Abstract
The aim of the present study was to investigate the presence of sympathetic innervation in human muscle spindles, using antibodies against neuropeptide Y (NPY), NPY receptors and tyrosine hydroxylase (TH). A total of 232 muscle spindles were immunohistochemically examined. NPY and NPY receptors were found on the intrafusal fibers, on the blood vessels supplying muscle spindles and on free nerve endings in the periaxial space. TH-immunoreactivity was present mainly in the spindle nerve and vessel. This is, to our knowledge, the first morphological study concerning the sympathetic innervation of the human muscle spindles. The results provide anatomical evidence for direct sympathetic innervation of the intrafusal fibers and show that sympathetic innervation is not restricted to the blood vessels supplying spindles. Knowledge about direct sympathetic innervation of the muscle spindle might expand our understanding of motor and proprioceptive dysfunction under stress conditions, for example, chronic muscle pain syndromes.
Keywords: chronic muscle pain, human skeletal muscles, muscle spindle, neuropeptide Y, neuropeptide Y receptors, sympathetic innervation, tyrosine hydroxylase
Introduction
Muscle spindles are specialized sensory receptors found in most skeletal muscles. Being sensitive to changes in muscle length, they play a central role in the control of movement and posture. A muscle spindle comprises an encapsulated bundle of small diameter muscle fibers, the intrafusal fibers, receiving both sensory (group Ia and group II) and motor innervation. The intrafusal fibers are classified as nuclear bag1, bag2 and nuclear chain fibers on the basis of their morphological, structural and physiological properties (Ovalle & Smith, 1972; Liu et al. 2002, 2003; Banks & Barker, 2004). The sensory innervation occupies the equatorial region of the fibers, whereas both gamma (fusimotor) and beta (skeletofusimotor) neurons are distributed to the polar regions (Banks & Barker, 2004). Muscle spindles are supplied by a spindle nerve and a spindle vessel that enter the capsule (Cooper & Daniel, 1963; Peikert et al. 2014).
Autonomic innervation of muscle spindles has been reported in some animal species. Fluorescence microscopy revealed noradrenergic innervation distributed to some muscle spindles by axons supplied either through the spindle nerve or from nearby perivascular nerves in the cat hind limb (Barker & Saito, 1981). In the rabbit masseter muscle, tyrosine hydroxylase (TH)-positive nerve fibers are present among the intrafusal fibers, in the capsule and the periaxial space of approximately 1/3 of the muscle spindles. Moreover, alpha1a-adrenoreceptor immunoreactivity on the surface of the intrafusal fibers is present in the polar region of a high percentage of muscle spindles in the rabbit masseter muscle (Bombardi et al. 2006). However, data on the possible presence of sympathetic innervation on human muscle spindles is lacking. It has been proposed that the sympathetic nervous system may participate in the control of spindle output (Roatta et al. 2002; Hellström et al. 2005; Passatore & Roatta, 2006). In animal models, it has been shown that sympathetic activation reduces spindle sensitivity and might affect motor control under stress conditions (Passatore & Roatta, 2006). Thus, muscle spindles have been hypothesized to be involved in the pathophysiology of chronic muscle pain syndromes (Johansson et al. 1999; Passatore & Roatta, 2006). However, electrophysiological studies in animal models and in humans have revealed conflicting results under different experimental conditions (Bombardi et al. 2006).
In order to provide a morphological basis for the physiological concepts, the aim of the present study was to investigate the presence of sympathetic innervation in human muscle spindles, using antibodies against neuropeptide Y (NPY), NPY receptors and TH.
Neuropeptide Y is an amidated 36-amino acid peptide that is stored and released with noradrenaline in many sympathetic nerves (Lundberg et al. 1982; Ekblad et al. 1984; Potter, 1988). In the peripheral nervous system, NPY is often co-localized in neurons with catecholamines (Ekblad et al. 1984; Wan & Lau, 1995), and it interacts with norepinephrine as a sympathetic co-transmitter, mainly in the regulation of vascular tone (Wan & Lau, 1995). Data suggest that NPY also produces long-lasting, dose-dependent changes in tension controlled by muscle spindles (Grassi et al. 1996). Different receptor subtypes mediate NPY effects (Michel et al. 1998; Ekstrand et al. 2003). TH is a catecholamine-synthesizing enzyme and is a well-established marker for sympathetic nerve fibers (Shibamori et al. 2004; Bombardi et al. 2006).
Materials and methods
Tissue preparation
Specimens from lumbrical, biceps, Ievator scapulae and deep muscles of the neck were obtained from previously healthy subjects shortly after death, following the Swedish transplantation law and with the approval of the Medical Ethical Committee, Umeå University, Sweden. Serial cross-sections and longitudinal sections (5 μm thick) of the frozen muscle samples were processed for immunohistochemistry (Liu et al. 2009).
Antibodies
Rabbit polyclonal antibodies (PAb) against NPY (Amersham International, Amersham, UK; Norevall & Forsgren, 1999), NPY receptors Y1 (Uddman et al. 2002), Y2, Y4 and TH (Code P40101-0; Biologicals Divisions, Rogers, Arkansas, USA) were used. A mouse monoclonal antibody (MAb) against neurofilament protein (NF) was used to identify nerves (Clone NR4, Code M0762, Dako, Glostrup, Denmark). Antibodies against myosin heavy chain isoforms were used for the purpose of intrafusal fiber typing: mouse MAb ALD19 (kind gift from Donald A. Fischman, Cornell University, New York, USA) and rabbit PAb MYH7b (kind gift from Stefano Schiaffino, CNR Inst. of Neuroscience, Padova, Italy) against slow tonic myosin were used to detect nuclear bag fibers (Liu et al. 2002, 2003; Rossi et al. 2010; Janbaz et al. 2014), mouse MAb A4.74 (Developmental Studies Hybridoma Bank, Iowa City, IA, USA) against fast adult myosin was used to detect nuclear chain fibers (Liu et al. 2002, 2003; Österlund et al. 2013). The MAbs against laminin alpha5-chain (sold as MAb 1924; Chemicon, Temecula, CA, USA) and against laminin alpha2-chain (kindly provided by Eva Engvall, La Jolla, CA, USA) were used to label basement membranes (Pedrosa-Domellöf et al. 2000).
The PAb against the Y2 receptor (kind gift of Susanne Nyström formerly at AstraZeneca R&D, Sweden and Jonas Ekstrand formerly at Medivir AB, Sweden) was raised by synthesizing two peptides, KKVACTEKWPGEEKSIYGTVYS, corresponding to amino acid residues 200–220 in the third extracellular loop, and CKAKKNLEVRKNSGPNDSFTE, corresponding to amino acid residues 357–377 located in the C-terminal tail of human Y2 receptor (KJ Ross−Petersen AS, Hörsholm, Denmark). The peptides were each conjugated to bovine serum albumin (BSA) and administered to rabbits (AgriSera AB, Vännäs, Sweden). Serum from boosted rabbits was collected and lgG was isolated by chromatography on Protein G Sepharose according to the manufacturer's recommendation (Amersham-Pharmacia Biotech, Uppsala, Sweden). The PAbs were further affinity-purified on Sulfo-Link columns (Pierce, Rockford, Il, USA) to which the corresponding peptide antigen had been coupled by its cysteine residue. A PAb for the human Y4 receptor (kind gift of Susanne Nyström formerly at AstraZeneca R&D, Sweden and Jonas Ekstrand formerly at Medivir AB, Sweden at the time of antibody development) was similarly produced using a synthetic peptide, KKKENRSKPLGTPYNFSEHCQDS, corresponding to amino acid residues 18–30 located in the N-terminal domain of human Y4 receptor. The selectivity of the affinity-purified PAbs was tested on Western blots containing lysates of cells producing recombinant hY1, hYz and hY4, respectively.
Immunofluorescence
Selected air-dried sections were postfixed in 3% (for NPY) and 2% (for TH) paraformaldehyde, respectively, and rehydrated in 0.01 m phosphate-buffered saline (PBS) containing 0.05% Tween. Air-dried sections designated to be treated with the other primary antibodies were rehydrated in 0.01 m PBS. Then, all sections were immersed in 5% normal donkey serum (Dakopatts, Glostrup, Denmark) prior to incubation with the primary antibody – appropriately diluted in 0.01 m PBS containing 0.1% BSA – overnight at 4 °C. After washing in PBS, an appropriate secondary antibody was added for 30 min at 37 °C: donkey anti-rabbit FITC for green fluorescence, donkey anti-mouse rhodamine Red-X for red fluorescence (Jackson Immunoresearch Laboratories, West Grove, PA, USA). After washing with PBS, the sections were mounted with Vectashield DAPI mounting medium (Vector Laboratories, Burlingame, CA, USA). The use of DAPI allows detection of nuclei. Double-staining was performed using sequential and/or simultaneous procedure protocols combining NPY/laminin, Y2 receptor/laminin, TH/NF and MYH7b/A4.74.
Control sections were prepared by replacing the primary antibody with 0.01 m PBS containing 0.1% BSA. No staining was observed in the control sections.
Analysis and data collection
The sections were examined and digital images were taken using a Spot camera (RT KE slider, Diagnostic Instruments, MI, USA) connected to a Nikon microscope (E800; Eclipse, Tokyo, Japan). A total of 232 muscle spindles were examined: 52 muscle spindles were examined for NPY; 43 for NPY receptors; and 137 for TH. All muscle spindles were randomly surveyed in the A, B or C region. The A region is defined by the presence of the periaxial space, the B region is found on either side of the A region, where the intrafusal fibers are closely surrounded by the capsule, and the C region comprises the extracapsular portions of the spindles (Banks & Barker, 2004). Some selected muscle spindles were followed and studied on a series of consecutive sections.
In the initial survey rather equivalent staining patterns were observed with all different antibodies against NPY receptors. However, the PAbs against Y2 were predominantly used because of more clear-cut and stronger staining.
Results
NPY and NPY receptors
Specific fluorescence was found in 12 out of 52 muscle spindles treated with the PAb against NPY (Fig.1). In six muscle spindles, the NPY labeling was found on the bag fiber either on the surface of the fiber or slightly indented into the fiber. NPY labeling was seen on a chain fiber in three muscle spindles, between the fibers in one muscle spindle, and on a capillary (in the periaxial space and in the capsular wall) in two other muscle spindles. NPY staining was seen in the muscle spindles sectioned in the A or B region, but it was not seen in the C region of the eight muscle spindles stained outside the capsule.
Fig 1.
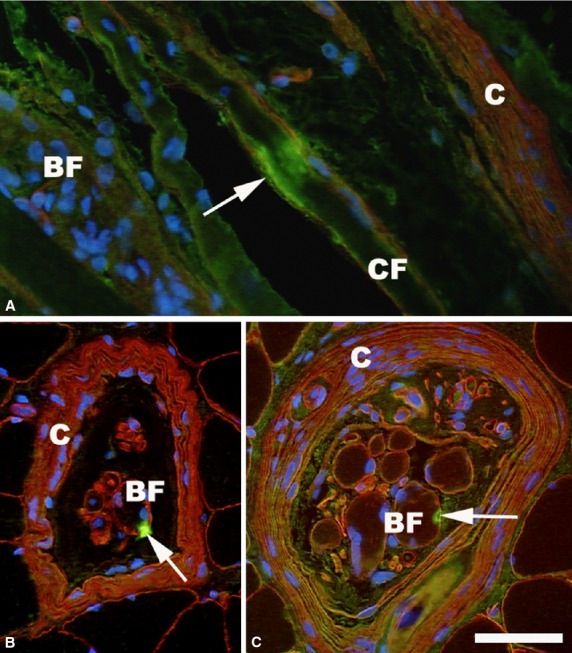
Immunofluorescence micrographs of three human lumbrical muscle spindles in the equatorial region double-stained for demonstration of laminin (red) and NPY (green). (A) Longitudinal section where specific labeling for NPY (arrow) appears on the surfaces of a nuclear chain fiber (CF). The capsule (C) is stained with laminin (red) and the nuclear bag fiber (BF) is filled with nuclei labeled blue with DAPI. (B and C) Cross-sections showing specific staining (arrow, green) on the surface of a bag fiber. Scale bar: 50 μm.
The PAbs against NPY receptors specifically labeled 17 out of 43 muscle spindles studied (Fig.2). In seven muscle spindles, staining was found on the surface of a bag fiber, in four muscle spindles on the surface of a chain fiber. In six muscle spindles, specific fluorescence was located between the fibers where no blood vessels were seen.
Fig 2.
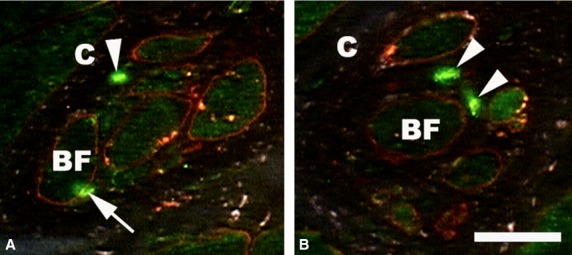
Immunofluorescence micrographs of two human lumbrical muscle spindles cross-sectioned in the equatorial region and double-stained for demonstration of laminin (red) and NPY Y2 receptors (green). Specific fluorescence for Y2 receptors (arrow) appears as a strong dot on the surface of a bag fiber (BF) and in between the fibers (arrowheads, A and B), presumably on free nerve endings. C, capsule. Scale bar: 25 μm.
Staining with NPY was often, but not always, present only in a single section of a given consecutive series, likely reflecting the varicose morphology of these thin axons. Specific fluorescence was also found on the surface of a small number of extrafusal fibers. Staining for NPY and NPY receptors was sporadically seen in the space between small extrafusal fibers, in the vicinity of nerves. The media of arterioles and small arteries was strongly stained by all PAbs against NPY receptors and NPY (Fig.3). Finally, strong specific fluorescence was also found in some axons inside nerve trunks and in streaks located in the perineurium of some sporadic nerves in the nerve trunks and in their perineurium.
Fig 3.
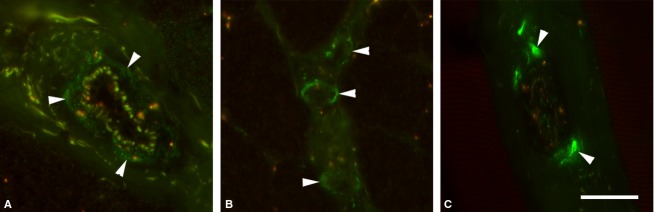
Examples of immunolabeling (arrowheads) with antibodies against NPY (A), Y2 (B) and TH (C) on the walls of arterioles. Scale bar: 25 μm.
TH
Specific fluorescence was found in 31 out of 137 muscle spindles (Figs4 and 5). Strong TH-immunoreactivity was present in the NF-positive spindle nerve within the capsule of 12 muscle spindles, mostly in their A region. In the C or far B region of six muscle spindles, very fine specific TH-positive reactions could be found closely associated with both nuclear chain and nuclear bag fibers. In two muscle spindles in the outer A region, a similar pattern was observed. However, in most of the muscle spindles in the A and B region, there was no clear evidence for TH-immunoreactivity among the intrafusal fibers. In longitudinally sectioned muscle spindles, the positively stained axons were thin, running along with the intrafusal fibers and had a varicose morphology, as shown in Fig.4D–F. TH labeling was only present in the nuclei of some fibers and this was considered as unspecific staining. In a total of 11 muscle spindles (A and B region), specific fluorescence was located around the spindle vessel or capillaries found within the periaxial space and/or in small vessels in the capsular layers.
Fig 4.
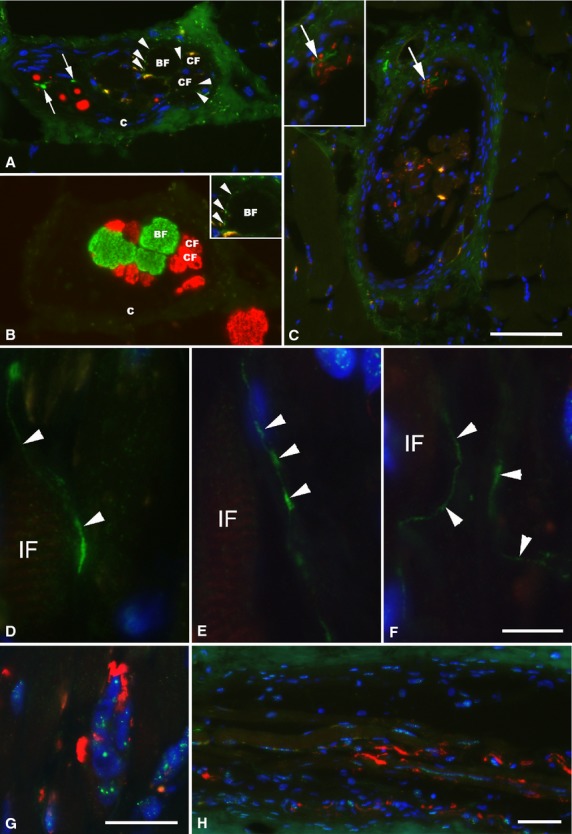
Immunofluorescence micrographs of human muscle spindles of the deep neck muscles. (A and B) Two consecutive sections of a muscle spindle in the far A region. The NF-positive (red) spindle nerve contains TH-immunoreactive axons (green, arrow). Around both the MYH7b-positive (green) bag fibers (BF) and A4.74-positive (red) chain fiber (CF), very fine but specific labeling with TH (arrowheads) is present and shown at higher magnification in the inset in B. (C) Cross-section of a muscle spindle in the A region showing TH-positive axon (green, arrow) within a spindle nerve alongside with axons labeled with NF (red). In the inset in C, the spindle nerve is shown at higher magnification. (D–F) Longitudinally running axons, with varicose morphology and strongly labeled with the antibody against TH (green, arrowheads) are shown running in parallel with intrafusal fibers (IF) along different parts of the A region of one single muscle spindle from the deep neck muscles. (G and H) Annulospiral endings, strongly labeled with the antibody against NF (red), in the same muscle spindle as above, are shown for comparison. Nuclei are labeled blue with DAPI. C, capsule. Scale bar: 50 μm (A–C and H); 10 μm (D–F); 25 μm (G).
Fig 5.
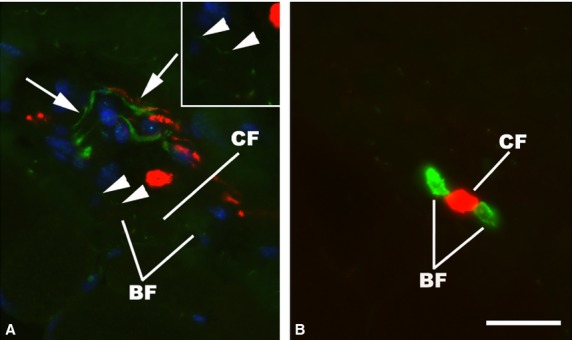
Immunofluorescence micrographs of a human muscle spindle of the deep neck muscles. Two consecutive cross-sections in the C region, double-stained for demonstration of TH (green) and NF (red) (A) and MyHC slow tonic (green) and MyHC fast (red) (B). The C region fibers – two MyHC slow tonic positive bag fibers (BF) and one MyHC fast positive chain fiber (CF) – are closely associated to a TH- and NF-positive nerve (arrows). Very fine but specific labeling with TH (arrowheads) is present on the surface of a BF and shown at higher magnification in the inset. Scale bar: 25 μm.
Moreover, specific reactions were sporadically present in between the extrafusal muscle fibers, as well as in axons inside nerve trunks and in small nerves around blood vessels (Fig.3) in the muscle specimens.
Discussion
This is, to our knowledge, the first study describing the presence of sympathetic innervation in human muscle spindles. The antibodies against NPY, NPY receptors and TH are well characterized, affinity-purified and the appropriate controls were negative. Therefore, our data provide anatomical evidence for direct sympathetic innervation of intrafusal fibers. These findings confirm previous results in the cat and the rabbit regarding the presence of the autonomic innervation of the muscle spindle (Ballard, 1978; Barker & Saito, 1981; Bombardi et al. 2006).
In muscle spindles labeled with antibodies against NPY, NPY receptor and TH, specific staining was found between the fibers where no blood vessels were seen. Based upon previous studies (Ballard, 1978; Barker & Saito, 1981; Bombardi et al. 2006), we conclude that this staining is most Iikely located on free nerve endings/nerves with varicose appearance. Autonomic innervation was present only in a low number of intrafusal fibers, but the proportion of muscle spindles receiving autonomic axons found in the present study was in agreement with previous data on the cat (Barker & Saito, 1981). In the rabbit masseter muscle, TH-positive axons were found in a higher percentage of muscle spindles. This might be due to methodical differences as the muscle spindles have been examined in serial sections along the entire length of the capsule (Bombardi et al. 2006). In the present study, however, a higher number of spindles have been studied only randomly in A, B or C regions. Nevertheless, the finding of sympathetic free nerve endings among the intrafusal fibers, the presence of TH-positive axons in the spindle nerve and the detection of NPY receptors on the intrafusal fibers provide strong evidence concerning the presence of the sympathetic nervous system in human muscle spindles. However, at this point, it is not possible to speculate whether only a subgroup of human muscle spindles receives sympathetic innervation or whether the low number of intrafusal fibers receiving autonomic innervation detected here only reflects a sampling problem due to sparse innervation.
Over a century ago, Ruffini suggested that the existence of sympathetic innervation of the muscle spindle implied the possibility of additional sympathetic modulation of muscle spindle afferent discharge (Ruffini, 1898). This would enable the autonomic system to influence functions attributed to the muscle spindles, i.e. motor reflex functions, coordination and proprioception. lt has been suggested that the action of sympathetic innervation on spindle afferents may be one of the mechanisms involved in adjusting a motor act during states of physical and emotional stress (Roatta et al. 2002). An important implication of the sympathetic innervation is that an increase in sympathetic outflow depresses the feedback control of muscle length (Schwartzman & Kerrigan, 1990; Roatta et al. 2002; Hellström et al. 2005; Passatore & Roatta, 2006). Low feedback gain may be useful in a motor condition typically associated with sympathetic activation, such as the fight-or-flight reaction, in which precision and fine control of movements can be sacrificed for stability and reliability of fast running or fighting movements. Otherwise, when motor tasks requiring precision and continuous proprioceptive feedback are performed under conditions of strong excitement and stress, the enhanced sympathetic outflow may affect accurate motor performance output.
The present data showing direct sympathetic innervation of the human muscle spindle fibers are of crucial importance for the understanding of motor and proprioceptive dysfunction seen under conditions of enhanced sympathetic activity, for example, during stress, which may lead to work-related myalgia and chronic muscle pain syndromes (Johansson et al. 1999; Passatore & Roatta, 2006), as well as the specific conditions grouped under the name of sympathetically maintained pain (e.g. Schwartzman & Kerrigan, 1990). Because previous electrophysiological studies on jaw and neck muscles of felines (Hellström et al. 2005) and rabbits (Roatta et al. 2002) have shown that sympathetic outflow influences the activity of muscle spindle afferents whereas studies performed on human toe and ankle muscles (Macefield et al. 2003; Matre & Knardahl, 2003) do not show such effect, the present data are of importance as they provide the morphological basis of sympathetic innervation of muscle spindles in a variety of human muscles.
Conclusion
This study provides for the first time clear morphological evidence of sympathetic innervation of human muscle spindles. Further studies are needed to determine whether this occurs in only a proportion of muscle spindles, or there is a sparse innervation of all spindles.
Acknowledgments
This study was supported by grants from the Swedish Research Council (K2012- 63x-20399-06-3; Stockholm, Sweden), the County Council of Västerbotten (Cutting Edge Medical Research and Central ALF; Umeå, Sweden) and the Medical Faculty, Umeå University, Sweden. The authors thank Prof. Sture Forsgren for kindly providing NPY antibody, Ulla Hedlund and Anna-Karin Olofsson for excellent technical assistance, and Vahid M. Harandi for assistance preparing figures. Furthermore, the authors thank Prof. Lars-Eric Thornell and Prof. Christian Albrecht May for support and expertise.
Author contributions
Dina Radovanovic performed experiments, evaluated the data and prepared the manuscript. Kevin Peikert performed experiments, evaluated the data and prepared the manuscript. Mona Lindström performed experiments, evaluated the data and reviewed the manuscript. Fatima Pedrosa Domellöf was the PI, supervised experiments, data evaluation and manuscript preparation.
References
- Ballard KJ. Typical sympathetic noradrenergic endings in a muscle spindle of the cat [proceedings] J Physiol. 1978;285:61P. [PubMed] [Google Scholar]
- Banks RW, Barker D. The muscle spindle. In: Engel AG, Franzini-Armstrong C, editors. Myology: Basic and Clinical. 3rd edn. Vol. 1. New York: McGraw-Hill; 2004. pp. 489–509. [Google Scholar]
- Barker D, Saito M. Autonomic innervation of receptors and muscle fibres in cat skeletal muscle. Proc R Soc Lond B Biol Sci. 1981;212:317–332. doi: 10.1098/rspb.1981.0042. [DOI] [PubMed] [Google Scholar]
- Bombardi C, Grandis A, Chiocchetti R, et al. Immunohistochemical localization of alpha(1a)-adrenoreceptors in muscle spindles of rabbit masseter muscle. Tissue Cell. 2006;38:121–125. doi: 10.1016/j.tice.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Cooper S, Daniel PM. Muscle spindles in man; their morphology in the lumbricals and the deep muscles of the neck. Brain. 1963;86:563–586. doi: 10.1093/brain/86.3.563. [DOI] [PubMed] [Google Scholar]
- Ekblad E, Edvinsson L, Wahlestedt C, et al. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul Pept. 1984;8:225–235. doi: 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- Ekstrand AJ, Cao R, Bjorndahl M, et al. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci USA. 2003;100:6033–6038. doi: 10.1073/pnas.1135965100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi C, Deriu F, Roatta S, et al. Sympathetic control of skeletal muscle function: possible co-operation between noradrenaline and neuropeptide Y in rabbit jaw muscles. Neurosci Lett. 1996;212:204–208. doi: 10.1016/0304-3940(96)12835-4. [DOI] [PubMed] [Google Scholar]
- Hellström F, Roatta S, Thunberg J, et al. Responses of muscle spindles in feline dorsal neck muscles to electrical stimulation of the cervical sympathetic nerve. Exp Brain Res. 2005;165:328–334. doi: 10.1007/s00221-005-2309-7. [DOI] [PubMed] [Google Scholar]
- Janbaz AH, Lindström M, Liu JX, et al. Intermediate filaments in the human extraocular muscles. Invest Ophthalmol Vis Sci. 2014;55:5151–5159. doi: 10.1167/iovs.14-14316. [DOI] [PubMed] [Google Scholar]
- Johansson H, Sjölander P, Djupsjöbacka M, et al. Pathophysiological mechanisms behind work-related muscle pain syndromes. Am J Ind Med. 1999;36:104–106. doi: 10.1002/(sici)1097-0274(199909)36:1+<104::aid-ajim37>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Liu JX, Eriksson PO, Thornell LE, et al. Myosin heavy chain composition of muscle spindles in human biceps brachii. J Histochem Cytochem. 2002;50:171–184. doi: 10.1177/002215540205000205. [DOI] [PubMed] [Google Scholar]
- Liu JX, Thornell LE, Pedrosa-Domellöf F. Muscle spindles in the deep muscles of the human neck. A morphological and immunocytochemical study. J Histochem Cytochem. 2003;51:175–186. doi: 10.1177/002215540305100206. [DOI] [PubMed] [Google Scholar]
- Liu JX, Willison HJ, Pedrosa-Domellöf F. Immunolocalisation of GQ1b and related gangliosides in human extraocular neuromuscular junctions and muscle spindles. Invest Ophthalmol Vis Sci. 2009;50:3226–3232. doi: 10.1167/iovs.08-3333. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Terenius L, Hökfelt T, et al. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand. 1982;116:477–480. doi: 10.1111/j.1748-1716.1982.tb07171.x. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Sverrisdottir YB, Wallin BG. Resting discharge of human muscle spindles is not modulated by increases in sympathetic drive. J Physiol. 2003;551:1005–1011. doi: 10.1113/jphysiol.2003.040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matre D, Knardahl S. Sympathetic nerve activity does not reduce proprioceptive acuity in humans. Acta Physiol Scand. 2003;178:261–268. doi: 10.1046/j.1365-201X.2003.01122.x. [DOI] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, et al. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- Norevall L, Forsgren S. NPY/sympathetic and NPY/VIP innervation of the blood vessels supplying rat tooth-related structures: effects of sympathectomy. Neuropeptides. 1999;33:216–226. doi: 10.1054/npep.1999.0758. [DOI] [PubMed] [Google Scholar]
- Österlund C, Liu JX, Thornell LE, et al. Intrafusal myosin heavy chain expression of human masseter and biceps muscles at young age shows fundamental similarities but also marked differences. Histochem Cell Biol. 2013;139:895–907. doi: 10.1007/s00418-012-1072-7. [DOI] [PubMed] [Google Scholar]
- Ovalle WK, Smith RS. Histochemical identification of three types of intrafusal muscle fibers in the cat and monkey based on the myosin ATPase reaction. Can J Physiol Pharmacol. 1972;50:195–202. doi: 10.1139/y72-030. [DOI] [PubMed] [Google Scholar]
- Passatore M, Roatta S. Influence of sympathetic nervous system on sensorimotor function: whiplash associated disorders (WAD) as a model. Eur J Appl Physiol. 2006;98:423–449. doi: 10.1007/s00421-006-0312-8. [DOI] [PubMed] [Google Scholar]
- Pedrosa-Domellöf F, Tiger CF, Virtanen I, et al. Laminin chains in developing and adult human myotendinous junctions. J Histochem Cytochem. 2000;48:201–210. doi: 10.1177/002215540004800205. [DOI] [PubMed] [Google Scholar]
- Peikert K, Kasper M, May CA. Distribution of caveolin in the muscle spindles of human skeletal muscles. J Anat. 2014;224:681–687. doi: 10.1111/joa.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter EK. Neuropeptide Y as an autonomic neurotransmitter. Pharmacol Ther. 1988;37:251–273. doi: 10.1016/0163-7258(88)90028-9. [DOI] [PubMed] [Google Scholar]
- Roatta S, Windhorst U, Ljubisavljevic M, et al. Sympathetic modulation of muscle spindle afferent sensitivity to stretch in rabbit jaw closing muscles. J Physiol. 2002;540(Pt 1):237–248. doi: 10.1113/jphysiol.2001.014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AC, Mammucari C, Argentini C, et al. Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles. J Physiol. 2010;588:353–364. doi: 10.1113/jphysiol.2009.181008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini A. On the minute anatomy of the neuromuscular spindles of the cat, and on their physiological significance. J Physiol. 1898;23:190–208. doi: 10.1113/jphysiol.1898.sp000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman RJ, Kerrigan J. The movement disorder of reflex sympathetic dystrophy. Neurology. 1990;40:57–61. doi: 10.1212/wnl.40.1.57. [DOI] [PubMed] [Google Scholar]
- Shibamori Y, Saito T, Tokuriki M, et al. The terminal of the sympathetic nerve fibers in the facial nerve. Acta Otolaryngol Suppl. 2004;553:61–64. doi: 10.1080/03655230410017698. [DOI] [PubMed] [Google Scholar]
- Uddman R, Möller S, Nilsson T, et al. Neuropeptide Y Y1 and neuropeptide Y Y2 receptors in human cardiovascular tissues. Peptides. 2002;23:927–934. doi: 10.1016/s0196-9781(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Wan CP, Lau BH. Neuropeptide Y receptor subtypes. Life Sci. 1995;56:1055–1064. doi: 10.1016/0024-3205(95)00041-4. [DOI] [PubMed] [Google Scholar]


