DOI] [PubMed] [Google Scholar]
- 3.Rutgeerts P, Sandborn W, Feagan B, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. [DOI] [PubMed] [Google Scholar]
- 4.Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am J Gastroenterol. 2009;104:760–767. [DOI] [PubMed] [Google Scholar]
- 5.Haens GRDA, Panaccione R, Higgins PDR, et al. The London position statement of the world congress of gastroenterology on biological therapy for IBD with the European Crohn's and colitis organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2010;106:199–212. [DOI] [PubMed] [Google Scholar]
- 6.Wright EK, De Cruz P, Gearry R, et al. Fecal biomarkers in the diagnosis and monitoring of Crohn's disease. Inflamm Bowel Dis. 2014;20:1668–1677. [DOI] [PubMed] [Google Scholar]
- 7.Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the lichtiger index, c-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis. 2013;19:332–341. [DOI] [PubMed] [Google Scholar]
- 8.af Björkesten C-G, Nieminen U, Turunen U, et al. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn's disease. Scand J Gastroenterol. 2012;47:528–537. [DOI] [PubMed] [Google Scholar]
- 9.Kopylov U, Rosenfeld G, Bressler B, et al. Clinical utility of fecal biomarkers for the diagnosis and management of inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:742–756. [DOI] [PubMed] [Google Scholar]
- 10.Bortlik M, Duricova D, Malickova K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn's disease. J Crohns Colitis. 2013;7:736–743. [DOI] [PubMed] [Google Scholar]
- 11.Bendtzen K, Ainsworth M, Steenholdt C, et al. Individual medicine in inflammatory bowel disease: monitoring bioavailability, pharmacokinetics and immunogenicity of anti-tumour necrosis factor-alpha antibodies. Scand J Gastroenterol. 2009;44:774–781. [DOI] [PubMed] [Google Scholar]
- 12.Velayos FS, Kahn JG, Sandborn WJ, et al. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn's disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol. 2013;11:654–666. [DOI] [PubMed] [Google Scholar]
- 13.Steenholdt C, Brynskov J, Thomsen OO, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn's disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014;63:919–927. [DOI] [PubMed] [Google Scholar]
- 14.Harvey R, Bradshaw J. A simple index of Crohn's disease activity. Lancet. 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 15.Regueiro M, Loftus E, Jr, Steinhart A, et al. Medical management of left-sided ulcerative colitis and ulcerative proctitis: critical evaluation of therapeutic trials. Inflamm Bowel Dis. 2006;12:979–994. [DOI] [PubMed] [Google Scholar]
- 16.D'Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. [DOI] [PubMed] [Google Scholar]
- 17.Simren M, Axelsson J, Gillberg R, et al. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol. 2002:97:389–396. [DOI] [PubMed] [Google Scholar]
- 18.Jelsness-Jørgensen L-P, Bernklev T, Moum B. Calprotectin is a useful tool in distinguishing coexisting irritable bowel-like symptoms from that of occult inflammation among inflammatory bowel disease patients in remission. Gastroenterol Res Pract. 2013;2013:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn's disease. Aliment Pharmacol Ther. 2011;33:987–995. [DOI] [PubMed] [Google Scholar]
- 20.Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685–698. [DOI] [PubMed] [Google Scholar]
- 21.Khanna R, Sattin BD, Afif W, et al. Review article: a clinician's guide for therapeutic drug monitoring of infliximab in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:447–459. [DOI] [PubMed] [Google Scholar]
- 22.Vos MD, Louis EJ, Jahnsen J, et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis. 2013;19:2111–2117. [DOI] [PubMed] [Google Scholar]


