Abstract
Objective
In 2011, the Centers for Medicare and Medicaid Services (CMS) replaced fee-for-service reimbursement for erythropoiesis stimulating agents (ESAs) with a fixed-sum bundled payment for all dialysis-related care and pay-for-performance incentives to discourage maintaining patients' hematocrits above 36 percent. We examined the impact of the new payment policy on the use of ESAs.
Data Sources
CMS's Renal Information Management System.
Study Design
Regression discontinuity design assessing the use of ESAs by hematocrit level before and after the implementation of the payment policy change.
Data Extraction
Secondary data from 424,163 patients receiving hemodialysis treatment between January 2009 and June 2011.
Principal Findings
The introduction of bundled payments with pay-for-performance initiatives was associated with an immediate and substantial decline in the use of ESAs among patients with hematocrit >36 percent and little change in the use of ESAs among patients with hematocrit ≤36 percent. In the first two quarters of 2011, the use of ESAs during dialysis fell by about 7–9 percentage points among patients with hematocrit levels >36 percent. No statistically significant differences in ESA use were observed at the thresholds of 30 or 33 percent.
Conclusions
CMS's payment reform for dialysis care reduced the use of ESAs in patients who may not benefit from these agents.
Keywords: Renal dialysis, bundled payments, incentive, Medicare
Despite widespread concern that fee-for-service (FFS) payments may motivate providers to maximize the volume and intensity of services while providing little financial incentive to provide effective care, FFS remains the dominant mode of paying providers in the United States (MedPAC 2011). The Centers for Medicare and Medicaid Services and others have therefore experimented with alternative payment models to FFS, including bundling and pay for performance (P4P). Under bundling, one provider or a group of providers are paid a single consolidated reimbursement for an episode of care instead of disaggregated payment for each test, procedure, or visit (Iglehart 2011; Mechanic 2011; Winkelmayor and Chertow 2011). P4P schemes tie payments to a provider's ability to meet specific clinical performance targets.
The Medicare program's payment policy for dialysis care provides a unique opportunity to examine the effects of transitioning from a limited bundle with FFS payments for ancillary tests and drugs to an expanded bundled payment and pay-for-performance incentives. Until December 31, 2010, Medicare reimbursed dialysis providers separately for each dose of administered erythropoiesis stimulating agents (ESAs), synthetic versions of the endogenous hormone erythropoietin used to treat anemia. This policy may have led to excessive use of ESAs since the per-unit cost of acquiring the drugs was often less than the per-unit Medicare reimbursement (Besarab et al. 1998). From 1991 to 2008, Medicare spending on ESAs increased nearly 10-fold from $200 million to about $2 billion (U.S. Renal Data System 2009). This growth occurred in spite of emerging clinical trial evidence demonstrating lack of benefit or an increased risk for death or stroke among patients treated with ESAs to normal or high levels of hematocrit (Besarab et al. 1998; Drüeke et al. 2006; Singh et al. 2006; Pfeffer et al. 2009; Brookhart et al. 2010). In 2007, the National Kidney Foundation (NKF) issued clinical practice guidelines recommending that hematocrit levels in ESRD patients range from 33 to 36 percent (NKF 2007), and the Food and Drug Administration (FDA) released “black-box” warnings suggesting a risk when the target hematocrit level exceeds 36 percent (FDA 2007).
Effective January 1, 2011, Medicare introduced an “expanded bundled” payment with performance incentives that altered how providers are paid for dialysis care (Federal Register 2010, 2012). Under the new bundle, Medicare pays providers a fixed sum for dialysis treatments, supplies, laboratory tests, and injectable drugs or their equivalent, including ESAs (MedPAC 2011). This meant that, all else remaining equal, providers could not increase revenue by using more ESAs while reducing ESA use would decrease per-patient provider expenditures. Although the uptake of bundling was expected to be staggered through 2014, over 95 percent of the providers chose to adopt the new payment model immediately (Nissenson, Mayne, and Krishnan 2011). Along with the expanded bundled payment, the Medicare program initiated payment penalties that cut reimbursements by up to 2 percent for dialysis facilities that did not meet a prespecified standard on a composite of three performance indicators in 2012; one pertained to maintaining hematocrit levels above 30 percent, a second pertained to maintaining hematocrit below 36 percent, and a third measure pertained to the Urea Reduction Ratio, a measure of the adequacy of dialysis (Swaminathan et al. 2012). The indicators that are included in the calculation of the payment penalties continue to change and evolve. For example, in 2013, CMS dropped the second measure mentioned above (i.e., hematocrit >36 percent).
In this study, we examined the immediate impact of Medicare's dialysis payment reforms on the use of ESAs among ESRD patients undergoing hemodialysis.
Methods
Policy Context
In this section, we provide an overview of the incentives and constraints that dialysis providers faced before and after the dialysis payment reform in January 2011. We will focus this discussion only on the reimbursements by CMS, since the recent changes in payment reform pertain exclusively to Medicare patients who comprise over three-fourths of all dialysis patients nationally.
Fee for Service Prior to January 2011
Prior to January 2011, CMS paid dialysis providers on a FFS basis. There were two main categories of FFS payments to dialysis providers. The first was the reimbursement for dialyzing the patient (typically three times a week). This payment rate, which remained virtually unchanged since 1983, was approximately $138 per treatment The second category of reimbursement was payment for each administered dose of medication, including ESAs. In 2007, CMS reimbursed dialysis providers $10 per 1,000 units of administered ESAs while the acquisition cost of ESAs was $8 per 1,000 units for the large dialysis organizations (Swaminathan et al. 2012). This margin gave providers strong incentives to maximize their use of ESAs.
Bundled Payments with Payment Penalties from January 2011
Beginning in January 2011, Medicare reformed dialysis payments to remove the incentive to overprescribe ESAs. CMS reimbursements for dialysis and all administered medications (including ESAs) were bundled together at a rate of $230 per treatment. In addition, CMS imposed modest payment penalties if the mean hematocrit level of a center's dialysis patients exceeded 36 percent or was lower than 30 percent (Swaminathan et al. 2012). Thus, under this new payment regime, providers could maximize profits by lowering their use of ESAs since they received the same flat bundled fee irrespective of the amount of administered ESAs. One countervailing force to this incentive may have been that at extremely low hematocrit levels patients may experience worse health outcomes or require hospitalization or blood transfusion. Further, consistently lower hematocrit levels may expose a dialysis provider to modest payment cuts.
If providers were solely motivated by maximizing monetary returns, the optimal outcome to the changed payment system would be to reduce ESA use at all hematocrit levels. However, the nephrology community (NKF) has consistently urged that providers target a hematocrit level of 36 percent, though there was more financial benefit in targeting levels even exceeding 36 percent. We hypothesize that CMS's bundled dialysis payments with payment penalties reduced the use of ESAs at hematocrit levels exceeding 36 percent.
Empirical Strategy
Before providing a detailed description of our statistical model, we provide a conceptual empirical framework. Each dialysis encounter in the dataset includes the most recent hematocrit value of the patient and whether an ESA was administered. Since ESAs are provided to regulate patient hematocrit levels within a given range, they may be prescribed on some visits, but not on others. The FDA-mandated label on ESAs, and clinical guideline from NKF stipulate caution in targeting hematocrit levels higher than 36 percent. Further, the CMS mandated potential payment penalties for maintaining patients' hematocrits above 36 percent. We hypothesize that prior to dialysis payment reform, there was a strong financial incentive to maximize the use of ESAs, irrespective of a patient's hematocrit level. Following the CMS's bundling and P4P payment reforms, dialysis providers instead faced an incentive to minimize their use of ESAs, particularly among patients with hematocrits exceeding 36 percent.
We used a regression discontinuity approach (Thistlewaite and Campbell 1960; Rubin 1977) nested within a pre-post design to evaluate the impact of the new bundled payment policy on the use of ESAs at specific hematocrit levels. In particular, we compared differences in the use of ESAs at hematocrit levels just above and just below the thresholds of 30, 33, and 36 percent, both before and after the institution of the expanded bundled payment policy. The regression discontinuity design is appropriate because unmeasured characteristics of patients that are potentially correlated with ESA use (i.e., unmeasured health status) are likely to be very similar for patients with hematocrit levels just below and above any given threshold (for example, 36 percent), a hypothesis that we further investigate. Further, the pre-post design allows us to empirically determine whether changes in ESA prescribing before and after January 2011 reflected established secular trends or a discrete break at the precise period of the policy change. Hence, this study design seeks to establish whether changes in the use of ESAs at three hematocrit thresholds (30, 33, and 36 percent) between periods before and after January 1, 2011, were plausibly caused by the new payment policy.
Study Population and Data Sources
We obtained data from the Renal Management Information System (REMIS) on all ESRD patients undergoing hemodialysis between January 1, 2009, and June 30, 2011, through the Centers for Medicare and Medicaid Services (CMS.gov 2012). Providers must report REMIS data to CMS for all patients receiving dialysis under their care. The data contain individual-level demographic and clinical information for a particular dialysis session, and the data are reported at least once per quarter for each patient. The CMS use these data to assess the quality of care provided to patients in the ESRD program.
For each reported dialysis session, the provider indicates whether an ESA was administered and the most recent hematocrit level that was available prior to the decision to prescribe or not prescribe ESA.
In our main analysis, we used data from 424,163 hemodialysis patients that underwent at least one dialysis treatment between January 1, 2009, and June 30, 2011, in a dialysis facility in the United States. In total, these patients generated a total of 2,539,209 observations. The CMS Privacy Board and Brown University's Research Protection Office approved the study protocol; informed consent from the patients was not required.
Variables
Our main dependent variable was the use of ESAs (yes or no) during a dialysis session. The main independent variables were the most recent hematocrit level at the time that ESA was administered, the time period of the measurement (year-quarter), and an interaction between hematocrit and time period (see Statistical Analysis section below).
Additional patient-level covariates included age, sex, race (white, black, and other), albumin and hematocrit levels at time of entry into ESRD, primary insurance status (Medicare, Medicaid, group insurance, other insurance, and no insurance) at the start of dialysis therapy, body mass index (BMI), time (in years) since entering the ESRD program (vintage), and the presence or absence of the following comorbid conditions at the time of initiation of dialysis: congestive heart failure, ischemic heart disease, diabetes, and hypertension. Comorbid conditions were assessed at the time of entry into the ESRD program. We included piecewise linear splines of age (with nodes at 19, 45, 65, and 74), a dummy variable for whether the albumin less was ≥3.5 g/dl or <3.5 g/dl, and continuously measured BMI.
Statistical Analysis
We constructed a linear regression model to estimate the relationship between hematocrit level and the use of ESAs. Each regression included observations from at least two quarters, where T0 denoted observations from one quarter and T1 denoted observations from a second quarter. In our regressions, we included the following terms: (i)  , an indicator variable for whether the observation was made in period T1; (ii) hematocrit level (hemat); (iii) Dhemat>τ, an indicator variable if hematocrit was greater than some threshold τ; (iv) an interaction term between T1 and hemat; and (v) an interaction term between Dhemat>τ and T1. The coefficients α4 and α5 are our primary estimates of interest and measure the difference in ESA use in period T1 relative to the period T0.
, an indicator variable for whether the observation was made in period T1; (ii) hematocrit level (hemat); (iii) Dhemat>τ, an indicator variable if hematocrit was greater than some threshold τ; (iv) an interaction term between T1 and hemat; and (v) an interaction term between Dhemat>τ and T1. The coefficients α4 and α5 are our primary estimates of interest and measure the difference in ESA use in period T1 relative to the period T0.
| 1 |
The vector denoted by x includes patient-level covariates previously mentioned. We clustered the standard errors by patient hematocrit level.
We explicitly tested for the presence of ESA treatment “discontinuities” at three hematocrit values. To do this, we performed analyses using 30, 33, and 36 percent as the thresholds of interest (τ).
All analysis were performed in STATA 11.0 (StataCorp 2011).
Results
Table1 presents the differences in the mean characteristics of individuals with hematocrit levels >36 percent, and those with hematocrit ≤36 percent, in the prebundling and postbundling periods. There was no difference in the characteristics of patients with hematocrit levels just above and below 36 percent. However, in the first two quarters of 2011 (post payment-reform), the use of ESAs was about 10 percentage points lower at hematocrit levels exceeding 36 percent compared to at levels ≤36 percent.
Table 1.
Demographic and Clinical Characteristics of Study Population, by Level of Hematocrit and Time Period
| 2011q1q2 | 2010q3q4 | 2010q1q2 | 2009q3q4 | 2009q1q2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | 483,131 | 501,245 | 527,329 | 513,026 | 514,478 | |||||
| Hct (%) | ≤36 | >36 | ≤36 | >36 | ≤36 | >36 | ≤36 | >36 | ≤36 | >36 |
| ESA use (%) | 56 | 46 | 57 | 56 | 56.1 | 57.2 | 58.1 | 57.7 | 59.1 | 58.3 |
| Socio-demographics | ||||||||||
| Age, year (SD) | 62.8 (15.1) | 63 (15.1) | 62.8 (15.1) | 63.3 (15.1) | 62.8 (15.1) | 63 (15.1) | 62.8 (15.1) | 63.3 (15.1) | 62.8 (15.1) | 63.3 (15.1) |
| Vintage, year (SD) | 3.5 (2) | 3.5 (2) | 3.5 (2) | 3.5 (2) | 3.5 (2) | 3.5 (2) | 3.5 (2) | 3.5 (2) | 3.5 (2) | 3.5 (2) |
| Male (%) | 54 | 53 | 54 | 53 | 54 | 54 | 54 | 54 | 54 | 53 |
| Race (%) | ||||||||||
| White | 54 | 55 | 55 | 54 | 54 | 55 | 55 | 54 | 55 | 54 |
| Black | 38 | 37 | 37 | 39 | 38 | 37 | 38 | 39 | 38 | 39 |
| Other race | 8 | 8 | 8 | 7 | 8 | 8 | 7 | 7 | 7 | 7 |
| Health insurance (%) | ||||||||||
| Medicare | 54 | 54 | 54 | 54 | 54 | 54 | 54 | 54 | 54 | 54 |
| Medicaid | 29 | 28 | 29 | 30 | 29 | 28 | 30 | 30 | 29 | 30 |
| Group coverage | 22 | 22 | 22 | 21 | 22 | 22 | 21 | 20 | 21 | 21 |
| Other coverage | 11 | 11 | 11 | 12 | 11 | 11 | 11 | 11 | 11 | 12 |
| No insurance | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Co-morbidities (%) | ||||||||||
| CHF | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 27 |
| IHD | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Hypertension | 42 | 42 | 42 | 42 | 42 | 42 | 42 | 42 | 42 | 42 |
| Diabetes | 44 | 44 | 45 | 45 | 44 | 44 | 45 | 44 | 44 | 44 |
| Clinical variables | ||||||||||
| Mean Albumin, g/dl (SD) | 2.9 (1) | 2.8 (1) | 3.0 (1) | 3.0 (1) | 2.9 (1) | 2.8 (1) | 3.0 (1) | 3.0 (1) | 3.0 (1) | 3.0 (1) |
| Hemoglobin, g/dl (SD) | 10 (1.7) | 10 (1.7) | 9.9 (1.7) | 9.9 (1.7) | 10 (1.7) | 10 (1.7) | 9.9 (1.7) | 9.9 (1.7) | 9.9 (1.7) | 9.9 (1.7) |
| Body mass index (SD) | 36.7 (6.9) | 36.5 (6.9) | 36.3 (6.9) | 36.5 (6.9) | 36.7 (6.9) | 36.5 (6.9) | 36.3 (6.9) | 36.5 (6.9) | 36.3 (6.9) | 36.5 (6.9) |
Note. All variables in table except for ESA use and Hct (hematocrit) and Vintage are measured at the time the patient begins dialysis. The variable vintage is simply the average number of years since beginning dialysis. Body mass index is measured as the ratio of patient weight (in kg) to the square of patient height (m2). Albumin and hemoglobin are measured in grams per deciliter (g/dl).
CHF, congestive heart failure; IHD, ischemic heart disease.
Figure1 plots trends in the use of ESA in each quarter between the first quarter of 2009 and the fourth quarter of 2011 for patients with hematocrit >27 and ≤30 percent; hematocrit >30 and ≤33 percent; hematocrit >33 and ≤36 percent; and patients with hematocrit >36 and ≤42 percent. From 2009 to 2010, there was little change in the use of ESAs for patients with hematocrit ≤36 percent. However, among patients with hematocrit >36 percent, there was a greater discontinuation in ESA use right after the introduction of bundling. In panel A of Figure2, the relationship between use of ESAs and the most recent hematocrit is plotted for successive half-year periods beginning with January 2009 and ending with June 2011. The results demonstrate little relationship between hematocrit and ESA administration in the prepayment reform period, but a greater discontinuation in the use of ESA at hematocrit levels >36 percent in the postpayment reform period. Further, there has been virtually no change in the relationship between ESA use and hematocrit at levels less than 36 percent. In panel B of Figure2, we plot the change in the relationship between ESA use and hematocrit between successive half-year periods. The figure suggests that, relative to the prebundling period, there is about an 8–10 percentage point decrease in the use of ESAs at hematocrit levels greater than 36 percent in the period between the first half of 2011 (2011q1q2), and the last half of 2010 (2010q3q4) but no change at levels ≤36 percent. There was no discernible trend between the periods 2009q3q4 (quarters 3 and 4 of 2009) and 2009q1q2 (quarters 1 and 2 of 2009), 2010q1q2 (quarters 1 and 2 of 2010) and 2009q3q3 (quarters 3 and 4 of 2009), and 2010q3q4 (quarters 3 and 4 of 2010) and 2010q1q2.
Figure 1.

Trends in the Use of ESA in Varying Ranges of Hematocrit LevelsNote. Scale on Y-axis ranges from 30 to 70 percent. The percentage rates refer to the use of any ESA during dialysis encounters that occurred during the specified time period.
Figure 2.

(A) Use of ESA by Hematocrit Levels. (B) Temporal Changes in Discontinuation in Use of ESA by Hematocrit Levels and PeriodNote. Scale on Y-axis ranges from 30 to 70 percent. The percentage rates refer to the use of any ESA during dialysis encounters that occurred during the specified time period.
Figure3 presents estimates of the coefficient α3 (estimated in equation 1) using three different thresholds (τ): 36 percent, 30, and 33 percent. In panel A, we graphically present the regression-adjusted estimates of the change in ESA use at hematocrit levels >36 percent (relative to those ≤36 percent) between successive quarters. The results demonstrate a 7.1 percentage point (95 percent CI: 5.6–8.6) decrease in the use of ESA in the first quarter of 2011 (relative to the fourth quarter of 2010) and a 9.3 percentage point (95 percent CI: 7.8–10.8) decrease in the quarter 2 of 2011 (relative to the fourth quarter of 2010). In contrast, there was no significant decrease in the use of ESAs across any of the prebundling time periods. In Figure3, panels B and C, we plot graphs depicting the changes in ESA use at hematocrit levels of 30 and 33 percent, respectively. We find that there was only limited and statistically insignificant change in the use of ESAs at these thresholds in both the pre- and postpayment reform periods.
Figure 3.

(A) Discontinuation in ESA Use at Hematocrit >36 percent Relative to Hematocrit ≤36 percent: Temporal Changes between Successive Quarters. (B) Discontinuation at Hematocrit >30 percent Relative to Patients with Hematocrit ≤30 percent. (C) Discontinuation at Hematocrit >33 percent Relative to Patients with Hematocrit ≤33 percentNotes. (A) Each point estimate shown in the figure represents the temporal change in ESA use at hematocrit >36 percent relative to the same change in patients with hematocrit ≤36 percent. For example, the first estimate presented is the (rate of ESA use in 2009Q2 for Patients with hematocrit >36 percent minus the rate in 2009Q1 for patients with hematocrit >36 percent) minus (Rate in 2009Q2 for Patients with hematocrit ≤36 percent minus the rate in 2009Q1 for patients with hematocrit ≤36 percent). Estimates shown in figure are regression-adjusted for age, sex, race, insurance status, initial hemoglobin, comorbid conditions, time since beginning dialysis (vintage), BMI, and albumin. Standard errors are clustered by patient hematocrit level and provider. The vertical bars denote the 95 percent confidence intervals around the point estimate.(B) Each point estimate shown in the figure represents the temporal change in ESA use at hematocrit >30 percent relative to the same change in patients with hematocrit ≤30 percent. For example, the first estimate presented is the (rate of ESA use in 2009Q2 for Patients with hematocrit >30 percent minus the rate in 2009Q1 for patients with hematocrit >30 percent) minus (Rate in 2009Q2 for Patients with hematocrit ≤30 percent minus the rate in 2009Q1 for patients with hematocrit ≤30 percent). Estimates shown in figure are regression-adjusted for age, sex, race, insurance status, initial hemoglobin, comorbid conditions, time since beginning dialysis (vintage), BMI, and albumin. Standard errors are clustered by patient hematocrit level and provider. The vertical bars denote the 95 percent confidence intervals around the point estimate.(C) Each point estimate shown in the figure represents the temporal change in ESA use at hematocrit >33 percent relative to the same change in patients with hematocrit ≤33 percent. For example, the first estimate presented is the (rate of ESA use in 2009Q2 for Patients with hematocrit >33 percent minus the rate in 2009Q1 for patients with hematocrit >33 percent) minus (rate in 2009Q2 for Patients with hematocrit ≤33 percent minus the rate in 2009Q1 for patients with hematocrit ≤33). Estimates shown in figure are regression-adjusted for age, sex, race, insurance status, initial hemoglobin, comorbid conditions, time since beginning dialysis (vintage), BMI, and albumin. Standard errors are clustered by patient hematocrit level and provider. The vertical bars denote the 95 percent confidence intervals around the point estimate.
Finally, using data from the first two quarters of 2011, in Figure4 we examines the changes in ESA use at 36 percent for different subgroups of patients. We find that the decline in ESA use at 36 percent is robust across a wide range of patient demographic (race, gender, and age) and clinical characteristics (presence of hypertension, congestive heart failure, or diabetes).
Figure 4.
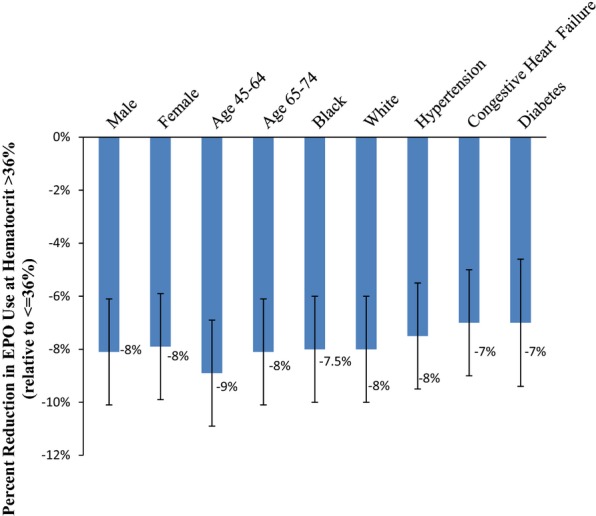
Postbundling Discontinuation in ESA Use at Hematocrit >36 percent Relative to Hematocrit ≤36 percent, by Gender, Age, Race, and Presence of Hypertension, Congestive Heart Failure, and DiabetesNotes. Each point estimate shown in the figure represents the difference in ESA use at hematocrit >36 percent relative to ESA use in patients with hematocrit ≤36 percent. Estimates shown in figure are regression-adjusted for age, sex*, race*, insurance status, initial hemoglobin, comorbid conditions, time since beginning dialysis (vintage), BMI, and albumin. Standard errors are clustered by patient hematocrit level and provider. The vertical bars denote the 95 percent confidence intervals around the point estimate. *sex: not included in gender-specific estimates; race* not included in race-specific estimates.
Discussion
In 2011, Medicare fundamentally changed the method for paying for dialysis care by bundling reimbursements for ESAs used to treat anemia in patients with ESRD, along with payments for all other dialysis-related services. In this study of all patients with ESRD receiving hemodialysis, we find that the introduction of bundled payments with pay-for-performance initiatives was associated with an immediate and substantial decline in the use of ESAs among patients with hematocrit >36 percent and little change in the use of ESA among patients with hematocrit ≤36 percent. Specifically, in the first two quarters of 2011, the use of ESA fell by about 7–9 percentage points among patients with hematocrit levels >36 percent. No statistically significant difference in ESA use was noted at thresholds of either 30 or 33 percent. Further, there was no change in the characteristics of the patients treated in the pre- and postpayment-reform periods. Taken together, the results suggest that bundled ESRD payments and performance-based incentives led to a better alignment between clinical practice and guidelines advocated both by the FDA and the NKF. Further, we did not observe a decrease in the use of ESA among patients with hematocrit levels less than 36 percent; suggesting that providers continued to administer ESAs to patients who may benefit from the drug, despite financial incentives to the contrary.
Our results are broadly consistent with recent data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) monitor, which suggest that the percent of patients with hematocrit levels exceeding 36 percent declined from 31.4 to 28.0 percent between August 2010 and April 2011 (Robinson et al. 2012). We find that ESA use among patients with ESRD fell in the first half of 2011, but primarily among patients with a hematocrit >36 percent. Previous studies have shown that under FFS reimbursement for ESA use, for-profit dialysis providers increased the use of ESAs relative to their not-for-profit counterparts (Thamer et al. 2007). We found that the mean hematocrit levels in the time period immediately preceding payment reforms was 35.7 percent with a standard deviation of about 3.5. This suggests that, on average, dialysis providers are not motivated solely by profits. If so, then payment reforms, by shifting ESAs from a profit-maximizing to a cost-minimizing component of the provider's objective function, could result in a decline in the use of ESAs at hematocrit levels exceeding 36 percent but preserve the use of ESAs at hematocrit levels less than 36 percent. Our hypothesis is that the introduction of CMS's payment reform for dialysis care will incentivize providers to reduce the use of ESAs, especially at hematocrit levels exceeding 36 percent. We found no immediate discontinuation in ESA use at hematocrit levels less than 36 percent, possibly a result of the (arguably mild) pay-for-performance initiatives that penalized providers when their patients' hematocrit levels fell below 30 percent.
Although the payment change for dialysis care provides a unique opportunity to examine the effects of transitioning to a expanded bundled payment with pay-for-performance incentives, necessary caution must be exercised in understanding whether the effects are generalizable to other contexts. The actual implementation of the bundled payments with payment penalties was done after almost a decade (CMS 2004) of discussions and debates on the potential benefits and negative unintended consequences of payment reform. The metrics that CMS adopted were aligned with clinical guidelines promulgated by the NKF and broadly consistent with evidence from randomized clinical trials. In addition, the market for ESRD services is highly consolidated with a substantial portion of ESRD care provided by two major providers (DaVita and Fresenius). This consolidation may facilitate rapid changes in clinical practice in response to a change in the payment mechanism. Finally, CMS implemented detailed data collection methods to document providers, adherence to performance metrics pertaining to hematocrit levels. For each patient on a quarterly basis, dialysis providers must submit the patient's most recent hematocrit level along with his or her use or nonuse of ESA. This provided CMS with the necessary data to monitor performance and implement payment penalties.
This study had important limitations. First, given the observational nature of the data, we cannot exclude the alternative explanation that another event in early 2011 contributed to the patterns in ESA administration that we observed. Second, the study does not address the important question of whether the decrease in the use of ESAs (at hematocrit levels >36 percent) was associated with changes in health outcomes. Third, bundling of payments might also have brought about changes in ESA doses conditional on use. Our data do not include information on ESA dose. It is entirely possible that at hematocrits of 33–36 percent, providers are maintaining ESA therapy but reducing dosages and thereby maximizing the profits obtained from their bundled payment. Above 36 percent, providers might perceive little risk of transfusion or other complications, and therefore recognize that there is neither financial nor patient harm in discontinuing ESA treatment.
Fourth, our data cover the period through June 2011 and therefore only represent the early effects of bundled payments on ESA use. However, isolating the effects of bundled payments separate from other policy and regulatory changes requires that we focus on the first two quarters of 2011. In particular, in the third and fourth quarters of 2011, the FDA issued a new advisory that removed the requirement that providers target a hematocrit level of at least 30 percent in hemodialysis patients (FDA 2011). The CMS followed suit, and in November 2011, they eliminated the quality indicator assessing the proportion of dialysis patients with hematocrit below 30 percent from the composite used to calculate payment penalties for dialysis providers (CMS 2011). Fifth, the hematocrit values used in this paper are based on the value prior to the decision to administer ESAs; we cannot say whether a particular ESA administration decision is guided by a particular hematocrit value. Sixth, data on several variables such as comorbid conditions are only present at the time of initiating into ESRD. Changes in these conditions over time may introduce measurement error in our independent variables. Nevertheless, we do not expect that such error would cause any difference in the estimated effect of bundled payments on the use of ESAs since such error should be equally prevalent in patients with hematocrit levels just above and just below 36 percent.
The NKF has consistently maintained that the optimal hematocrit levels of dialysis patients should lie between 33 and 36 percent. In the wake of the shift in reimbursement in January 2011, concerns may have been raised that the health of patients may be adversely affected (Iglehart 2011). Our findings, to a limited degree, allay some of those fears. We find that the introduction of bundled payments for dialysis care was associated with substantial and immediate discontinuation in the use of ESAs among patients with a hematocrit level of greater than 36 percent, but no significant discontinuation in ESA use at lower hematocrit levels. The findings suggest that the implementation of alternative provider payment methods to FFS reimbursement may accelerate evidence-based practice and reduce the use of clinically inappropriate health services.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: We acknowledge support from the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (Grant No. R21 DK095485 02). Rajnish Mehrotra has received grant support and/or honorarium from Amgen, Baxter Healthcare, and DaVita Inc.
Disclosures: None.
Disclaimers: None.
Supporting Information
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
References
- Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The Effects of Normal as Compared with Low Hematocrit Values in Patients with Cardiac Disease Who Are Receiving Hemodialysis and Epoetin. New England Journal of Medicine. 1998;339:584–90. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- Brookhart AM, Schneeweiss S, Avorn J, Bradbury BD, Liu J. Winkelmayer WC. Comparative Mortality Risk of Anemia Management Practices in Incident Hemodialysis Patients. Journal of the American Medical Association. 2010;303(9):857–64. doi: 10.1001/jama.2010.206. [DOI] [PubMed] [Google Scholar]
- CMS. 2004. “ Centers for Medicare and Medicaid Services: CMS Bundled Payment for Care Initiative. September 2012 ” [accessed on September 15, 2012]. Available at http://www.innovations.cms.gov/initiatives/bundled-payments/index.html.
- CMS. 2011. “ Centers for Medicare and Medicaid Services: Fact Sheet. November 2011 ” [accessed on September 22, 2012]. Available at http://www.cms.gov/apps/media/press/factsheet.asp?counter=4148.
- Renal Management Information System (REMIS) [Internet] Baltimore, MD: Centers for Medicare and Medicaid Services; 2012. CMS.gov [accessed on July 25, 2012]. Available at http://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/IdentifiableDataFiles/RenalManagementInformationSystem.html. [Google Scholar]
- Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU. Scherhag A for the CREATE Investigators. Normalization of Hemoglobin Levels in Patients with Chronic Kidney Disease and Anemia. New England Journal of Medicine. 2006;355(20):2071–84. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- Federal Register. Medicare Program; End-Stage Renal Disease Prospective Payment System; Final Rule and Proposed Rule. Federal Register. 2010;75(155):49030. [PubMed] [Google Scholar]
- Federal Register. Medicare Program; End-Stage Renal Disease Quality Incentive Program. Final Rule. Federal Register. 2012;76(3):628. [PubMed] [Google Scholar]
- Food and Drug Administration [FDA] 2007. [accessed on September 12, 2012]. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm109024.htm.
- Food and Drug Administration [FDA] 2011. [accessed on September 20, 2012]. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm260670.htm.
- Iglehart JK. Bundled Payment for ESRD-Including ESAs in Medicare's Dialysis Package. New England Journal of Medicine. 2011;364(7):593–5. doi: 10.1056/NEJMp1014187. [DOI] [PubMed] [Google Scholar]
- Mechanic RE. Opportunities and Challenges for Episode-Based Payment. New England Journal of Medicine. 2011;365(9):777–9. doi: 10.1056/NEJMp1105963. [DOI] [PubMed] [Google Scholar]
- MedPAC. Report to the Congress: Medicare Payment Policy [Internet] Washington, DC: Medicare Payment Advisory Commission [MedPAC]; 2011. [accessed on July 24, 2012]. Available at http://medpac.gov/documents/Mar11_EntireReport.pdf. [Google Scholar]
- National Kidney Foundation [NKF] KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease: 2007 Update of Hemoglobin Target. American Journal of Kidney Diseases. 2007;50(3):471–530. doi: 10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Nissenson AR, Mayne TJ. Krishnan M. The 2011 ESRD Prospective Payment System: Perspectives from DaVita, a For-Profit Large Dialysis Organization. American Journal of Kidney Diseases. 2011;57(4):550–2. doi: 10.1053/j.ajkd.2011.01.009. [DOI] [PubMed] [Google Scholar]
- for TREAT Investigators. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJV, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD. Toto RR. A Trial of Darbepoetin Alpha in Type 2 Diabetes and Chronic Kidney Disease. New England Journal of Medicine. 2009;361(21):2019–32. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- Robinson BM, Fuller DS, Bieber BA, Turenne MN. Pisoni RL. The DOPPS Practice Monitor for US Dialysis Care: Trends through April 2011. American Journal of Kidney Diseases. 2012;59(2):309–14. doi: 10.1053/j.ajkd.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Rubin D. Assignment to Treatment on the Basis of a Covariate. Journal of Educational and Behavioral Statistics. 1977;2:11–26. [Google Scholar]
- Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M. Reddan D for CHOIR Investigators. Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease. New England Journal of Medicine. 2006;355:2085–98. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- Swaminathan S, Mor V, Mehrotra R. Trivedi A. Medicare's Payment Strategy for End-Stage Renal Disease Now Embraces Bundled Payment and Pay-for-Performance to Cut Costs. Health Affairs. 2012;31(9):2051–8. doi: 10.1377/hlthaff.2012.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamer M, Zhang Y, Kaufman J, Cotter D, Dong F. Hernan MA. Dialysis Facility Ownership and Epoetin Dosing in Patients Receiving Hemodialysis. Journal of the American Medical Association. 2007;297(15):1667–74. doi: 10.1001/jama.297.15.1667. [DOI] [PubMed] [Google Scholar]
- Thistlewaite DL. Campbell DT. Regression-Discontinuity Analysis: An Alternative to the Ex Post Facto Experiment. Journal of Educational Psychology. 1960;51:309–17. [Google Scholar]
- U.S. Renal Data System. USRDS 2009 Annual Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive Kidney Diseases; 2009. [Google Scholar]
- Winkelmayor WC. Chertow GM. The 2011 ESRD Prospective Payment System: An Uncontrolled Experiment. American Journal of Kidney Diseases. 2011;57(4):542–6. doi: 10.1053/j.ajkd.2011.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


