Abstract
Signaling for limb bone development usually precedes that for muscle development, such that cartilage is generally present before muscle formation. It remains obscure, however, if: (i) tetrapods share a general, predictable spatial correlation between bones and muscles; and, if that is the case, if (ii) such a correlation would reflect an obligatory association between the signaling involved in skeletal and muscle morphogenesis. We address these issues here by using the results of a multidisciplinary analysis of the appendicular muscles of all major tetrapod groups integrating dissections, muscle antibody stainings, regenerative and ontogenetic analyses of fluorescently-labeled (GFP) animals, and studies of non-pentadactyl human limbs related to birth defects. Our synthesis suggests that there is a consistent, surprising anatomical pattern in both normal and abnormal phenotypes, in which the identity and attachments of distal limb muscles are mainly related to the topological position, and not to the developmental primordium (anlage) or even the homeotic identity, of the digits to which they are attached. This synthesis is therefore a starting point towards the resolution of a centuries-old question raised by authors such as Owen about the specific associations between limb bones and muscles. This question has crucial implications for evolutionary and developmental biology, and for human medicine because non-pentadactyly is the most common birth defect in human limbs. In particular, this synthesis paves the way for future developmental experimental and mechanistic studies, which are needed to clarify the processes that may be involved in the elaboration of the anatomical patterns described here, and to specifically test the hypothesis that distal limb muscle identity/attachment is mainly related to digit topology.
Keywords: birth defects, comparative anatomy, development, evolution, homology, myology, polydactyly
Introduction
The limbs of tetrapods enabled the spectacular transition from water to land habitats (Romer, 1933; Bowler, 1996, 2007; Wagner & Chiu, 2001; Fabrezi et al. 2007; Wagner & Larsson, 2007; Weatherbee & Niswander, 2007; Laurin, 2011). A major goal of research on tetrapods is to explain the evolutionary patterns – and their causes and consequences – of the considerable morphological variation in the forelimbs and hindlimbs of tetrapods, both within and diverging from the pentadactyl bauplan of extant tetrapods (N.B., more basal, fossil, taxa such as Ichthyostega and Acanthostega had up to eight digits: Coates et al. 2002; Laurin, 2011). The somatic limb muscle progenitor cells apparently do not carry intrinsic positional information (Duprez, 2002). The early development of condensations that will give rise to bones may provide the positional signaling for the subsequent development of muscles (Manzano et al. 2013). Muscles can also play an important role in at least some aspects of skeletal morphogenesis. For example, muscle contraction might help regulate chondrocyte intercalation and skeletal elongation, thus facilitating coordination between muscle and skeletal development (Shwartz et al. 2012).
Information obtained from non-pentadactyl limbs is crucial to clarify how the functional and spatial associations between bones and muscles change during the evolution of morphological variation in limbs as well as the development of the common limb birth defects found in humans and other species (Dunlap, 1967; Muntz, 1975; Shubin & Alberch, 1986; Kardon, 1998; Wagner & Chiu, 2001; Coates et al. 2002; Duprez, 2002; Wagner & Larsson, 2007; Weatherbee & Niswander, 2007; Ponssa et al. 2010; Laurin, 2011; Shwartz et al. 2012; Manzano et al. 2013). However, myological data about non-pentadactyl limbs are relatively rare in the literature. The scarcity of such information is paradoxical because limb reduction is a topic that has long attracted researchers' attention (Owen, 1849; Presch, 1975; Caputo et al. 1995; Diogo et al. 2013a,b). The interest in limb reduction can be explained by the suitability of the existing patterns for elucidating broader evolutionary themes, such as the occurrence of evolutionary trends, the frequency of anatomical convergence and the existence of evolutionary reversals that violate ‘Dollo's law’ (Diogo & Abdala, 2010; Diogo & Wood, 2012; Diogo & Tanaka, 2014). Inferences of evolutionary patterns in limbs and the study of human limb birth defects are therefore usually based either on external morphology or osteology, and almost always ignore muscular anatomy. Anatomical and developmental studies usually focus on pentadactyl autopodia (hands/feet), and those dealing with deviations from the norm focus on reduction or gain of cartilages and/or bones.
Importantly, the scarcity of data available about the relationship between hard and soft tissues in non-pentadactyl limbs affects not only the knowledge of broad evolutionary subjects but also human evolution and human medicine (Diogo & Tanaka, 2014). Indeed, changes in the number of digits are the most common anomalies of human limbs at birth (e.g. the presence of an extra toe and/or finger has a 0.2% incidence, i.e. 1 in 500 births), but information about the soft tissue changes occurring in these anomalies is extremely scarce (Castilla et al. 1996). Non-pentadactyly, especially when it concerns the complete duplication of, or failure to form, a digit, profoundly affects the functioning of the limbs and principally of the hand and digits, which are used for complex and fine tasks in daily life (Waters & Bae, 2012). Surgery is therefore often recommended and performed within the first years of life to improve the biomechanical function of the limb, and to provide the most natural look, feel and function of the corrected limb for the infant or child (Watt & Chung, 2009). Surgical options depend on the specific type of defect, and the level of duplication/reduction of both the hard and soft tissues guides the operative treatment of poly- and oligodactyly (Tonkin & Bulstrode, 2007). Therefore, such surgeries are often very complex and, due to the scarcity of muscle studies, the specific muscle configuration and attachments found in such limbs is poorly known and thus difficult to predict, particularly in the less studied types of defects (Waters & Bae, 2012).
Many limb anomalies are attributable to homeotic transformation (replacement of a normal body part by one that normally forms in another region of the body) – one of the most popular current topics in evolutionary developmental biology. For instance, in preaxial polydactyly, one of the most common congenital anomalies of the human hand, the duplication of the thumb, leads to the two most radial digits having an homeotic identity of digit 1 (Castilla et al. 1996). Homeotic transformations have also played an important role in the evolution of normal phenotypes. For example, it is now commonly accepted that the digits of the adult bird wing derive from the second, third and fourth developmental primordia (embryonic condensations), but that homeotically and morphologically these digits correspond to digits 1, 2 and 3 of other tetrapods; a similar homeotic transformation seemingly also occurred in the hand of the three-toed Italian skink Chalcides chalcides (Young et al. 2009).
In order to study the spatial associations between limb bones and muscles, we have completed a long term, multidisciplinary project (Diogo & Abdala, 2010; Diogo & Wood, 2012; Diogo & Molnar, 2014; Diogo & Tanaka, 2014) that describes and compares the appendicular muscles of all major groups of tetrapods in order to reconstruct their evolution, including dissections and developmental and regenerative studies. For the purpose of this paper, we have combined the results of these studies with new investigations of how human birth defects involving the formation of non-pentadactyl limbs influence muscle attachments (Fig.1; see Materials and methods). By doing this, we were able to test if the anatomical patterns seen in those birth defects follow the patterns seen in wild-type non-human tetrapods with non-pentadactyl limbs and to infer if the study of birth defects could therefore reciprocally illuminate those patterns under unusual conditions. This work therefore has far-reaching implications for evolutionary and developmental biology, and for human medicine by providing a broad discussion obtained in regenerative, developmental, comparative and teratological works, and paves the way for future developmental experimental and mechanistic studies.
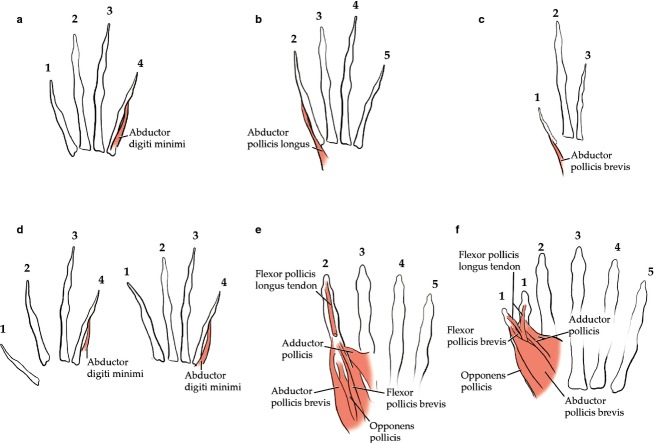
Fig 1.
Scheme of muscle insertions supporting the hypothesis presented in the present work, compiled from our previous studies of both the normal phenotype of members of various tetrapod clades and the abnormal phenotype of humans with birth defects. (a) Salamander (Ambystoma mexicanum) hand showing that the abductor digiti minimi (which usually goes to digit 5 of the pentadactyl hands of other tetrapods) goes to digit 4, which is the most ulnar digit in the hand of this species (for more details, see Diogo & Tanaka, 2014). (b) Frog (Eleutherodactylus caqui) hand, illustrating that the abductor pollicis longus (which usually goes to digit 1 of the pentadactyl hands of other tetrapods) goes to digit 2, which is the most radial digit in the hand of this species (for more details, see Diogo & Ziermann, 2014). (c) Bird (Gallus domesticus) hand, illustrating that the abductor pollicis brevis (which usually goes to digit 1 of the pentadactyl hands of other tetrapods) goes to digit 1, which is the most radial digit in the hand of this species but is developed from the primordium of digit 2 (for more details, see Diogo & Abdala, 2010; Diogo & Molnar, 2014). (d) Bird (Gallus domesticus; left) and crocodylian (Caiman latirostris; right) feet showing that the abductor digiti minimi (which usually goes to digit 5 of the pentadactyl feet of other tetrapods) goes to digit 4, which is the most fibular digit in the foot of these species (for more details, see Diogo & Abdala, 2010; Diogo & Molnar, 2014). (e) Hand of four-digit hand of a human newborn with trisomy 18 (the other hand having 6 digits and being shown in Fig.1f) that represents an extreme case of limb birth defects. Our hypothesis is supported because, despite the absence of a thumb, all the muscles normally associated with the thumb are present and attach to digit 2, which is the most radial digit. The only exception to our hypothesis is that in this hand the most radial tendon of the flexor digitorum superficialis, which usually goes to digit 2 (i.e. to a digit that is not the most radial digit) goes to digit 2 of this hand (i.e. to its most radial digit; for more details, see Smith et al. 2015). (f) Hand of six-digit hand of the same human newborn with trisomy 18. Our hypothesis is supported because, despite the presence of a partially duplicated thumb (with no duplication of metacarpal 1 but with two partial thumbs with identity of digit 1), the muscles normally associated with the thumb are not duplicated. Instead, the muscles that normally insert, respectively, onto the radial and ulnar sides of the thumb insert onto the radial and ulnar sides of the most radial and most ulnar digits with identity of digit 1, respectively, as predicted, i.e. as if the muscles were ‘unaware of/blind about’ the partial duplication of the thumb. Interestingly, the tendon of the flexor digitorum profundus, which usually goes to the central (so, not ulnar and not radial) portion of the thumb, bifurcates to go to both the most ulnar and most radial digits with identity of digit 1 (for more details, see Smith et al. 2015).
Materials and methods
Tetrapod comparative anatomy
As explained above, we have integrated data from our previous studies based on dissections, histological sectioning and imaging, and embryological and regenerative works of wild-type and fluorescently-labeled (GFP) animals with new data about skeletal and muscle human limb birth defects compiled from on our dissections and an extensive literature review. In recent works, we provided a comprehensive synthesis of the evolution and homologies of all the forearm, hand, leg and foot muscles of each major tetrapod clade (urodeles, anurans, turtles, lepidosaurs, crocodilians, birds and mammals) based on comparative (Diogo & Abdala, 2010; Diogo et al. 2010, 2013a,b; Diogo & Wood, 2012; Diogo & Molnar, 2014), developmental (Diogo & Tanaka, 2014; Diogo & Ziermann, 2014) and regenerative (Diogo et al. 2013a,b, 2014) studies. The methodology used in those publications to study the development and adult anatomy, and to compare and infer the homologies between the musculature of the taxa shown in Fig.1 and discussed in the text is described in detail in each respective publication. The ontogenetic studies, either using wild-type or GFP animals, allow us to follow and compare the development of muscles from the early stages of their appearance to their adult configuration (e.g. the primordium from which they form and the specific position within that primordium, the configuration of their fibers, how they expand, their attachments, etc.), and thus are extremely useful to study muscle homology in different taxa. A list of the studied non-primate vertebrate specimens is given by Diogo & Abdala (2010), Diogo et al. (2010, 2013a,b, 2014), Diogo & Molnar (2014), Diogo & Ziermann (2014), and Diogo & Tanaka (2014), while a list of the studied primates is given by Diogo & Wood (2012).
Human birth defects
We recently compiled information about the musculoskeletal system of 65 human trisomic individuals (27, 30 and 8 with trisomies 13, 18 and 21, respectively) dissected by us (see, e.g. Figs 3, 4 to Fig 1) and by others (muscle data collected from Barash et al. 1970; Bersu & Ramirez-Castro, 1977; Colacino & Pettersen, 1978; Ramirez-Castro & Bersu, 1978; Aziz, 1979, 1980, 1981a,b; Pettersen, 1979; Pettersen et al. 1979; Dunlap et al. 1986; Urban & Bersu, 1987). This dataset is the result of a huge effort by a team of anatomists and developmental biologists, and provides a unique opportunity to study broader evolutionary, developmental and medical issues (Smith et al. 2015). A detailed list of these 65 human trisomic individuals, organized by age, sex and type of trisomy, and major type of birth defects, including non-pentadactyly – which is often found in individuals with trisomies 13 and 18 (see, e.g. Figs 3, 4) – is given in Smith et al. (2015). As explained in more detail in Smith et al. (2015), all human individuals with birth defects dissected by us are part of the collection of birth defects of Diogo's lab, which was obtained through the Department of Pathology of Howard's College of Medicine/Hospital (for more details, see Smith et al. 2015). Apart from taking into account the information we compiled and provided about muscle defects in humans with trisomy in Smith et al. (2015), for the specific purpose of the present paper we additionally undertook a detailed review of the scarce number of other publications providing detailed distal limb muscle data for individuals with non-pentadactyl limbs due to birth defects (Heiss, 1957; Light, 1992; Tonkin & Bulstrode, 2007; Waters & Bae, 2012; see section below). It is important to stress that our determination of the identity and homology of the muscles in the humans with both normal and abnormal phenotypes is made from an analysis of all data available (e.g. developmental primordium, innervation, orientation of the fibers, origin, insertion, divisions, topological relationship to other muscles and bones: see Smith et al. 2015 for more details), and therefore is not based only on topology, which could lead to a circular reasoning (see Results and discussion below). As explained above, for more information about the specific criteria used to study the homology of each muscle of each taxon discussed in this paper and shown in Fig.1, the readers should refer to the original publications where we presented and discussed those criteria (see, e.g. caption of Fig.1).
Results and discussion
The unique combination of the results obtained from our analyses of wild-type non-human tetrapods and of human birth defects provides a starting point towards the resolution of a long-standing evolutionary question raised by authors such as Owen (1849): do tetrapods share a general, predictable spatial correlation between limb bones and muscles? This is because we found a surprisingly consistent pattern, both in the non-pentadactyl limbs of wild-type taxa such as frogs, salamanders and chickens, and in the humans with birth defects: the identity and attachments of the distal forelimb and hindlimb muscles are mainly related to the physical (topological) position, and not the number of the primordium or even the homeotic identity, of the digits to which the muscles are attached. Figure1, compiled from our previous studies (see Materials and methods and caption of Fig.1), provides numerous examples of wild-type tetrapods illustrating that the loss of digit 1 and/or 5 in the foot or hand is related to changes in which the muscles that normally go to these digits in pentadactyl taxa change their insertions to attach onto digit(s) 2 and/or 4, respectively. For instance, the hand digits of urodeles (salamanders) such as axolotls derive from the primordia, and have a homeotic identity, of digits 1–4 (Young et al. 2009), but as shown in Fig.1a, digit 4 is the attachment point of the abductor digiti minimi muscle that usually goes to digit 5 in pentadactyl tetrapods.
At this point, it is important to clarify what is meant by ‘topological position’ vs. ‘number of primordium’ vs. ‘homeotic identity’ of the digits. Topological position refers to the adult relationship with other structures and to adult spatial data, not necessarily to the position of the developmental primordia. For instance, the topological position of the adult avian digit that derives from the second developmental primordium is digit 1, because this is the most radial (medial/preaxial) digit in the adult (Fig.1c). In this case, the topological position (digit 1) and homeotic identity (digit 1) are the same and are different from the developmental primordium from which the digit develops (the second primordium; Young et al. 2009).
Accordingly, and as predicted by our hypothesis, birds do usually have an abductor pollicis brevis going to this most radial wing digit, while in pentadactyl taxa this muscle is always inserted onto digit 1, which derives from the first, and not the second, primordium (Fig.1c). This contrasts with the above-mentioned case of axolotls, where digit 4 of the hand develops from the primordium of digit 4 and has a homeotic identity of digit 4, but its topological position is similar to that of digit 5 in pentadactyl tetrapods because this is the most ulnar (lateral/axial) digit of the hand. The axolotl case therefore illustrates and supports our hypothesis, because although the homeotic identity of the most ulnar digit of the axolotl hand is that of digit 4, the digit is associated with muscles that normally go to digit 5 in pentadactyl tetrapods, such as the abductor digiti minimi.
Another example corroborating that the identity and attachments of muscles is mainly related to the topological position and not the ontogenetic primordium or homeotic identity of the digits concerns the hands of frogs. It is fairly well accepted that the hand digits of these amphibians are derived from the primordia, and have a homeotic identity, of digits 2–5 (Young et al. 2009). However, frogs usually have a muscle that goes to digit 1 in pentadactyl tetrapod hands: the abductor pollicis longus (Fig.1b). In frogs, this muscle develops ontogenetically in connection to digit 2 exactly as it develops in connection with digit 1 in pentadactyl tetrapods – i.e. lying radial and somewhat deep to the extensor digitorum and running distoradially to attach onto metacarpal II/digit 2. The detailed study of non-pentadactyl tetrapod feet also consistently supports our hypothesis because in taxa such as crocodylians and birds that have only digits 1, 2, 3 and 4 in the foot, digit 4 is the insertion site for the abductor digiti minimi, which in pentadactyl feet always goes to digit 5 (Fig.1d).
Our hypothesis and the specific patterns observed in all non-pentadactyl autopodia of the wild-type tetrapods we examined are consistent with evolutionary and biomechanical theory because the most pre-/post-axially extreme digits (i.e. digits 1 and 5) are often specialized anatomically, and thus have increased mobility and/or are moved by peculiar muscles, such as the abductor pollicis longus and the abductor digiti minimi. Therefore, on the one hand it is sensible that the loss of, for example, digit 1 in the forelimb of birds is accompanied by a homeotic transformation in which the most radial digit of the wing (derived from the primordium of digit 2) recovers the identity of digit 1. On the other hand, it also is logical that even in those cases of digit reduction where there are no such homeotic transformations there is a developmental mechanism (configuration/identify of muscles related to position, and not identity, of digits) ensuring that the digits of the extremities still keep the peculiar muscles that are related to the specialized functions of digits 1 and/or 5.
In order to ascertain whether the patterns that we found in the non-pentadactyl autopodia of wild-type tetrapod taxa are also consistently found in cases of birth defects, we compiled data about distal limb muscle patterns in humans with non-pentadactyl hands, based on our own dissections and review of the literature (see Materials and methods). We undertook a project aimed to study in detail both the soft and hard tissues of non-pentadactyl human autopodia from individuals with different genetic backgrounds, so this study allows correlation of the observed patterns and birth defects with specific genetic conditions. Strikingly, the predictions made by our hypothesis were consistently supported by our dissections, even in the most extreme cases, such as that seen in the trisomy-18 human newborn illustrated in Fig.1e,f, which had four digits on one of the hands (thumb missing) and six digits on the other hand (partial duplication of the thumb). In the hand with four digits, all the muscles that are normally associated with the thumb are present but attach onto digit 2 (Fig.1e), while in the hand with six digits the muscles that normally attach onto the radial and ulnar sides of the thumb attach, respectively, onto the radial and ulnar sides of the most radial and the most ulnar thumbs (Fig.1f), as predicted. As explained in the Materials and methods, our determination of the identity and homology of the distal limb muscles in both normal and abnormal phenotypes is made from an analysis of all data available (e.g. developmental primordium, innervation, orientation of the fibers, origin, insertion, divisions, topological relationship to other muscles and bones). So, for instance, the muscle identified as adductor pollicis in the hands shown in Fig.1e,f is, for example, innervated by the deep ulnar nerve, has fibers directed disto-radially, originates from the contrahens fascia, and is divided into oblique and transverse heads, as is usually the case with the adductor pollicis of the normal human phenotype, the only difference being thus that in the hand shown in Fig.1e it goes to digit 2 and not to the thumb and that in the hand shown in Fig.1f it goes to the ulnar side of the partially duplicated thumb.
Cases such as the one found in the hand with six digits have been described in the scarce medical data available in the literature about the associations between soft and hard tissues in non-pentadactyl human limbs (Light, 1992), which also provide, in general, support for our hypothesis. That is, in the case of a hand with six digits, which is designated as preaxial polydactyly, the partial duplication of digit 1 (leading to the two partial thumbs having an homeotic identity of digit 1) is not accompanied by duplication of metacarpal 1 – it is therefore only a partial duplication of the thumb – nor by a duplication of the muscles that normally go to the thumb. Instead, the muscles abductor pollicis brevis and flexor pollicis brevis, which are the most radial thumb muscles in the normal phenotype, go to the most radial of the two partial thumbs with identity of digit 1; and the adductor pollicis, which is the most ulnar thumb muscle in the normal phenotype, goes to the most ulnar of the two partial thumbs with identity of digit. In other words, the muscles are not simply duplicated, as are the phalanges of digit 1. Instead, the muscles go to each of the two partial thumbs with identity of digit as if they were ‘unaware of/blind about’ the partial duplication of the thumb; that is, topologically they attach to the partially duplicated thumb as if there is a single thumb. This pattern thus conforms to the pattern seen in the hand with 4 digits shown in Fig.1e and in the normal phenotype of the non-pentadactyl limbs of the taxa shown in Fig.1f, in the sense that the muscles seem to be ‘unaware of/blind about’ genetic and developmental changes and to mainly follow topology, as explained above.
Importantly, our studies of human birth defects also support the idea that developmental or morphological constraints are so important that even in cases of extreme anatomical defects there is a ‘logical’ pattern that can be predicted and that moreover often mirrors the patterns seen in the normal phenotype of other taxa (Alberch, 1989). However, it is important to stress that there are apparently a few exceptions to the rule postulated by our hypothesis. For instance, Heiss (1957) described a peculiar case in which a human subject had two pentadactyl hands that had no thumbs and in which, contrary to the cases referred to above, there were no major topological changes of the resulting muscles. That is, in both hands of this human subject the normal thumb muscles were all reported as missing. In general, this configuration seems to be characteristic of the rare human disorder named ‘tri-phalangeal thumb’, which is a malformation of digit 1 including a perfect homeotic transformation of the thumb into an index finger and in which the muscles that are normally associated with the thumb are absent (e.g. abductor opponens/adductor pollicis; Heiss, 1957). If future dissections of humans with ‘tri-phalangeal thumbs’ confirm these reports and the premise that there are few exceptions to the rule postulated by our hypothesis, this will show that in some cases genetic and/or epigenetic factors lead to peculiar cases where the identity and attachments of the muscles are mainly related to the homeotic identity of the digits to which they attach, and not to their topological position. This would have important implications because it would elucidate a hitherto-unrecognized developmental plasticity regarding the patterning of hard and soft tissues, and therefore would open new lines of research within the field of developmental biology. It is, however, interesting to note that we did not find any similar exceptions in any of the non-pentadactyl limbs (both hands and feet) of the wild-type tetrapods studied by us so far (Fig.1; Materials and methods). This might indicate that such plasticity would be more the result of extreme defects than a true developmental plasticity seen during normal ontogeny in tetrapods.
In summary, our new comparative synthesis has implications for developmental and evolutionary biology and for human medicine, because it addresses questions concerning normal and abnormal development, and paves the way to future mechanistic developmental studies about the ontogenetic and the specific genetic and/or epigenetic causes of the developmental evolutionary patterns we have inferred here. For instance, a recent study showed that the cells that give rise to the limb bone eminences where the muscles attach are descendants of a unique set of Scx- and Sox9-positive progenitors, and that these bony eminences emerge only after the primary cartilage rudiments have formed (Blitz et al. 2013). That is, the cells that give rise to these eminences are not descendants of chondrocytes, and the formation of bony eminences is thus external, and independent, to the formation of the developing bone; these cells are added onto the developing long bone in a modular fashion (Blitz et al. 2013). This developmental modularity might therefore help explain the anatomical and evolutionary patterns that we have observed in the non-pentadactyl limbs of wild-type tetrapods and of humans with birth defects. For example, it would explain why the muscles that normally go to digits 1 and/or 5 in pentadactyl taxa become attached to digits 2 and/or 4 of these limbs. A test of this hypothesis would be to block the expression of Scx and Sox9 in the developing non-pentadactyl limbs of a certain taxon in order to investigate if this blocking would interfere with the modular pattern change of muscle attachments observed by us in the wild-type members of that taxon. Blitz et al. (2013) blocked the expression of Scx and Sox9, but did not study in detail how the blocking affected the attachments of each muscle. This synthesis thus paves the way for future developmental experimental and mechanistic studies, which are needed to clarify the processes that may be involved in the elaboration of the anatomical patterns described here, and to specifically test the hypothesis that distal limb muscle identity/attachment is mainly related to digit topology. This synthesis also paves the way for subsequent comparisons between the patterns and processes found in the distal limb with those found in the proximal limb and in the head. Importantly, the detailed knowledge of these patterns and processes, and of their possible exceptions, is also of great value for the medical community because they will allow physicians and surgeons to have a much better knowledge of the configuration of the soft tissues of the non-pentadactyl limbs that are so commonly found in human birth defects. In fact, because the pattern described by us (Fig.1e,f) is not always found in humans with non-pentadactyl limbs (see, e.g. reference to Heiss, 1957, above), further studies of humans with birth defects are needed to clarify in which specific cases/syndromes/defects the pattern does not apply, so this information is available for, and useful to, clinicians, surgeons, and the medical and scientific community in general.
Acknowledgments
The authors particularly thank B. Wood, B. Richmond, M. Ashley-Ross, P. Johnston and J. Hutchinson for discussions about appendicular muscles. This research was supported by the Howard University College of Medicine.
References
- Alberch P. The logic of monsters: evidence for internal constraint in development and evolution. Geobios Mem Spec. 1989;12:21–57. [Google Scholar]
- Aziz MA. Muscular and other abnormalities in a case of Edwards' syndrome (18-Trisomy) Teratology. 1979;20:303–312. doi: 10.1002/tera.1420200214. [DOI] [PubMed] [Google Scholar]
- Aziz MA. Anatomical defects in a case of Trisomy 13 with a D/D translocation. Teratology. 1980;22:217–227. doi: 10.1002/tera.1420220211. [DOI] [PubMed] [Google Scholar]
- Aziz MA. Muscular anomalies caused by delayed development in human aneuploidy. Clin Genet. 1981a;19:111–116. doi: 10.1111/j.1399-0004.1981.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Aziz MA. Possible atavistic structures in human aneuploids. Am J Phys Anthropol. 1981b;54:347–353. doi: 10.1002/ajpa.1330540308. [DOI] [PubMed] [Google Scholar]
- Barash BA, Freedman L, Optizj JM. Anatomic studies in the 18-Trisomy syndrome. Birth Defects Orig Artic Ser. 1970;4:3–15. [PubMed] [Google Scholar]
- Bersu ET, Ramirez-Castro JL. Anatomical analysis of the developmental effects of aneuploidy in man – the 18-trisomy syndrome: I. Anomalies of the head and neck. Am J Med Genet. 1977;1:173–193. doi: 10.1002/ajmg.1320010204. [DOI] [PubMed] [Google Scholar]
- Blitz E, Sharir A, Akiyama H, et al. Tendon–bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development. 2013;140:2680–2690. doi: 10.1242/dev.093906. [DOI] [PubMed] [Google Scholar]
- Bowler PJ. Life's Splendid Drama: Evolutionary Biology and the Reconstruction of Life's Ancestry, 1860–1940. Chicago: The University of Chicago Press; 1996. [Google Scholar]
- Bowler PJ. Fins and limbs and fins into limbs: the historical context, 1840–1940. In: Hall BK, editor. Fins into Limbs. Evolution, Development, and Transformation. Chicago: The University of Chicago Press; 2007. pp. 7–14. [Google Scholar]
- Caputo V, Lanza B, Palmieri R. Body elongation and limb reduction in the genus Chalcides Laurenti 1768 (Squamata Scincidae): a comparative study. Trop Zool. 1995;8:95–152. [Google Scholar]
- Castilla EE, da Fonseca RL, da Graça Dutra M, et al. Epidemiological analysis of rare polydactylies. Am J Med Genet. 1996;65:295–303. doi: 10.1002/(SICI)1096-8628(19961111)65:4<295::AID-AJMG10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Coates MI, Jeffery JE, Ruta M. Fins to limbs: what the fossils say. Evol Dev. 2002;4:390–401. doi: 10.1046/j.1525-142x.2002.02026.x. [DOI] [PubMed] [Google Scholar]
- Colacino S, Pettersen J. Analysis of the gross anatomical variations found in four cases of Trisomy 13. Am J Med Genet. 1978;2:31–50. doi: 10.1002/ajmg.1320020106. [DOI] [PubMed] [Google Scholar]
- Diogo R, Abdala V. Muscles of Vertebrates – Comparative Anatomy, Evolution, Homologies and Development. Oxford: Taylor and Francis; 2010. [Google Scholar]
- Diogo R, Molnar JL. Comparative anatomy, evolution and homologies of the tetrapod hindlimb muscles, comparisons with forelimb muscles, and deconstruction of the forelimb–hindlimb serial homology hypothesis. Anat Rec. 2014;297:1047–1075. doi: 10.1002/ar.22919. [DOI] [PubMed] [Google Scholar]
- Diogo R, Tanaka EM. Development of fore- and hindlimb muscles in GFP-transgenic axolotls: morphogenesis, the tetrapod bauplan, and new insights on the forelimb–hindlimb enigma. J Exp Zool. 2014;322B:106–127. doi: 10.1002/jez.b.22552. [DOI] [PubMed] [Google Scholar]
- Diogo R, Wood B. Comparative Anatomy and Phylogeny of Primate Muscles and Human Evolution. Oxford: Taylor and Francis; 2012. [Google Scholar]
- Diogo R, Ziermann JM. Development of fore- and hindlimb muscles in frogs: morphogenesis, homeotic transformations, digit reduction, and the forelimb–hindlimb enigma. J Exp Zool B Mol Dev Evol. 2014;322:86–105. doi: 10.1002/jez.b.22549. [DOI] [PubMed] [Google Scholar]
- Diogo R, Potau JM, Pastor JF. Photographic and Descriptive Musculoskeletal Atlas of Gorilla. Oxford: Taylor and Francis; 2010. [Google Scholar]
- Diogo R, Linde-Medina M, Abdala V, et al. New, puzzling insights from comparative myological studies on the old and unsolved forelimb/hindlimb enigma. Biol Rev. 2013a;88:196–214. doi: 10.1111/j.1469-185X.2012.00247.x. [DOI] [PubMed] [Google Scholar]
- Diogo R, Murawala P, Tanaka EM. Is salamander hindlimb regeneration similar to that of the forelimb? Anatomical and morphogenetic analysis of hindlimb muscle regeneration in GFP-transgenic axolotls as a basis for regenerative and developmental studies. J Anat. 2013b;10:459–468. doi: 10.1111/joa.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo R, Nacu E, Tanaka EM. Is salamander limb regeneration really perfect? Anatomical and morphogenetic analysis of forelimb muscle regeneration in GFP-transgenic axolotls as a basis for regenerative, developmental and evolutionary studies. Anat Rec. 2014;297:1076–1089. doi: 10.1002/ar.22906. [DOI] [PubMed] [Google Scholar]
- Dunlap DG. The development of the musculature of the hindlimb in the frog, Rana pipiens. J Morphol. 1967;119:241–258. doi: 10.1002/jmor.1051190210. [DOI] [PubMed] [Google Scholar]
- Dunlap SS, Aziz MA, Rosenbaum KN. Comparative anatomical analysis of human Trisomies 13, 18, and 21: I. The forelimb. Teratology. 1986;33:159–186. doi: 10.1002/tera.1420330204. [DOI] [PubMed] [Google Scholar]
- Duprez D. Signals regulating muscle formation in the limb during embryonic development. Int J Dev Biol. 2002;46:915–925. [PubMed] [Google Scholar]
- Fabrezi M, Abdala V, Oliver MIM. Developmental basis of limb homology in lizards. Anat Rec. 2007;290:900–912. doi: 10.1002/ar.20522. [DOI] [PubMed] [Google Scholar]
- Heiss H. Beiderseitige kongenitale daumenlose Fünffingerhand bei Mutter und Kind. Z Anat Entw-Gesch. 1957;120:226–231. [PubMed] [Google Scholar]
- Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125:4019–4032. doi: 10.1242/dev.125.20.4019. [DOI] [PubMed] [Google Scholar]
- Laurin M. Paleontological evidence: origin and early evolution of limbed vertebrates. In: Bels V, Casinos A, Davenport J, Gasc JP, Jamon M, Laurin M, Renous S, editors. How Vertebrates Moved onto Land. Paris: Museum national d'Histoire naturelle; 2011. pp. 33–73. [Google Scholar]
- Light TR. Thumb reconstruction. Hand Clin. 1992;8:161–175. [PubMed] [Google Scholar]
- Manzano A, Abdala V, Ponssa ML, et al. Ontogeny and tissue differentiation of the pelvic girdle and hind limbs of anurans. Acta Zool. 2013;94:420–436. [Google Scholar]
- Muntz L. Myogenesis in the trunk and leg during development of the tadpole of Xenopus laevis (Daudin 1802) J Embryol Exp Morphol. 1975;33:757–774. [PubMed] [Google Scholar]
- Owen R. On the Nature of Limbs. London: John Van Voorst; 1849. [Google Scholar]
- Pettersen JC. Anatomical studies of a boy trisomic for the distal portion of 13q. Am J Med Genet. 1979;4:383–400. doi: 10.1002/ajmg.1320040409. [DOI] [PubMed] [Google Scholar]
- Pettersen JC, Koltis GG, White MG. An examination of the spectrum of anatomic defects and variations found in eight cases of Trisomy 13. Am J Med Genet. 1979;3:183–210. doi: 10.1002/ajmg.1320030209. [DOI] [PubMed] [Google Scholar]
- Ponssa ML, Goldberg J, Abdala V. Sesamoids in anurans: new data, old issues. Anat Rec. 2010;293:1646–1668. doi: 10.1002/ar.21212. [DOI] [PubMed] [Google Scholar]
- Presch W. The evolution of limb reduction in the teiid lizard genus Bachia. Bull Sci Calif Acad Sci. 1975;74:113–121. [Google Scholar]
- Ramirez-Castro JL, Bersu ET. Anatomical analysis of the developmental effects of aneuploidy in Man – The 18 trisomy syndrome: II. Anomalies of the upper and lower limbs. Am J Med Genet. 1978;2:285–306. doi: 10.1002/ajmg.1320020309. [DOI] [PubMed] [Google Scholar]
- Romer AS. The Osteology of the Reptiles. Chicago: The University of Chicago Press; 1933. 772 pp. [Google Scholar]
- Shubin NH, Alberch P. A morphogenetic approach to the origin and basic organization of the tetrapod limb. Evol Biol. 1986;20:319–387. [Google Scholar]
- Shwartz Y, Farkas Z, Stern T, et al. Muscle contraction controls skeletal morphogenesis through regulation of chondrocyte convergent extension. Dev Biol. 2012;370:154–163. doi: 10.1016/j.ydbio.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Smith CM, Molnar JL, Ziermann JM. Muscular and Skeletal Anomalies in Human Trisomy in an Evo-devo Context: Description of a T18 Cyclopic Newborn and Comparison Between Edwards (T18), Patau (T13) and Down (T21) Syndromes using 3-D Imaging and Anatomical Illustrations. Oxford: Taylor and Francis; 2015. [Google Scholar]
- Tonkin MA, Bulstrode NW, et al. Bilhaut-Cloquet procedure for Wassel types III, IV and VII thumb duplication. J Hand Surg Eur. 2007;32:684–693. doi: 10.1016/J.JHSE.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Urban B, Bersu ET. Chromosome 18 aneuploidy: anatomical variations observed in cases of full and mosaic Trisomy 18 and a case of deletion of the short arm of Chromosome 18. Am J Med Genet. 1987;27:425–434. doi: 10.1002/ajmg.1320270221. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Chiu CH. The tetrapod limb: a hypothesis on its origin. J Exp Zool (Mol Dev Evol) 2001;291:226–240. doi: 10.1002/jez.1100. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Larsson HCE. Fins and limbs in the study of the evolutionary novelties. In: Hall BK, editor. Fins into Limbs. Evolution, Development, and Transformation. Chicago: The University of Chicago Press; 2007. pp. 49–61. [Google Scholar]
- Waters PM, Bae DS. Pediatric Hand and Upper Limb Surgery: A Practical Guide. London: Lippincott Williams and Wilkins; 2012. [Google Scholar]
- Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25:215–227. doi: 10.1016/j.hcl.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Weatherbee SD, Niswander LA. Mechanisms of chondrogenesis and osteogenesis in limbs. In: Hall BK, editor. Fins into Limbs. Evolution, Development, and Transformation. Chicago: The University of Chicago Press; 2007. pp. 93–102. [Google Scholar]
- Young RL, Caputo V, Giovannotti M, et al. Evolution of digit identity in the three-toed Italian skink Chalcides chalcides: a new case of digit identity frame shift. Evol Dev. 2009;11:647–658. doi: 10.1111/j.1525-142X.2009.00372.x. [DOI] [PubMed] [Google Scholar]