Abstract
Objectives
To quantify end-of-life (EOL) medical costs for adult Medicaid beneficiaries diagnosed with cancer.
Data Sources
We linked Medicaid administrative data with 2000–2003 cancer registry data to identify 3,512 adult Medicaid beneficiaries who died after a cancer diagnosis and matched them to a cohort of beneficiaries without cancer who died during the same period.
Study Design
We used multivariable regression analysis to estimate incremental per-person EOL cost after controlling for beneficiaries' age, race/ethnicity, sex, cancer site, and state of residence.
Principal Findings
End-of-life costs during the final 4 months of life were about $10,000 higher for Medicaid cancer patients than for those without cancer. Medicaid cancer patients are more intensive users of inpatient and ambulatory services than are Medicaid patients without cancer. Medicaid cancer patients who die soon after diagnosis have higher costs of care and use inpatient services more intensely than do Medicaid patients without cancer.
Conclusions
Medicaid cancer patients incur substantially higher EOL costs than noncancer patients. This increased cost may reflect the cost of palliative care. Future studies should assess the types and timing of services provided to Medicaid cancer patients at the EOL.
Keywords: Econometrics, health care costs, Medicaid, chronic disease, cancer, end-of-life cost
Background
Cancer has been the leading cause of death among men and women younger than 85 years of age in the United States since 2005 (Jemal et al. 2010). Medicaid is the nation's single largest source of health insurance for low-income young and middle-aged men and women, and the program plays a critical role in providing health insurance coverage for many low-income beneficiaries who are diagnosed with cancer. On average, states expend about $2 billion in cancer care costs and Medicaid pays about 5 percent of these costs (Tangka et al. 2013). Implementation of the Breast and Cervical Cancer Prevention and Treatment Act of 2000 has further extended the role of Medicaid in providing coverage for cancer treatment (enrollment, though, represents less than 0.5 percent of Medicaid enrollees) (Lantz, Weisman, and Itani 2003; French et al. 2004). With health care reform, some state Medicaid programs will cover low-income individuals earning up to 138 percent of the poverty level, which will significantly increase enrollment, but these rates will differ, as states have the option of not expanding Medicaid coverage (Kaiser Family Foundation 2010).
Significant disparities exist in cancer outcomes between persons with higher socioeconomic status and persons with lower socioeconomic status, including Medicaid beneficiaries (Eggleston et al. 2006; Clegg et al. 2009). Across a wide range of cancer sites, Medicaid beneficiaries have a higher probability of being diagnosed with late-stage cancers than do people who are insured privately (Chen et al. 2007; Halpern et al. 2008). In addition, low-income men and women have significantly lower overall survival rates after a cancer diagnosis than men and women with higher socioeconomic status, even after controlling for cancer stage at diagnosis (Bradley, Given, and Roberts 2002; Du et al. 2006; Byers et al. 2008).
Managing end-of-life (EOL) care appropriately is important to ensure that patients receive high-quality care that is also cost effective. EOL health care can be extremely expensive. Costs are highest for Medicare cancer patients, for example, during the first year of treatment and the last year of life (Yabroff et al. 2008). Patients who are hospitalized or who receive life-sustaining medical care at the EOL incur high costs, whereas some cases, giving palliative care instead of curative therapy can reduce costs and improve patients' quality of life (Abrahm 2011). A recent study concluded that initiation of palliative care can result in significant savings to Medicaid (Morrison et al. 2011). Higher treatment costs in the final week before death are associated with worse quality of life near the EOL (Zhang et al. 2009).
In this study, we compared the EOL medical costs among adult Medicaid patients with and without cancer to generate the incremental EOL cost attributable to cancer. Cancer care is expensive, and understanding the resources expended on services for patients with cancer at the EOL will allow Medicaid programs to assess whether better care management processes can be implemented to reduce costs while maintaining or even improving survival time and quality of life.
Methods
The conceptual framework used to guide the design of this study is one that has been implemented in previous studies on the cost of cancer patients (Yabroff et al. 2008). Cancer costs are estimated to occur along the continuum of cancer care across three phases: treatment, continuing, and EOL. In this study, the focus is on EOL cost; costs of cancer patients are compared with those of noncancer patients to determine incremental EOL costs to the Medicaid program. Prior studies based on Medicare patients have shown that costs are generally highest during the treatment phase and then during the EOL period. Research on overall EOL care has shown that there is unnecessary use of intensive treatments during the EOL phase and that shifting to palliative care earlier during the course of EOL care will reduce costs (Neuberg 2009). We therefore, hypothesize that cancer patients will potentially incur even higher cost than patients with other conditions because cancer treatments such as chemotherapy can be very expensive. Intensive treatments may be provided even to patients who are diagnosed at a late stage, when the expected lifespan can be just a few months (Zhang et al. 2009). Because utilization of health services and thus costs can vary by the demographic characteristics of the patient and also site of the tumor, we control for these factors in our estimation of the cancer-related EOL costs. Another factor that can affect utilization and cost is insurance status. This analysis includes only Medicaid beneficiaries, so other insurance groups are not included.
Linking Medicaid and Cancer Registry Data to Identify Cancer Cohort
We analyzed Medicaid administrative data linked with cancer registry data from 2000 through 2003, obtaining the Medicaid data from the Centers for Medicare and Medicaid Services and the cancer registry data from the state cancer registries in Georgia and Illinois. The Institutional Review Board (IRB) at RTI International approved the research plan for this study and granted waivers of informed consent and of Health Insurance Portability and Accountability Act (HIPAA) authorization. The IRBs of the Georgia and Illinois state health departments also reviewed and approved the project. All data were linked and analyzed using SAS software (SAS Institute, Cary, NC, USA).
Georgia and Illinois were selected because of their high-quality Medicaid and cancer registry data and low managed care penetration within Medicaid beneficiaries. Georgia did not have any enrollees in capitated managed care plans during the study period (about 65 percent were enrolled in primary care case management, and these individuals are included in our analysis because they have fee-for-service claims for services) and Illinois had about 12 percent enrolled in capitated plans. Both states provided comprehensive care to Medicaid beneficiaries diagnosed with cancer; adult beneficiaries generally included low-income parents and disabled individuals (similar to other state Medicaid programs). Both states had no copayments for cancer-specific treatments but had small copayments of 50 cents to $1 for prescription medications. Although copayments can be implemented, Medicaid beneficiaries cannot be denied services if they are unable to pay. As mandated by the federal regulations, during the study period both states covered low-income disabled adults and parents. In Georgia, the definition of low income was based on the federal minimum requirements, while Illinois used a more generous qualification based on income thresholds. Neither state covered childless adults (only a limited number of states had waivers for covering this group during the study period), but—like more than half the states—both had medically needy programs and would have covered adults with cancers once they had spent down earnings and savings to meet the Medicaid eligibility criteria.
Adult Medicaid beneficiaries aged 21–64 years who were diagnosed with cancer during the 4-year period from 2000 to 2003 were selected from the Medicaid enrollment data for linkage with the cancer registry data. Staff of the cancer registries linked the data with a deterministic match process using Social Security numbers, date of birth, and gender. The Medicaid enrollment file contains beneficiary eligibility information, demographic characteristics, and indicators of monthly enrollment, whereas Medicaid claim files provide information on services used and the payment associated with these services. Key variables from the cancer registry data include date of cancer diagnosis, stage at diagnosis, cancer site, and date of death. To ensure we had complete utilization data to estimate cost, we excluded from our study sample any beneficiaries enrolled in both Medicare and Medicaid (approximately 17 percent of Medicaid beneficiaries are dual enrollees). Dual enrollees are low-income beneficiaries for whom Medicare is the primary payer with Medicaid covering services not covered by Medicare; hence, Medicaid claims data alone do not provide complete utilization information for these individuals. Dual enrollees were excluded before the linkage with cancer registry data was performed. In addition, we retained only the cases of people who died during the years included in the study and who had continuous fee-for-service enrollment for the 4-month period before death (420 individuals on the basis of these criteria).
Selecting the Medicaid Noncancer Cohort
We also used Medicaid claims information to select a cohort of patients without cancer who were enrolled and died during the same period from 2000 through 2003, to assess incremental EOL medical cost resulting from a cancer diagnosis. The noncancer cohort, similar to the cancer cohort, included only Medicaid beneficiaries ages 21–64 years. In addition, enrollees of both Medicare and Medicaid were excluded, and only people with at least 4 months of continuous enrollment in fee-for-service Medicaid before their death were retained for analysis. Each patient with cancer (3,512) was matched by age groupings, gender, race, and state with two Medicaid recipients without cancer (7,024). When a patient with cancer could be matched with more than two patients without cancer, two matches were selected at random. We performed three separate iterations of the selection process to determine whether there were underlying differences in the comparison cohort selected. Our descriptive and multivariate assessments using these comparison cohorts indicated that our selection process was quite robust, as there were no compelling differences in the results using any of the three noncancer cohorts.
Defining Variables for the Analysis
We generated a series of variables to use in descriptive and multivariable analysis using the linked Medicaid and cancer registry data. We created the same variables for the cancer and noncancer cohort except for variables specifically related to cancer (for example, stage at diagnosis). A variable for age at diagnosis was created in three age groupings (21–35, 36–50, and 51–64 years) as specified in previous Medicaid studies (Subramanian et al. 2010, 2011). Beneficiaries were categorized as white, black, or other race on the basis of the information on race provided in the Medicaid eligibility file. Date of death was obtained from the Medicaid administrative data; we also used the death information provided in the cancer registry to supplement the Medicaid information if it was not available for the cancer cohort. We assessed cost related to all-cause mortality for patients with cancer (i.e., cost related to cancer and other causes of death combined) as presented in previous analyses (Abrahm 2011). We decided on this approach because the stated cause of death is not always accurate and because cancer, even when not the primary cause of death, could be a contributing factor or one of multiple causes (Bregg and Schrag 2002; Lund et al. 2010). In addition, we created four categories to assess differences based on time from cancer diagnosis to death: less than 3 months, 3–6 months, 7–12 months, and more than 12 months. Using the cancer staging information available in the cancer registry data, we identified the stage at diagnosis as local, regional, distant, or unknown (North American Association of Central Cancer Registries 2012). We also used the information on site of the cancer available in the cancer registry data to categorize prevalent cancer sites among Medicaid beneficiaries.
Medicaid payments were aggregated over the 4-month period before death. No consistent definition exists for the EOL period because the time remaining until death is based on individual patient prognosis. Studies in the peer-reviewed literature have varying EOL periods, ranging from a week to 1 year (Neuberg 2009; Abrahm 2011). Hospice care is generally reserved for patients believed to have 6 or fewer months to live (Kuebler, Esper, and Heidrich 2006). We decided to use the 4-month period before death as our EOL period, because Medicaid cancer patients are often diagnosed at a late stage and therefore may die within a short period of time from diagnosis (Chen et al. 2007; Halpern et al. 2008). We did attempt to perform sensitivity analysis using longer timeframes, including 6-month and 1-year periods, but the sample size available for analysis was reduced significantly because of the short periods of Medicaid enrollment (or gaps in enrollment) before death. The 4-month cost included all expenditures incurred in the month of death and the previous 3 months. For example, even if cancer patients died within 3 months of their cancer diagnosis, we still report cost for a 4-month period before death to be able to systematically compare with the noncancer cohort (for whom we cannot identify a specific diagnosis date from Medicaid claims). Payments were estimated separately for expenditures related to the following services: hospital admissions, ambulatory care services, prescription drugs, and long-term care. The costs reflect all services received by the Medicaid beneficiaries in the 4-month period before death including any palliative treatments received, such as chemotherapy, radiation, surgery, or care in intensive care units. All expenditures/costs were adjusted using the medical care services component of the Consumer Price Index and reported in 2003 dollars.
Assessing Incremental EOL Medical Costs for Patients with Cancer
We used a generalized linear model (GLM) with a gamma distribution and a log link to estimate incremental costs attributed to cancer—that is, the additional costs incurred for cancer patients compared with noncancer patients. The GLM accounts for skewness in the distribution of expenditures without requiring the retransformation of the results from log scale that is required when log costs are used (Manning and Mullahy 2001). In most iterations of the model, using ordinary least square regressions; controlling for age at diagnosis, gender, race, and state; and, as appropriate, controlling for stage at diagnosis, cancer sites, and time to death provided results very similar to those obtained with the GLM. The GLM was the more appropriate estimator in determining cost for selected prevalent cancer sites, especially sites for which the sample size was small. Therefore, we chose to report all cost estimates consistently using the GLM results. To test the robustness of the results, we also generated predictive marginals and found the results to be similar to those reported in the manuscript.
Results
Table1 presents descriptive statistics of the study population. The cancer cases and noncancer controls were similar in age, gender, and race, as would be expected from the matching process. Among the patients with cancer, almost half the cohort was diagnosed at distant stage (47.1 percent) and more than one quarter at regional stage (27.7%). The most common site of cancer was the lung, representing almost one third (32.3 percent) of the cancers. Other prevalent sites were the digestive track, oropharynx, female breast, female genital organs, and urinary track. Overall, 23.5 percent of the cancer cohort died within 3 months of diagnosis, and 27.6 percent died more than 12 months after diagnosis. The per-person unadjusted 4-month total medical cost was $34,749 for patients with cancer versus $24,109 for patients without cancer. Figure1 shows the unadjusted cost by type of service: hospital stays, ambulatory care visits, prescription drugs, and long-term care.
Table 1.
Patient Demographics, Cancer Characteristics, and End-of-Life Time and Cost for Adult Medicaid Beneficiaries with and without Cancer
| Cancer Cases* | Noncancer Controls* | |||
|---|---|---|---|---|
| n | % | n | % | |
| Sample size | 3,512 | 7,024 | ||
| Age groupings | ||||
| 21–35 | 235 | 6.7 | 470 | 6.7 |
| 36–50 | 1,199 | 34.1 | 2,398 | 34.1 |
| 51–64 | 2,078 | 59.2 | 4,156 | 59.2 |
| Females | 1,615 | 46.0 | 3,230 | 46.0 |
| Race groupings | ||||
| White | 1,529 | 43.5 | 3,058 | 43.5 |
| Black | 1,608 | 45.8 | 3,216 | 45.8 |
| Other | 375 | 10.7 | 750 | 10.7 |
| Stage groupings | ||||
| Local | 454 | 12.9 | ||
| Regional | 974 | 27.7 | ||
| Distant | 1,654 | 47.1 | ||
| Unknown | 430 | 12.2 | ||
| Cancer site | ||||
| Lung | 1,135 | 32.3 | ||
| Digestive organs | 816 | 23.2 | ||
| Oropharynx | 257 | 7.3 | ||
| Female breast | 222 | 6.3 | ||
| Female genital organs | 212 | 6.0 | ||
| Urinary tract | 114 | 3.3 | ||
| Other cancers | 755 | 21.5 | ||
| Time to death from cancer diagnosis | ||||
| Within 3 months | 825 | 23.5 | ||
| 3–6 months | 734 | 20.9 | ||
| 7–12 months | 982 | 28.0 | ||
| More than 12 months | 971 | 27.6 | ||
| Unadjusted 4-month cost ($)† | $34,749 | $24,109 | ||
Sample includes all cancer and noncancer patients with 4 months of continuous enrollment before death.
Unadjusted cost for the 4-month period before death in 2003 dollars.
Figure 1.
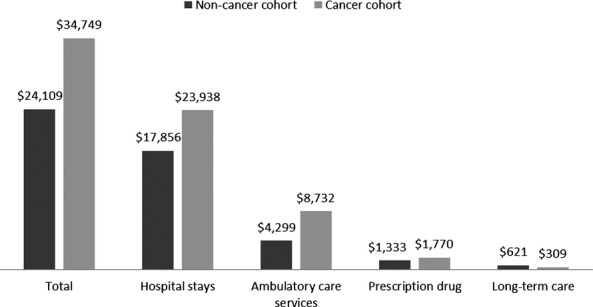
Unadjusted Average Four-Month Cost Prior to Death for the Cancer and Noncancer Cohort
Table2 presents the adjusted incremental per-person EOL cost for patients with cancer compared with patients without cancer. When all cancer cases are considered, the incremental cost of caring for cancer patients at the EOL is $9,814 per person, with both hospitalizations and ambulatory care contributing equally to the excess cost. When the sample is stratified by time to death from diagnosis, patients who die within 3 months of diagnosis have the highest total costs, and the costs decrease as time to death increases (range from $14,644 to $7,009). Among patients with cancer who die within 3 months of diagnosis, inpatient care accounts for most of the excess cost. Among patients with cancer who die within 3–6 months, both inpatient and ambulatory care contribute to excess costs. For patients who die more than 6 months from diagnosis, ambulatory care accounts for the majority of excess costs.
Table 2.
Adjusted Incremental Per-Person End-of-Life Cost among Nonelderly Medicaid Beneficiaries with Cancer Compared with Noncancer Controls*
| All Cancer Cases | Died within 3 months of Diagnosis | Died between 3 and 6 months of Diagnosis | Died between 7 and 12 months of Diagnosis | Died More Than 12 months of Diagnosis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC | 95% CI | IC | 95% CI | IC | 95% CI | IC | 95% CI | IC | 95% CI | |
| Hospital stays | 4,356† | 2,822–5,890 | 13,156† | 6,662–19,650 | 6,006† | 778–11,234 | 3,150 | −1,124 to 7,424 | 299 | −3,372 to 3,970 |
| Ambulatory care | 4,424† | 4,082–4,766 | 1,962† | 1,049–2,875 | 5,567† | 4,193–6,941 | 5,241† | 4,038–6,444 | 5,771† | 4,563–6,979 |
| Prescription drug | 378† | 256–500 | −237 | −504 to 30 | 338 | −32 to 708 | 830† | 383–1,277 | 722† | 316–1,128 |
| Long-term care | −333† | −410 to −256 | −162 | −344 to 21 | −132 | −331 to 66 | −304† | −432 to −176 | −301† | −414 to −188 |
| Total | 9,814† | 8,059–11,569 | 14,644† | 8,242–21,046 | 12,763† | 6,454–19,072 | 10,108† | 4,705–15,511 | 7,009† | 2,188–11,830 |
IC, incremental cost of care for cancer versus noncancer patients; sum of components does not equal total due to nonlinearities.
Regression analysis controlled for differences in age, sex, race, state, and stage at diagnosis. All measures reported are per person. Medicaid cost is reported for the 4-month period prior to death in 2003 dollars.
Indicates significantly different from zero at the 95% confidence level.
95% CI, confidence interval.
Table3 reports the incremental cost per person for selected cancer sites compared with incremental cost per person for the noncancer cohort. The total incremental cost for cancers of the lung, female breast, and female genital organs is about $8,500, and it is about $10,000 for cancers of the digestive organs and urinary tract. Cancer of the oropharynx has a substantially higher incremental cost per person of $22,547, as do cancers at other sites not reported separately ($27,784). Inpatient stays and ambulatory care services account for most of the excess costs.
Table 3.
Incremental per-Person End-of-Life Cost of Cancer Cases Compared to Noncancer Controls for Selected Tumor Sites*
| Lung | Digestive Organs | Oropharynx | Breast | Female Genital Organs | Urinary Tract | Other Sites | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC | SE | IC | SE | IC | SE | IC | SE | IC | SE | IC | SE | IC | SE | |
| Hospital stays | 4502† | 1,742 | 9090† | 2,331 | 21602† | 5,672 | 6,515 | 3,675 | 6,355† | 3,555 | 9,973† | 5,232 | 26,324† | 4,622 |
| Ambulatory care | 2,819† | 379 | 2,243† | 387 | 2,446† | 621 | 2,302† | 671 | 1,659† | 611 | 1,719† | 775 | 1,948† | 434 |
| Prescription drug | −231† | 100 | −431† | 95 | −442† | 136 | −131 | 189 | −366† | 157 | −325 | 206 | −127 | 130 |
| Long-term care | −194† | 96 | −306† | 103 | −215 | 160 | −111 | 173 | −79 | 171 | −199 | 217 | −80 | 117 |
| Total | 8,557† | 2,039 | 10,424† | 2,363 | 22,547† | 4,959 | 8,837† | 3,925 | 8,200† | 3,772 | 10,264† | 5,103 | 27,784† | 4,045 |
All measures reported are per person. Medicaid cost is reported for the 4-month period prior to death in 2003 dollars.
IC, incremental cost of care for cancer versus noncancer patients; sum of components does not equal total due to nonlinearities.
Analysis controlled for differences in age, sex, race, state, stage at diagnosis, and time between diagnosis and death.
Indicates significantly different from zero at the 95% confidence level.
SE, standard error.
Discussion
In this study, we analyzed Medicaid claims linked with cancer registry data to estimate EOL costs for young and middle-aged Medicaid beneficiaries diagnosed with cancer during the last 4 months before death. We found that costs of care during the final months of life for Medicaid patients with cancer are consistently higher than for Medicaid patients without cancer. EOL costs during the final 4 months of life were about $10,000 higher for Medicaid patients with cancer than those without cancer. Costs vary by cancer site, possibly reflecting differences in prognosis, disease course, number and type of recommended treatment modalities, and the costs of chemotherapeutics and other anticancer treatments. Medicaid patients with cancer are more intensive users of both inpatient and ambulatory services compared with Medicaid patients without cancer. Medicaid patients with cancer who die soon after diagnosis have higher costs of care and are more intensive users of inpatient services than are Medicaid patients without cancer. Several factors may contribute to the greater incremental cost found among those dying only a few months after cancer diagnosis compared with those dying after a longer time period. First, because we examined cost in the 4 months prior to death, for some patients dying shortly after diagnosis, costs of diagnostic testing, staging and initial treatment may be captured which may be less likely for those further out from diagnosis. Second, given evidence that many patients with advanced cancers may receive first- and second-line treatments (Temel et al. 2008), those closer to diagnosis may be more likely to have engaged in active treatment. For those living longer after diagnosis, preferences may have changed over time for some (Temel et al. 2008). Increasing time since diagnosis may also be associated with the likelihood of having earlier EOL discussions with providers (Mack et al. 2012), suggesting that such discussions for those living shorter durations after diagnosis may be more likely to occur closer to death. Given that EOL discussions are associated with less aggressive cancer treatment and earlier referrals to hospice care (Wright et al. 2008), then those dying sooner after diagnosis may be more likely to receive aggressive care for more of the 4 month EOL window in the current analysis. Other possibilities include inadequate comprehension of prognosis or communication with providers (Earle et al. 2008; Temel et al. 2008), and unrealistic expectations about treatment benefits (Earle et al. 2008).
Prior studies have shown that both physicians and patients have difficulty engaging in conversations about imminent death (Feeg and Elebiary 2005; Matsuyama, Reddy, and Smith 2006; Fadul et al. 2009; Peppercorn et al. 2011). Such conversations might be especially difficult in cases where a patient is newly diagnosed with a late-stage cancer, is predicted to have only a few months to live, and has to come to terms with the prognosis and decide whether to initiate palliative care instead of seeking treatments that are not likely to be curative or prolong life. This difficulty in initiating discussions surrounding EOL could explain the high cost reported in this study for cancer patients who die soon after their diagnosis; they may continue to receive aggressive multimodal treatments instead of undergoing palliative care. Oncologists and other health care professionals providing cancer care should receive palliative care training to guide patients through EOL conversations so patients do not feel they will be abandoned by health care professionals if they are not receiving active treatments (Parks and Winter 2009; Morrison et al. 2011). Zhang et al. (2009) showed that cancer patients who had EOL conversations with their health care professionals chose decreased use of life-sustaining treatments and had lower EOL costs, and better quality of life near the EOL. They also were more likely to receive outpatient hospice care and earlier hospice referrals. Lack of or very late enrollments in hospice are potential indicators of poor quality care at EOL (Earle et al. 2003). With better understanding and information about dying and palliative care, patients are more likely to hold meaningful EOL conversations with health care professionals that can guide decision making. Recent studies provide evidence that use of palliative care consultation teams lowered Medicaid health care costs without reducing appropriateness or quality of care (Zhang et al. 2009), but Medicaid patients with cancer are less likely to have discussed hospice care with providers (Huskamp et al. 2009).
To our knowledge, this is the first study to report the EOL costs for cancer patients enrolled in Medicaid. A few studies have reported cancer EOL cost for Medicare beneficiaries and those privately insured. The Medicare studies predominantly used data from the 1980s and estimated the incremental EOL cost for cancer patients to be $15,000 during the last year of life (Riley et al. 1995; Brown et al. 1999). Given the increases in Medicare cost of care, the estimated cost at the present time is likely to be much higher, but these estimates and Medicare enrollees may not be directly comparable to Medicaid beneficiaries who are younger but also have disabilities. A recent study using private insurance claims from 2002 to 2009 found overall cost for cancer patients during the last 6 months of life to be about $12,500 per month ($75,000 over 6 months) (Chastek et al. 2012). This is higher than the unadjusted cost of about $8,750 per month ($35,000 over 4 months) reported for Medicaid beneficiaries in this study and could potentially be due to restricted coverage of health care services or lower payment rates for services in Medicaid compared to private insurers.
Other studies on the cost of EOL care indicate that use of high-intensity, acute-care services at the EOL can contribute to increased costs of care (Fries et al. 1993; Sharma et al. 2008; Abrahm 2011; Bergman et al. 2011). Although our analysis did not examine intensity of care, increased inpatient costs for cancer patients observed in this study could be consistent with such findings. Another important finding from the current analysis is that costs related to ambulatory care services are significantly higher for patients with cancer than for patients without cancer. It is uncertain to what extent the higher ambulatory care costs for patients with cancer could be related to the use of high-cost chemotherapy in the last months or weeks of life (Matsuyama, Reddy, and Smith 2006). Future studies should investigate the types of services provided to cancer patients in both inpatient and ambulatory care settings to better understand the health care services provided at the EOL.
The rise in Medicaid enrollment expected to occur if health care reform is implemented increases the importance of accounting for EOL cancer care costs in the planning process for state Medicaid programs (Holahan et al. 2009). The incremental cost of cancer treatment for the 6-month postdiagnosis period is estimated to range from $25,000 to $45,000 for Medicaid beneficiaries diagnosed at a regional and distant stage (Subramanian et al. 2010, 2011). For those alive beyond the treatment phase and with a distinct EOL period, an additional cost of about $10,000 will be incurred during the last 4 months of life, a significant addition to the overall cost of cancer care. As of January 2013, 33 states limited Medicaid eligibility for parents to less than 100 percent of the federal poverty level and, in most states, other nondisabled adults were ineligible regardless of their income. In states that opt to expand Medicaid enrollments, Medicaid eligibility will be significantly increased for low-income parents and adults. Low-income uninsured individuals who will be enrolling in Medicaid may have significant unmet needs because of lack of access to usual sources of care through which preventive health care services, including cancer screening, could have been provided (Pizer, Frakt, and Iezzoni 2009). Therefore, these individuals may be diagnosed with late-stage cancers on enrollment in Medicaid. Prior analysis has shown that Medicaid beneficiaries who enroll around the time of diagnosis are more likely to be diagnosed with later-stage cancers than are Medicaid beneficiaries who are continuously enrolled in Medicaid before diagnosis (Bradley et al. 2005). Nevertheless, low-income individuals who previously would have faced substantial out-of-pocket cost and access-to-care issues will now be able to qualify for Medicaid and obtain insurance coverage for cancer treatment, EOL care, and other health care services.
Although claims data have been used extensively to assess costs of cancer care, these data are collected for administrative purposes and have limitations when used to estimate costs for specific cohorts. We pooled data from two states to ensure that adequate sample sizes were available for analysis, but state Medicaid programs have different cost structures, and therefore even though the overall pattern of cost increases is generalizable, the magnitude of the cost differences may not be the same. Thus, although we can conclude generally that EOL costs are higher for Medicaid patients with cancer than for those without cancer, the magnitude of the difference may not be the same among state Medicaid programs. Our study only included individuals who were continuously enrolled in Medicaid for 4 months before death. This cohort could differ from Medicaid patients with cancer overall because of significant turnover in enrollment (Ramsey et al. 2008). In addition, although we assessed incremental costs using a matched cohort of cancer patients, there could be underlying differences between these two groups, including comorbid conditions that we were unable to capture in our matching process. We also did not have information on cause of death and assessed all-cause mortality, which could mask differences in cost by cause of death.
In addition, our study excluded dual enrollees whose cost patterns may be different from those reported in this study. The focus of this study was on the adult Medicaid beneficiaries and these costs are likely to differ from aged-beneficiaries and our estimates are based on fee-for-service enrollees which may not be generalizable to managed care beneficiaries. We also did not directly address quality of care; the quality of care provided to Medicaid beneficiaries may differ from other insurance cohorts, including those with private coverage. Moreover, the accuracy of the cost estimates depends on the completeness of claims submitted by health care professionals for services provided, but because cancer and EOL care are relatively expensive, and claims must be complete for appropriate compensation to be received, we expect any bias caused by missing information to be minimal. Furthermore, because we assessed the incremental cost of cancer compared with the costs for the noncancer cohort, the study is unaffected by any bias that may have been caused by inaccuracies in the reporting of specific procedure codes, as we did not rely on these codes to identify patients receiving cancer care.
Another consideration is that our study presented results for cancers detected among the Medicaid population in the two states included in the study. Because state Medicaid programs can differ in their eligibility criteria, provider payment levels, and coverage policies, cancers diagnosed among the overall population of adult Medicaid enrollees may be different. Finally, our sample size was small for some of the types of cancer analyzed, so future studies should be conducted with larger sample sizes to confirm our study's findings related to costs of treatment for these types of cancer.
Conclusions
The findings from this study suggest that EOL medical costs for adult Medicaid patients with cancer are significantly higher than those of similarly aged Medicaid patients without cancer. Taking these higher costs into account is important for assessing the future cost of cancer care for Medicaid enrollees. Future studies should assess the quality of care provided to Medicaid cancer patients in the last few months of life (Zhang et al. 2009; Heist, Gallagher, and Temel 2012). Health care professionals should be strongly encouraged to discuss palliative care with cancer patients, when appropriate, as palliative care may not only lower Medicaid health care costs but, more important, can improve the quality and length of life of patients with cancer during the months preceding their death (Temel et al. 2010).
Acknowledgments
Joint Acknowledgment/Disclosure Statement: We thank the staff at the Georgia and Illinois cancer registries for their assistance with linking the Medicaid and cancer registry databases. This work was funded by Centers for Disease Control and Prevention Contract Number 200-2002-00575, Task Order 25.
Disclosures: None.
Disclaimers: None.
Supporting Information
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
References
- Abrahm JL. Advances in Palliative Medicine and End-of-Life Care. Annual Review of Medicine. 2011;62:187–99. doi: 10.1146/annurev-med-050509-163946. [DOI] [PubMed] [Google Scholar]
- Bergman J, Saigal CS, Lorenz KA, Hanley J, Miller DC, Gore JL, Litwin MS P. Urologic Diseases in America. Hospice Use and High-Intensity Care in men Dying of Prostate Cancer. Archives of Internal Medicine. 2011;171(3):204–10. doi: 10.1001/archinternmed.2010.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CJ, Given CW. Roberts C. Race, Socioeconomic Status, and Breast Cancer Treatment and Survival. Journal of the National Cancer Institute. 2002;94(7):490–6. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- Bradley CJ, Gardiner J, Given CW. Roberts C. Cancer, Medicaid Enrollment, and Survival Disparities. Cancer. 2005;103(8):1712–8. doi: 10.1002/cncr.20954. [DOI] [PubMed] [Google Scholar]
- Bregg CB. Schrag D. Attribution of Deaths Following Cancer Treatment. Journal of the National Cancer Institute. 2002;94(14):1044–5. doi: 10.1093/jnci/94.14.1044. [DOI] [PubMed] [Google Scholar]
- Brown ML, Riley GF, Potosky AL. Etzioni RD. Obtaining Long-Term Disease Specific Costs of Care: Application to Medicare Enrollees Diagnosed with Colorectal Cancer. Medical Care. 1999;37(12):1249–59. doi: 10.1097/00005650-199912000-00008. [DOI] [PubMed] [Google Scholar]
- Byers TE, Wolf HJ, Bauer KR, Bolick-Aldrich S, Chen VW, Finch JL, Fulton JP, Schymura MJ, Shen T, Van Heest S, Yin X G. Patterns of Care Study. The Impact of Socioeconomic Status on Survival after Cancer in the United States: Findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. 2008;113(3):582–91. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- Chastek B, Harley C, Kallich J, Newcomer L, Paoli CJ. Teitelbaum AH. Health Care Costs for Patients with Cancer at the End of Life. Journal of Oncology Practice. 2012;8(6):75s–80s. doi: 10.1200/JOP.2011.000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AY, Schrag NM, Halpern M, Stewart A. Ward EM. Health Insurance and Stage at Diagnosis of Laryngeal Cancer: Does Insurance Type Predict Stage at Diagnosis? Archives of Otolaryngology and Head Neck Surgery. 2007;133(8):784–90. doi: 10.1001/archotol.133.8.784. [DOI] [PubMed] [Google Scholar]
- Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD, Goodman MT, Lynch CF, Schwartz SM, Chen VW, Bernstein L, Gomez SL, Graff JJ, Lin CC, Johnson NJ. Edwards BK. Impact of Socioeconomic Status on Cancer Incidence and Stage at Diagnosis: Selected Findings from the Surveillance, Epidemiology, and End Results: National Longitudinal Mortality Study. Cancer Causes and Control. 2009;20(4):417–35. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XL, Fang S, Coker AL, Sanderson M, Aragaki C, Cormier JN, Xing Y, Gor BJ. Chan W. Racial Disparity and Socioeconomic Status in Association with Survival in Older Men with Local/Regional Stage Prostate Carcinoma: Findings from a Large Community-Based Cohort. Cancer. 2006;106(6):1276–85. doi: 10.1002/cncr.21732. [DOI] [PubMed] [Google Scholar]
- Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ. Block S. Identifying Potential Indicators of the Quality of End-of-Life Cancer Care from Administrative Data. Journal of Clinical Oncology. 2003;21(6):1133–8. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC. Ayanian JZ. Aggressiveness of Cancer Care Near the End of Life: Is It a Quality-of-Care Issue? Journal of Clinical Oncology. 2008;26(23):3860–6. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleston KS, Coker AL, Williams M, Tortolero-Luna G, Martin JB. Tortolero SR. Cervical Cancer Survival by Socioeconomic Status, Race/Ethnicity, and Place of Residence in Texas, 1995-2001. Journal of Womens Health. 2006;15(8):941–51. doi: 10.1089/jwh.2006.15.941. [DOI] [PubMed] [Google Scholar]
- Fadul N, Elsayem A, Palmer JL, Del Fabbro E, Swint K, Li Z, Poulter V. Bruera E. Supportive Versus Palliative Care: What's in a Name?: a Survey of Medical Oncologists and Midlevel Providers at a Comprehensive Cancer Center. Cancer. 2009;115(9):2013–21. doi: 10.1002/cncr.24206. [DOI] [PubMed] [Google Scholar]
- Feeg VD. Elebiary H. Exploratory Study on end-of-Life Issues: Barriers to Palliative Care and Advance Directives. American Journal of Hospice and Palliative Care. 2005;22(2):119–24. doi: 10.1177/104990910502200207. [DOI] [PubMed] [Google Scholar]
- French C, True S, McIntyre R, Sciulli M. Maloy KA. State Implementation of the Breast and Cervical Cancer Prevention and Treatment Act of 2000: A Collaborative Effort among Government Agencies. Public Health Reports. 2004;119(3):279–85. doi: 10.1016/j.phr.2004.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries JF, Koop CE, Beadle CE, Cooper PP, England MJ, Greaves RF, Sokolov JJ. Wright D. Reducing Health Care Costs by Reducing the Need and Demand for Medical Services. The Health Project Consortium. New England Journal of Medicine. 1993;329(5):321–5. doi: 10.1056/NEJM199307293290506. [DOI] [PubMed] [Google Scholar]
- Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J. Chen AY. Association of Insurance Status and Ethnicity with Cancer Stage at Diagnosis for 12 Cancer Sites: A Retrospective Analysis. Lancet Oncology. 2008;9(3):222–31. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- Heist RS, Gallagher ER. Temel JS. Effect of Early Palliative Care on Chemotherapy Use and End-of-Life Care in Patients with Metastatic Non-Small-Cell Lung Cancer. Journal of Clinical Oncology. 2012;30(4):394–400. doi: 10.1200/JCO.2011.35.7996. [DOI] [PubMed] [Google Scholar]
- Holahan J, Garrett B, Headen I. Lucas A. 2009. “ Health Reform: The Cost of Failure ” [accessed on May 1, 2009]. Available at http://www.urban.org/url.cfm?ID=411887.
- Huskamp HA, Keating NL, Malin JL, Zaslavsky AM, Weeks JC, Earle CC, Teno JM, Virnig BA, Kahn KL, He Y. Ayanian JZ. Discussions with Physicians about Hospice among Patients with Metastatic Lung Cancer. Archives of Internal Medicine. 2009;169(10):954–62. doi: 10.1001/archinternmed.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J. Ward E. Cancer Statistics, 2010. CA: A Cancer Journal for Clinicians. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Kaiser Family Foundation. 2010. “ Medicaid Coverage and Spending in Health Reform: National and State-by-State Results for Adults at or Below 133% FPL ” [accessed on May 1, 2010]. Available at http://www.kff.org/healthreform/8076.cfm.
- Kuebler KK, Esper P. Heidrich DE. Palliative and End-of-life Care: Clinical Practice Guidelines. St. Louis, MO: Elsevier Health Sciences; 2006. [Google Scholar]
- Lantz PM, Weisman CS. Itani Z. A Disease-Specific Medicaid Expansion for Women. The Breast and Cervical Cancer Prevention and Treatment Act of 2000. Womens Health Issues. 2003;13(3):79–92. doi: 10.1016/s1049-3867(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Lund JL, Harlan LC, Yabroff KR. Warren JL. Should Cause of Death from the Death Certificate be Used to Examine Cancer-Specific Survival? A Study of Patients with Distant Stage Disease. Cancer Investigation. 2010;28(7):758–64. doi: 10.3109/07357901003630959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack JW, Cronin A, Taback N, Huskamp HA, Keating NL, Malin JL, Earle CC. Weeks JC. End-of-Life Care Discussions among Patients with Advanced Cancer: A Cohort Study. Annals of Internal Medicine. 2012;156(3):204–10. doi: 10.1059/0003-4819-156-3-201202070-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning WG. Mullahy J. Estimating Log Models: To Transform or Not to Transform? Journal of Health Economics. 2001;20(4):461–94. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- Matsuyama R, Reddy S. Smith TJ. Why Do Patients Choose Chemotherapy Near the End of Life? A Review of the Perspective of Those Facing Death from Cancer. Journal of Clinical Oncology. 2006;24(21):3490–6. doi: 10.1200/JCO.2005.03.6236. [DOI] [PubMed] [Google Scholar]
- Morrison RS, Dietrich J, Ladwig S, Quill T, Sacco J, Tangeman J. Meier DE. Palliative Care Consultation Teams Cut Hospital Costs for Medicaid Beneficiaries. Health Affairs. 2011;30(3):454–63. doi: 10.1377/hlthaff.2010.0929. [DOI] [PubMed] [Google Scholar]
- Neuberg GW. The Cost of End-of-Life Care: A New Efficiency Measure Falls Short of AHA/ACC Standards. Circulation: Cardiovascular Quality and Outcomes. 2009;2(2):127–33. doi: 10.1161/CIRCOUTCOMES.108.829960. [DOI] [PubMed] [Google Scholar]
- North American Association of Central Cancer Registries. 2012. “ NAACCR Data Standards for Cancer Registries ” [accessed on May 1, 2012]. Available at http://www.naaccr.org/
- Parks SM. Winter L. End of Life Decision-Making for Cancer Patients. Primary Care. 2009;36(4):811–23. doi: 10.1016/j.pop.2009.07.006. table of contents. [DOI] [PubMed] [Google Scholar]
- Peppercorn JM, Smith TJ, Helft PR, Debono DJ, Berry SR, Wollins DS, Hayes DM, Von Roenn JH, Schnipper LE O. American Society of Clinical. American Society of Clinical Oncology Statement: Toward Individualized Care for Patients with Advanced Cancer. Journal of Clinical Oncology. 2011;29(6):755–60. doi: 10.1200/JCO.2010.33.1744. [DOI] [PubMed] [Google Scholar]
- Pizer SD, Frakt AB. Iezzoni LI. Uninsured Adults with Chronic Conditions or Disabilities: Gaps in Public Insurance Programs. Health Affairs. 2009;28(6):w1141–50. doi: 10.1377/hlthaff.28.6.w1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey SD, Zeliadt SB, Richardson LC, Pollack L, Linden H, Blough DK. Anderson N. Disenrollment from Medicaid after Recent Cancer Diagnosis. Medical Care. 2008;46(1):49–57. doi: 10.1097/MLR.0b013e318158ec7f. [DOI] [PubMed] [Google Scholar]
- Riley GF, Potosky AL, Lubitz JD. Kessler LG. Medicare Payments from Diagnosis to Death for Elderly Cancer Patients by Stage at Diagnosis. Medical Care. 1995;33(8):828–41. doi: 10.1097/00005650-199508000-00007. [DOI] [PubMed] [Google Scholar]
- Sharma G, Freeman J, Zhang D. Goodwin JS. Trends in End-of-Life ICU Use among Older Adults with Advanced Lung Cancer. Chest. 2008;133(1):72–8. doi: 10.1378/chest.07-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Trogdon J, Ekwueme DU, Gardner JG, Whitmire JT. Rao C. Cost of Cervical Cancer Treatment: Implications for Providing Coverage to low-Income Women under the Medicaid Expansion for Cancer Care. Womens Health Issues. 2010;20(6):400–5. doi: 10.1016/j.whi.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Trogdon J, Ekwueme DU, Gardner JG, Whitmire JT. Rao C. Cost of Breast Cancer Treatment in Medicaid: Implications for State Programs Providing Coverage for Low-Income Women. Medical Care. 2011;49(1):89–95. doi: 10.1097/MLR.0b013e3181f81c32. [DOI] [PubMed] [Google Scholar]
- Tangka FK, Trogdon JG, Ekwueme DU, Guy GP, Jr, Nwaise I. Orenstein D. State-Level Cancer Treatment Costs: How Much and Who Pays? Cancer. 2013;119(12):2309–16. doi: 10.1002/cncr.27992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel JS, McCannon J, Greer JA, Jackson VA, Ostler P, Pirl WF, Lynch TJ. Billings JA. Aggressiveness of Care in a Prospective Cohort of Patients with Advanced NSCLC. Cancer. 2008;113(4):826–33. doi: 10.1002/cncr.23620. [DOI] [PubMed] [Google Scholar]
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA. Lynch TJ. Early Palliative Care for Patients with Metastatic Non-Small-Cell Lung Cancer. New England Journal of Medicine. 2010;363(8):733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, Mitchell SL, Jackson VA, Block SD, Maciejewski PK. Prigerson HG. Associations between End-of-Life Discussions, Patient Mental Health, Medical Care Near Death, and Caregiver Bereavement Adjustment. Journal of American Medical Association. 2008;300(14):1665–73. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A. Brown ML. Cost of Care for Elderly Cancer Patients in the United States. Journal of the National Cancer Institute. 2008;100(9):630–41. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wright AA, Huskamp HA, Nilsson ME, Maciejewski ML, Earle CC, Block SD, Maciejewski PK. Prigerson HG. Health Care Costs in the Last Week of Life: Associations with End-of-Life Conversations. Archives of Internal Medicine. 2009;169(5):480–8. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


