Article first published online 24 March 2015.
Key Words: ulcerative colitis, step-up therapy, colectomy, extraintestinal manifestations
Abstract
Background:
Physicians traditionally treat ulcerative colitis (UC) using a step-up approach. Given the paucity of data, we aimed to assess the cumulative probability of UC-related need for step-up therapy and to identify escalation-associated risk factors.
Methods:
Patients with UC enrolled into the Swiss IBD Cohort Study were analyzed. The following steps from the bottom to the top of the therapeutic pyramid were examined: (1) 5-aminosalicylic acid and/or rectal corticosteroids, (2) systemic corticosteroids, (3) immunomodulators (IM) (azathioprine, 6-mercaptopurine, methotrexate), (4) TNF antagonists, (5) calcineurin inhibitors, and (6) colectomy.
Results:
Data on 996 patients with UC with a median disease duration of 9 years were examined. The point estimates of cumulative use of different treatments at years 1, 5, 10, and 20 after UC diagnosis were 91%, 96%, 96%, and 97%, respectively, for 5-ASA and/or rectal corticosteroids, 63%, 69%, 72%, and 79%, respectively, for systemic corticosteroids, 43%, 57%, 59%, and 64%, respectively, for IM, 15%, 28%, and 35% (up to year 10 only), respectively, for TNF antagonists, 5%, 9%, 11%, and 12%, respectively, for calcineurin inhibitors, 1%, 5%, 9%, and 18%, respectively, for colectomy. The presence of extraintestinal manifestations and extended disease location (at least left-sided colitis) were identified as risk factors for step-up in therapy with systemic corticosteroids, IM, TNF antagonists, calcineurin inhibitors, and surgery. Cigarette smoking at diagnosis was protective against surgery.
Conclusions:
The presence of extraintestinal manifestations, left-sided colitis, and extensive colitis/pancolitis at the time of diagnosis were associated with use of systemic corticosteroids, IM, TNF antagonists, calcineurin inhibitors, and colectomy during the disease course.
Ulcerative colitis (UC) is a chronic inflammatory disorder of the colorectum of unknown etiology. It is believed that UC develops due to a dysregulated mucosal immune response to commensal gut flora in genetically susceptible individuals.1 The risk of UC is the highest in North America and northern Europe with incidence rates varying from 9 to 20 cases per 100,000 person-years and prevalence rates from 156 to 291 cases per 100,000 inhabitants.1 Traditionally, physicians treat patients with UC using a step-up approach. Newly diagnosed individuals with mild-to-moderate UC are usually first treated with 5-aminosalicylic acid (5-ASA), which is considered to be an effective and inexpensive treatment that provides rapid symptom relief.2,3 In case of insufficient response or intolerance to 5-ASA, patients may require corticosteroids and/or immunomodulators, such as azathioprine or 6-mercaptopurine, as a step-up therapy.4,5 In case of insufficient response to corticosteroids and/or immunomodulators, therapeutic options, such as infliximab, adalimumab, cyclosporine, or tacrolimus, may be necessary.6,7 In case of a failure of all pharmacologic therapies, a (procto)colectomy can be performed.
Although several studies have evaluated the cumulative probability of surgery over time, the studies that have systematically analyzed the risk factors associated with the need for therapeutic escalation in patients with UC are rare.8–11 The knowledge of such risk factors may assist physicians in choosing the appropriate therapy for a given patient with UC depending on his/her risk profile.
In this study, we aimed to assess the cumulative probability of UC-related need for step-up in therapy over time and to identify specific risk factors associated with a therapy escalation.
MATERIALS AND METHODS
Patients
Starting in 2006, patients with IBD from all regions of Switzerland have been included into the Swiss Inflammatory Bowel Disease Cohort Study (SIBDCS). The SIBDCS is supported by a research grant from the Swiss National Science Foundation and approved by the local ethics committees. The cohort profile has been described by Pittet et al.12 To be included into the study, patients signed written informed consent. Permanent residency status in Switzerland and/or coverage by a Swiss health insurance plan were mandatory requirements for inclusion of patients. Upon inclusion, patients undergo a thorough clinical and laboratory assessment. Patients attend follow-up visits at least once a year. The enrollment and follow-up questionnaires capture clinical, socioeconomic, and psychosocial data. Detailed questionnaires designed to assess physician-determined measures are completed by the treating physicians, whereas questionnaires designed to assess patient-reported outcomes, such as quality of life, social impairment, and symptoms, are completed by patients with IBD themselves. The data from questionnaires of all patients included into the SIBDCS between November 2006 and June 2013 were used for the analysis performed in this article. Patients included in this study were recruited in the following health care settings: 61% at university hospitals, 13% at large nonuniversity hospitals, 6% at county hospitals, and 20% in private practice. In Switzerland, family physicians serve as primary care providers. If IBD is suspected, patients are then referred by family physicians to gastroenterologists.
Methods
The data obtained from questionnaires completed by patients and physicians were collected and validated by the staff at the SIBDCS data center. Rigorous rules were followed to ensure data quality. The prescription of the following therapies was analyzed over time: topical 5-ASA, oral 5-ASA, rectal budesonide (enema or foam), systemic corticosteroids (oral budesonide and prednisone), azathioprine, 6-mercaptopurin, methotrexate, infliximab, adalimumab, certolizumab pegol, cyclosporine, and tacrolimus.
We defined the following steps in the step-up approach to UC treatment: (1) use of 5-ASA (administered either topically [suppositories, enema] and/or orally) or rectal corticosteroids (budesonide enema or foam) or both, (2) use of systemic corticosteroids (oral budesonide or prednisone or equivalent drugs), (3) use of immunomodulators (azathioprine, 6-mercaptopurine, methotrexate), (4) use of TNF antagonists (infliximab or adalimumab), (5) use of calcineurin inhibitors (cyclosporine, tacrolimus), (6) undergoing UC-related surgery, which includes proctocolectomy and colectomy with ileorectal anastomosis.
Of note, infliximab was the only registered anti-TNF therapy for UC treatment until the company producing adalimumab obtained regulatory approval for use of this product in Switzerland in December 2013. Disease activity at inclusion into the SIBDCS was assessed using the modified Truelove and Witts activity index.13 A positive IBD family history was defined as having at least 1 first-degree relative with either UC or Crohn's disease.
Statistical Analysis
Clinical data were retrieved at the SIBDCS data center (institut universitaire de médecine sociale et préventive, University of Lausanne). All standard statistical analyses were performed using Stata (version 12.1; College Station, TX), whereas time-to-event analyses were performed using the R (version 2.15.1).14 Continuous data distribution was analyzed using Normal QQ-plots. For continuous data, results were presented as median, interquartile range and range in case data followed nonnormal distribution, and as mean ± SD and range in case of data that followed normal distribution. For categorical data, results were presented as absolute numbers and relative frequencies.
The Kaplan–Meier method was used to examine cumulative proportions of therapy use over time; the Turnbull's15 extension to Kaplan–Meier method was used for left-censored data.15 Logistic regression was used to identify factors associated with the following binary outcomes: use of at least systemic corticosteroids (use of systemic corticosteroids or IM or TNF antagonists or calcineurin inhibitors or surgery) versus no therapy or use of 5-ASA and/or rectal corticosteroids only; use of at least IM (use of IM or TNF antagonists or calcineurin inhibitors or surgery) versus no therapy or 5-ASA therapy and/or rectal corticosteroids or systemic corticosteroids; use of at least TNF antagonists (use of TNF antagonists or calcineurin inhibitors or surgery) versus no therapy or 5-ASA and/or rectal corticosteroids or systemic corticosteroids or IM; use of at least calcineurin inhibitors (use of calcineurin inhibitors or surgery) versus no therapy or 5-ASA and/or rectal corticosteroids or systemic corticosteroids or IM or TNF antagonists; undergoing UC-related surgery versus no therapy or all pharmacologic therapies. Results were presented as odds ratio (OR) and 95% confidence interval (CI). Factors with a P value <0.1 were entered into a multivariable logistic regression model. A P value <0.05 was considered as statistically significant.
RESULTS
Characteristics of the Study Population
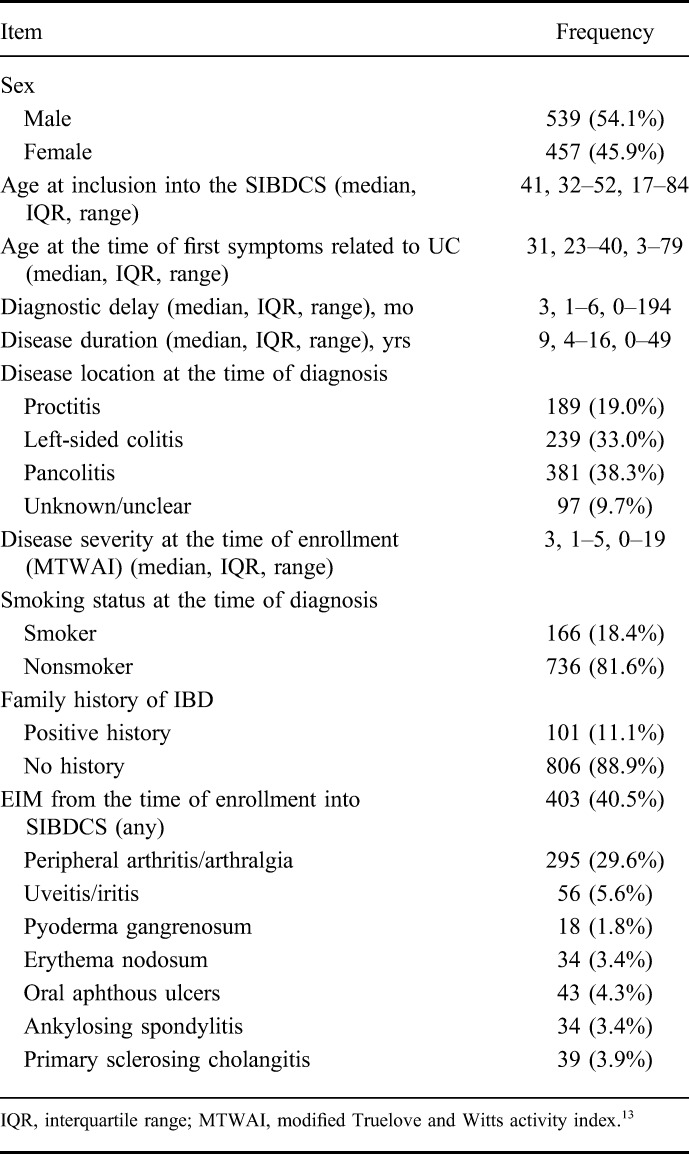
The characteristics of the study population are shown in Table 1. A total of 996 patients with UC were included. Median age at the time of inclusion into the SIBDCS was 41 years (interquartile range, 32–52). The disease location at the time of diagnosis was as follows: proctitis in 189 patients (19.0%), left-sided colitis in 239 patients (33.0%), extensive colitis/pancolitis in 381 patients (38.3%), and unknown in 97 patients (9.7%). Median disease duration was 9 (4–16) years. A total of 403 (40.5%) patients presented with extraintestinal manifestations (EIM) (evaluated from the time of enrollment into the SIBDCS until the time of last follow-up visit). Colectomy was performed in 94 patients with UC. Of these, 8 patients (8.5%) suffered from colorectal cancer and 7 (7.4%) from colonic dysplasia. Seventy-nine patients with UC (84%) underwent colectomy due to refractory disease not responding to medication regimens.
TABLE 1.
Characteristics of the UC Population
Cumulative Proportion of UC-related Drug Use and UC-related Surgery
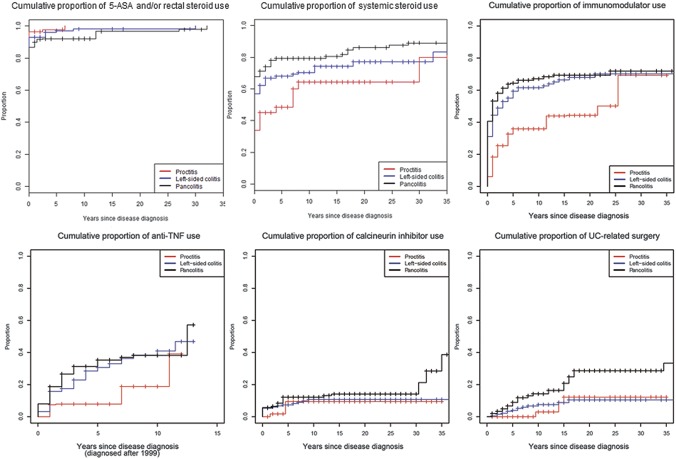
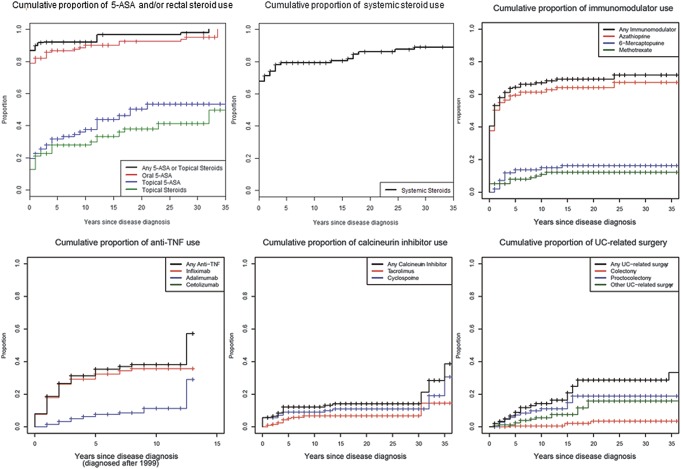
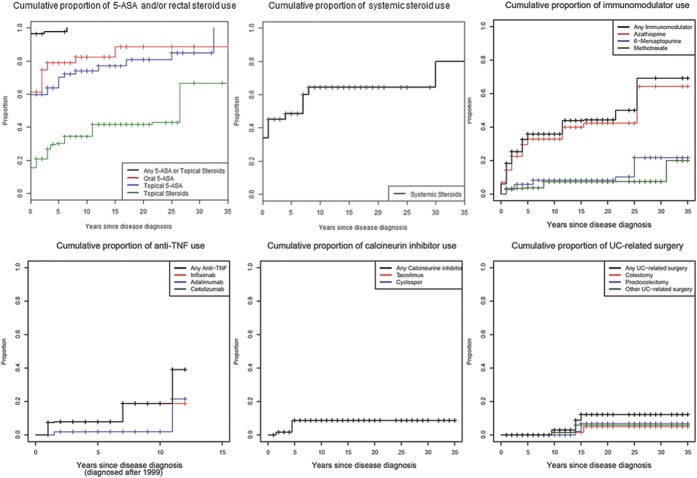
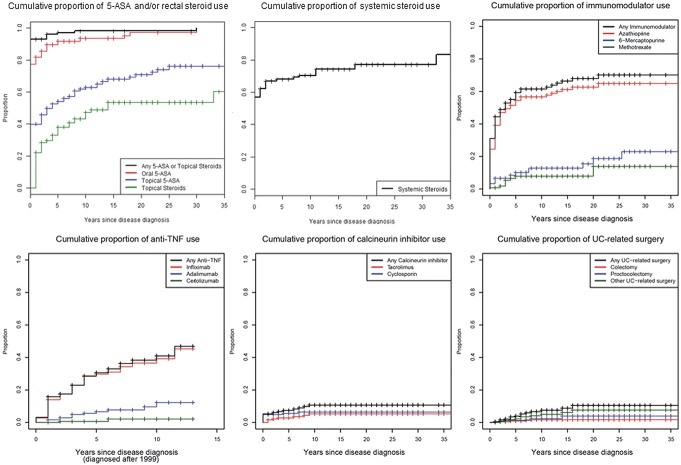
Figures 1–4 illustrate the cumulative probability of use of the different medications and undergoing UC-related surgery for all disease locations (Fig. 1), proctitis only (Fig. 2), left-sided colitis only (Fig. 3), and extensive/pancolitis only (Fig. 4).
FIGURE 1.
Cumulative probability for the use of 5-ASA and/or rectal corticosteroids, systemic corticosteroids, immunomodulators, TNF antagonists, calcineurin inhibitors, and for undergoing surgery for patients with all UC locations examined together.
FIGURE 4.
Cumulative probability for use of 5-ASA and/or rectal corticosteroids, systemic corticosteroids, immunomodulators, TNF antagonists, calcineurin inhibitors, and for undergoing surgery for patients with extensive colitis and pancolitis.
FIGURE 2.
Cumulative probability for use of 5-ASA and/or rectal corticosteroids, systemic corticosteroids, immunomodulators, TNF antagonists, calcineurin inhibitors, and for undergoing surgery for patients with ulcerative proctitis.
FIGURE 3.
Cumulative probability for use of 5-ASA and/or rectal corticosteroids, systemic corticosteroids, immunomodulators, TNF antagonists, calcineurin inhibitors, and for undergoing surgery for patients with left-sided colitis.
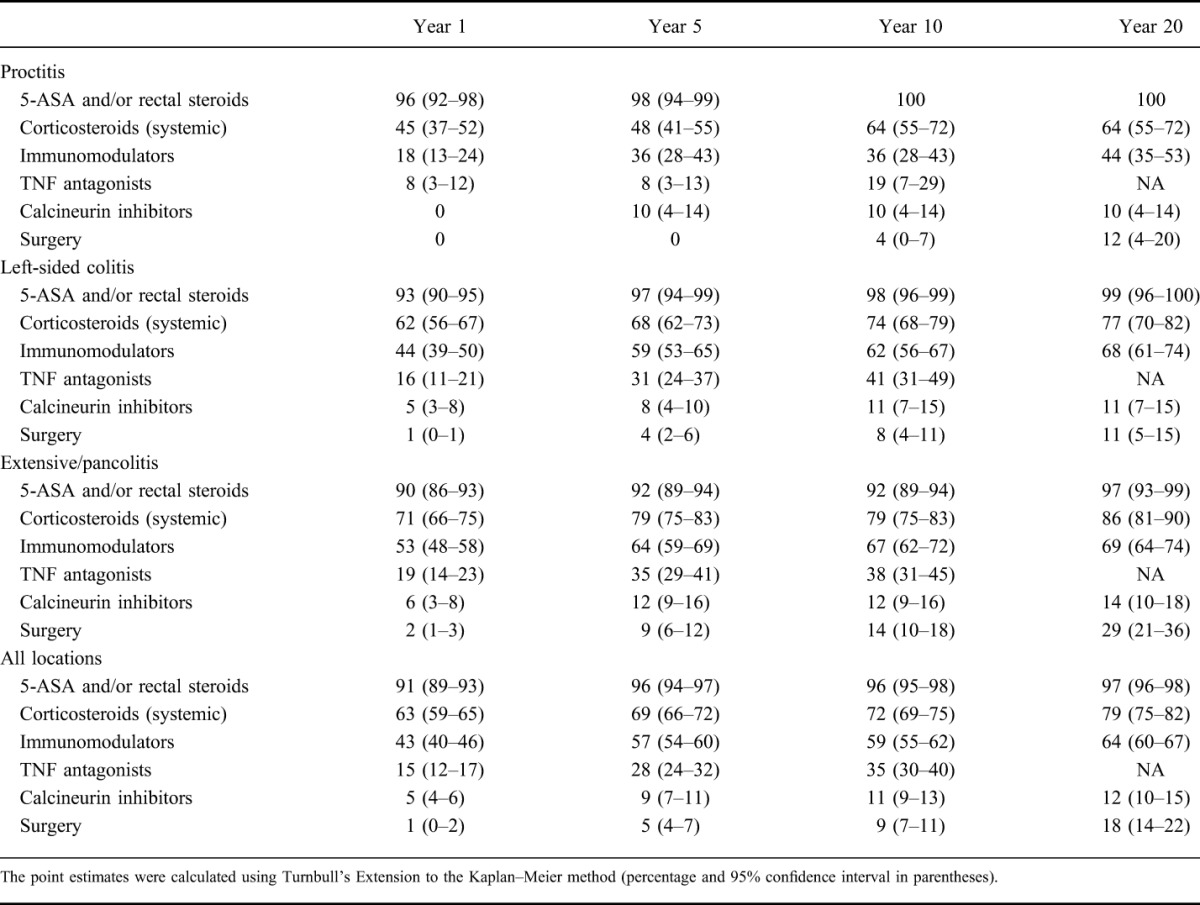
In Table 2, the point estimates of cumulative probability of UC-specific medication use/undergoing surgery at years 1, 5, 10, and 20 after UC diagnosis stratified according to disease location are shown. Our data indicate that more extensive UC at the time of diagnosis was associated with a higher probability of treatment with corticosteroids, TNF antagonists, calcineurin inhibitors, and undergoing UC-related surgery.
TABLE 2.
Proportion of Patients Treated with Various UC-specific Therapies and/or Undergoing Surgery at Years 1, 5, 10, and 20 After UC Diagnosis
Systematic Analysis of Risk Factors for Therapy Escalation
We further evaluated which factors might be associated with therapy escalation using logistic regression modeling. The following outcomes were analyzed: sex, age at the time of diagnosis, smoking status at the time of diagnosis, IBD family history, the presence of EIM from the time of enrollment into the SIBDCS, disease location at the time of diagnosis, and disease duration (OR per year of disease duration are shown). The results of this analysis are illustrated in Table 3. The following factors were found to be positively associated with the use of at least systemic corticosteroids during the disease course in the univariate model (Table 3): the presence of EIM (OR = 2.556, P = 0.001), left-sided colitis at the time of diagnosis (OR = 2.207, P < 0.001), and extensive/pancolitis at the time of diagnosis (OR = 6.064, P < 0.001). We observed a trend for the positive association between disease duration (per year) and the use of at least systemic corticosteroids (OR = 1.021, P = 0.057). In the multivariate model (Table 4), the following factors were significantly associated with at least systemic corticosteroid use: the presence of EIM (OR = 2.393, P < 0.001), left-sided colitis at the time of diagnosis (OR = 2.211, P < 0.001), and pancolitis at the time of diagnosis (OR = 5.806, P < 0.001).
TABLE 3.
Univariate Logistic Regression Modeling to Identify Risk Factors Associated with Particular Therapies in UC Patients
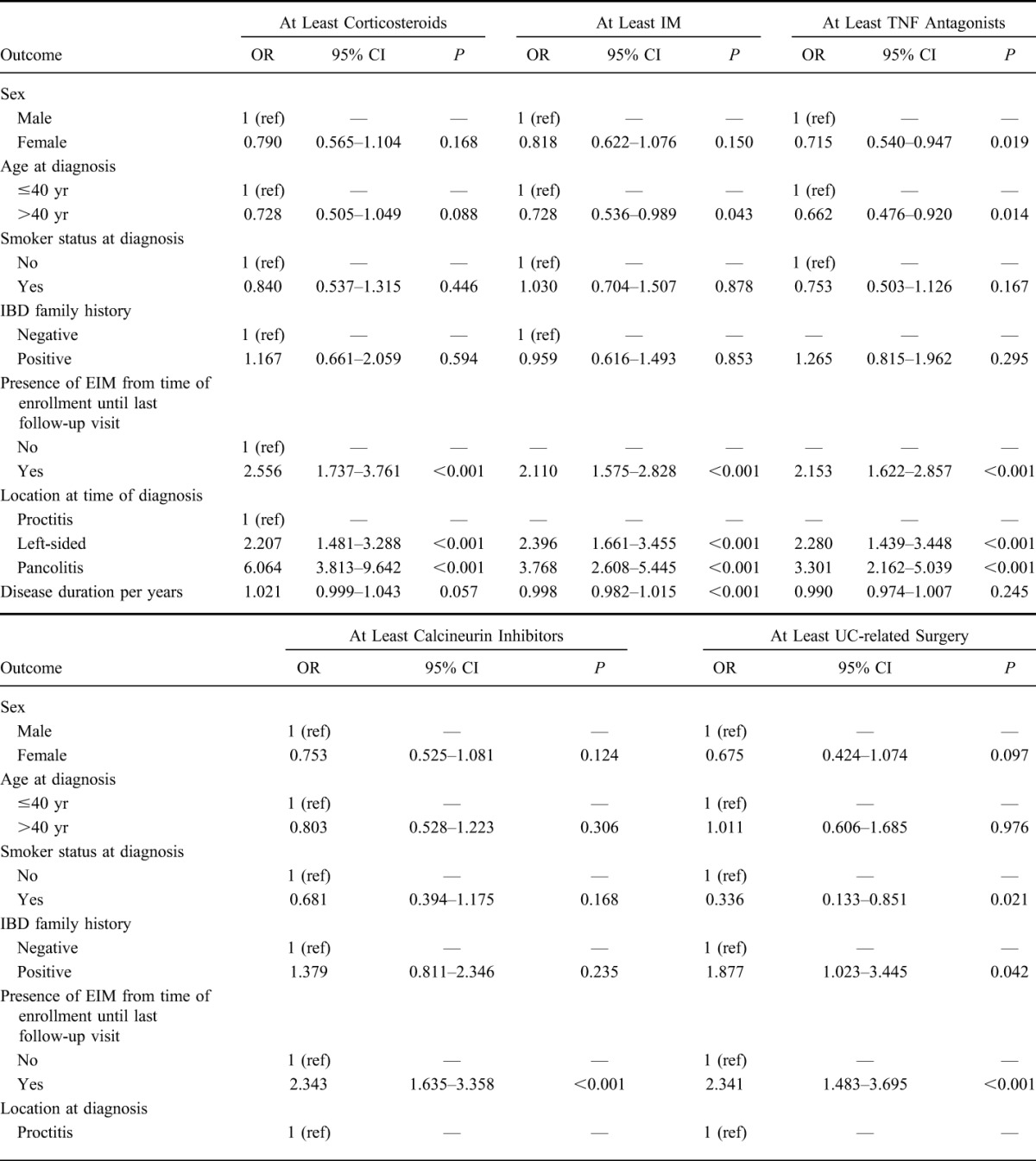
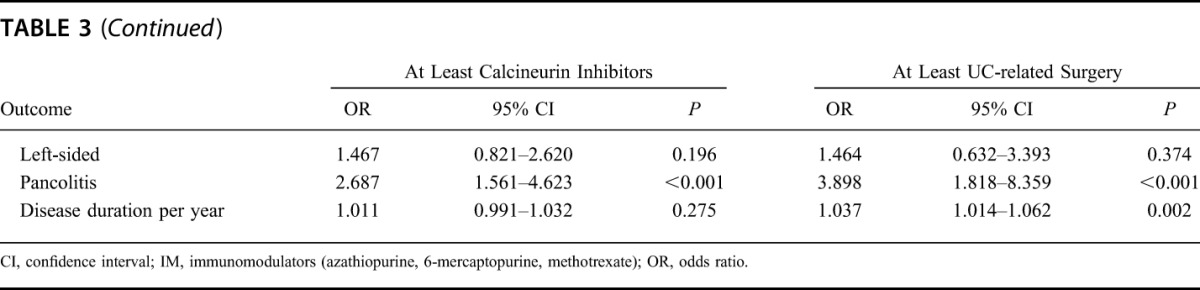
TABLE 4.
Multivariate Logistic Regression Modeling of the Factors with P value <0.1 as Presented in Table 3
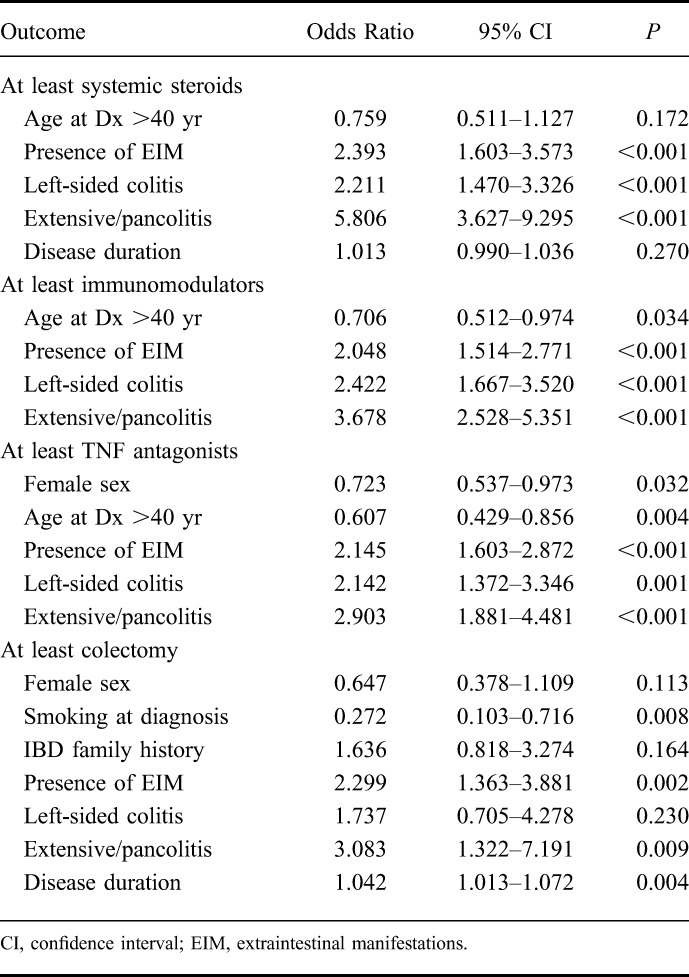
We identified the following factors to be associated with the use of at least immunomodulators during disease course (Table 3): age at diagnosis >40 years (OR = 0.728, P = 0.043), the presence of EIM (OR = 2.110, P < 0.001), left-sided colitis (OR = 2.396, P < 0.001), and pancolitis/extensive colitis (OR = 3.768, P < 0.001) at the time of diagnosis, and disease duration (OR = 0.998, P < 0.001). In the multivariate model (Table 4), the following factors were associated with at least IM use: age >40 years at diagnosis (OR 0.706, P = 0.034), the presence of EIM (OR = 2.048, P < 0.001), left-sided colitis (OR = 2.422, P < 0.001), and extensive/pancolitis (OR = 3.678, P < 0.001) at the time of diagnosis.
The following factors were found to be positively associated with the use of at least TNF antagonists during the disease course (Table 3): the presence of EIM (OR = 2.153, P < 0.001), left-sided colitis at the time of diagnosis (OR = 2.280, P < 0.001), and extensive/pancolitis at the time of diagnosis (OR = 3.301, P < 0.001). The following factors were protective for the use of at least TNF antagonists: female sex (OR = 0.715, P = 0.019) and older than 40 years at the time of UC diagnosis (OR = 0.662, P = 0.014). In the multivariate model (Table 4), the following factors were associated with at least TNF-antagonist use: female sex (OR = 0.723, P = 0.032) older than 40 years at diagnosis (OR = 0.607, P = 0.004), the presence of EIM (OR = 2.145, P < 0.001), left-sided colitis (OR = 2.143, P = 0.001), and extensive/pancolitis at the time of diagnosis (OR = 2.903, P < 0.001).
We identified the following factors to be positively associated with the use of at least calcineurin inhibitors during disease course (Table 3): the presence of EIM (OR = 2.343, P < 0.001) and extensive/pancolitis at the time of diagnosis (OR = 2.687, P < 0.001). Because of the limited number of patients treated with calcineurin inhibitors, no multivariate logistic regression modeling was performed.
The following factors were found to be positively associated with patients undergoing at least UC-related surgery in the univariate model (Table 3): positive family history for IBD (OR = 1.877, P = 0.042), presence of EIM (OR = 2.341, P < 0.001), extensive/pancolitis at diagnosis (OR = 3.898, P < 0.001), and disease duration (OR = 1.037, P = 0.002). Smoking at the time of UC diagnosis had a protective effect on UC-related surgery (OR = 0.336, P = 0.021). The following factors were significantly associated with at least UC-related surgery in the multivariate model (Table 4): smoking at the time of diagnosis (OR = 0.272, P = 0.008), the presence of EIM (OR = 2.29, P = 0.002), extensive/pancolitis at diagnosis (OR = 3.083, P = 0.009), and disease duration (OR = 1.042, P = 0.004).
DISCUSSION
In this study, we assessed the cumulative probability of patients undergoing therapy escalation in a large cohort of patients with UC and systematically analyzed risk factors associated with such a therapy escalation.
In accordance with the current European Crohn's and Colitis Organization guidelines on UC management, the proportion of patients using topical and/or oral 5-ASA products in our cohort was high.16 We observed that, at year 1 after diagnosis, 91% of all patients with UC use 5-ASA products irrespective of disease location at the time of diagnosis. When all disease locations were examined together, the use of 5-ASA products in our cohort increased from the 91% at year 1 to 96% (95% CI, 94%–97%) at year 10 after UC diagnosis. Given the fact that rectal budesonide therapy is used mainly in patients with UC with mild disease, we analyzed rectal steroid use over time together with 5-ASA drugs.
For all disease locations, the cumulative probability of systemic corticosteroid use was 63% (95% CI, 59%–65%) at year 1 and 69% (95% CI, 66%–72%) at year 5 after UC diagnosis. In our cohort, the cumulative probability of corticosteroid use at year 1 after UC diagnosis is higher compared with the 34% (63 out of 185 patients) reported by Faubion et al17 in a population-based study of patients with UC. The observed difference may be related to the fact that the SIBDCS is not population-based, and that the majority of patients (80%) are recruited in a hospital setting, which may be associated with the inclusion of more severe patients that are more frequently treated with corticosteroids. A much lower frequency of corticosteroid use was reported by Charpentier et al in a population-based cohort (EPIMAD registry) of 474 patients with UC with disease onset of >60 years of age. Authors reported that a cumulative probability of corticosteroid use of 21% (95% CI, 17%–25%) at year 1 after UC diagnosis and 34% (95% CI, 29%–38%) at year 5 after UC diagnosis were observed.11 In addition, the authors found that elderly onset IBD is characterized by a milder disease course when compared with young-onset IBD.11 As such, the difference between the rate of corticosteroids used in our cohort and in the EPIMAD registry can be explained by the fact that the EPIMAD registry is population-based and includes a higher proportion of patients recruited in private practice, when compared with the SIBDCS, as well as by a relatively mild disease course in the patients with elderly onset IBD. Lakatos et al18 reported that 30.4% of patients with UC (67/220) in a Hungarian incidence cohort used corticosteroids over a median follow-up of 5 years, which is much lower than the 69% (95% CI, 66%–72%) of corticosteroid use that we observed at 5 years in the SIBDCS. However, the 5-year cumulative probability of corticosteroid use in our cohort is comparable with the rates observed in patients with Crohn's disease in a population-based cohort from Cardiff and regional cohort from Copenhagen. In these cohorts, the rates of corticosteroid use reach almost 75% at 5 years after diagnosis.19,20 We could not identify studies reporting on the cumulative probability of corticosteroid use at years 10 and 20 after diagnosis in other populations of patients with UC.
The point estimates of cumulative probability of immunomodulator use for all disease locations together were as follows: 43% (95% CI, 40%–46%) at year 1 after diagnosis, 57% (95% CI, 54%–60%) at year 5 after diagnosis, and 59% (95% CI, 55%–62%) at year 10 after diagnosis. The point estimates of cumulative probability of immunomodulator use in our cohort are again higher when compared with those reported by Charpentier et al. Authors found a cumulative probability of immunomodulator use of 3% (95% CI, 1%–4%) at year 1 after diagnosis, 10% (95% CI, 7%–14%) at year 5 after diagnosis, and 15% (95% CI, 11%–20%) at year 10 after diagnosis in elderly onset UC patients. Lakatos et al reported on azathioprine exposure of only 5.9% in an incidence cohort of 220 Hungarian patients with UC with a median follow-up of 5 years.
For all disease locations together, the point estimates of cumulative probability of TNF antagonist use were as follows: 15% (95% CI, 12%–17%) at year 1 after diagnosis, 28% (95% CI, 24%–32%) at year 5 after diagnosis, and 35% (95% CI, 30%–40%) at year 10 after diagnosis. Because regulatory approval for adalimumab use in patients with UC in Switzerland was obtained in December 2013, the majority of patients with UC in this study were treated with infliximab and only a small fraction with adalimumab, which was prescribed after loss of response to infliximab. Charpentier et al documented a cumulative probability of TNF antagonist use of 0% at year 1, 0.4% (95% CI, 0.1%–0.6%) at year 5, and 25% (95% CI, 1%–3%) at year 10 after UC diagnosis. We did not identify other cohort studies in which the cumulative probabilities of anti-TNF treatment over time were examined.
The point estimates of the cumulative probability of calcineurin inhibitor (cyclosporine, tacrolimus) use for all disease locations together were as follows: 5% (95% CI, 4%–6%) at year 1 after diagnosis, 9% (95% CI, 7%–11%) at year 5 after diagnosis, and 11% (95% CI, 9%–13%) at year 10 after diagnosis. To the best of our knowledge, no data on the long-term follow-up of patients with UC treated with calcineurin inhibitors in other cohorts have been reported.
When all disease locations were examined together, the point estimates of cumulative probability of proctocolectomy or colectomy with ileorectal anastomosis were as follows: 1% (95% CI, 0%–2%) at year 1 after diagnosis, 5% (95% CI, 4%–7%) at year 5 after diagnosis, 9% (95% CI, 7%–11%) at year 10 after diagnosis, and 18% (95% CI, 14%–22%) at year 20 after UC diagnosis. As such, the annual rate of UC-related surgery was roughly 1%. The surgery rate in our cohort during the first 5 years of disease duration is comparable with the one reported by Lakatos et al.18 Authors reported on a colectomy rate of 2.3% in the incidence cohort of 220 patients with UC (over a median follow-up time of 5 yr).18 Our results compare well with those of other studies, in which a 25-year cumulative probability of colectomy ranging between 20% and 30% was reported. It is important to point out that higher point estimates of cumulative probability for UC-related surgery have been reported in studies conducted in referral centers when compared with population-based studies.10,21–23
When we conducted the analysis to identify risk factors for therapy escalation in UC, we found that EIM and extensive disease at the time of diagnosis (left-sided colitis and extensive colitis/pancolitis) were associated with an elevated risk for undergoing treatment with at least corticosteroids and at least immunomodulators. Furthermore, we found that EIM and extensive disease at the time of diagnosis were also risk factors for treatment with at least TNF antagonists, while female sex and age >40 years at the time of UC diagnosis were protective. The presence of EIM, extended disease location at the time of diagnosis (extensive colitis/pancolitis), and the disease duration were identified as risk factors for (procto)-colectomy. Cigarette smoking at UC diagnosis had a protective effect for undergoing UC-related surgery. Our results are in accordance with findings of a 10-year observational study, in which extensive colitis at the time of UC diagnosis was significantly associated with colectomy during the follow-up period (hazard ratio = 2.98, 95% CI, 1.25–7.08, P = 0.013), whereas cigarette smoking had a protective but insignificant effect on colectomy.24 To the best of our knowledge, no other study has shown that the presence of EIM during the disease course represents a risk factor for therapeutic step-up, including colectomy.
Our article has several strengths. We present data on the therapeutic escalation over time in a large and well-characterized cohort of patients with UC. Such data are urgently needed given the lack of comparable studies. In addition, the median duration of the follow-up period for patients in this study is 9 years, which means that our data are appropriate for evaluation of disease outcome over time. In addition to validating already known risk factors, such as disease extent, we discovered another important risk factor, namely the presence of EIM, which represents a risk factor for therapy escalation, including colectomy. However, the findings of our study should be interpreted with a number of considerations in mind. One important limitation of our study is that the SIBDCS is not a population-based study. As such, our results are subject to a referral center bias. Because 80% of patients with UC in the SIBDCS were recruited in referral centers and only 20% of patients by gastroenterologists in private practice, patients with UC suffering from mild disease are potentially underrepresented. Thus, the cumulative proportion of patients with UC in need of a therapy escalation could be lower in patients managed by primary care physicians. Therefore, the presented data is more likely to be applicable to patients seen at a Swiss referral center but not necessarily to a broader UC population nor perhaps to the patients in practices in other countries. In addition, given the fact that the presence of EIM in our study was examined during the entire disease course, this parameter does not necessarily represent a risk factor for therapy escalation but may represent per se an indication for step-up therapy.
In summary, we were able to characterize the requirement for step-up therapy over time in a large well-characterized cohort of patients with UC and to identify risk factors for such a therapy escalation. These results will allow the treating gastroenterologists to perform a risk assessment and to provide patients with the estimates of their future therapy use.
ACKNOWLEDGMENTS
Author contributions: Study concept and design, E. Safroneeva, S. R. Vavricka, N. Fournier, A. Straumann, G. Rogler, A. M. Schoepfer; Acquisition of data, S. R. Vavricka, A. Straumann, G. Rogler, A. M. Schoepfer; Statistical analysis, N. Fournier, A. M. Schoepfer; Analysis and interpretation of data, E. Safroneeva, S. R. Vavricka, N. Fournier, A. Straumann, G. Rogler, A. M. Schoepfer; Drafting of the manuscript, E. Safroneeva, S. R. Vavricka, N. Fournier, A. Straumann, G. Rogler, A. M. Schoepfer; Critical revision of the manuscript for important intellectual content, E. Safroneeva, S. R. Vavricka, N. Fournier, A. Straumann, G. Rogler, A. M. Schoepfer; obtained funding, E. Safroneeva, S. R. Vavricka, A. Straumann, G. Rogler, A. M. Schoepfer; Technical or material support, E. Safroneeva, A. Straumann, G. Rogler, A. M. Schoepfer; Study supervision, A. M. Schoepfer.
All members of the SIBDCS group involved in the acquisition of data: Claudia Anderegg; Peter Bauerfeind; Christoph Beglinger; Stefan Begré; Dominique Belli; José Bengoa; Luc Biedermann; Janek Binek; Mirjam Blattmann; Nadia Blickenstorfer; Stephan Boehm; Jan Borovicka; Christian Braegger; Patrick B¨hr; Bernard Burnand; Emmanuel Burri; Sophie Buyse; Matthias Cremer; Dominique Criblez; Philippe de Saussure; Lukas Degen; Joakim Delarive; Christopher Dörig; Barbara Dora; Gian Dorta; Tobias Ehmann; Ali El Wafa; Mara Egger; Matthias Engelmann; Christian Felley; Markus Fliegner; Nicolas Fournier; Montserrat Fraga; Alain Frei; Pascal Frei; Remus Frei; Michael Fried; Florian Froehlich; Raoul Furlano; Suzanne Gallot-Lavallée; Martin Geyer; Marc Girardin; Delphine Golay; Tanja Grandinetti; Beat Gysi; Horst Haack; Johannes Haarer; Beat Helbling; Peter Hengstler; Denise Herzog; Cyrill Hess; Klaas Heyland; Thomas Hinterleitner; Philippe Hiroz; Claudia Hirschi; Petr Hruz; Pascal Juillerat; Rosmarie Junker; Christina Knellwolf; Christoph Knoblauch; Henrik Köhler; Rebekka Koller; Claudia Krieger; Gerd A. Kullak-Ublick; Markus Landolt; Frank Lehmann; Valérie McLin; Philippe Maerten; Michel Maillard; Christine Manser; Andrew Macpherson; Michael Manz; George Marx; Rémy Meier; Christa Meyenberger; Jonathan Meyer; Pierre Michetti; Benjamin Misselwitz; Darius Moradpour; Patrick Mosler; Christian Mottet; Christoph Müller; Pascal Müller; Beat Müllhaupt; Claudia Münger; Leilla Musso; Andreas Nagy; Cristina Nichita; Jan Niess; Natacha Noël; Andreas Nydegger; Maliza Nzabonimpa; Nicole Obialo; Carl Oneta; Cassandra Oropesa; Céline Parzanese; Laetitia-Marie Petit; Franziska Piccoli; Julia Pilz; Gaëlle Pittet; Valérie Pittet; Bruno Raffa; Ronald Rentsch; Sophie Restellini, Jean-Pierre Richterich; Silvia Rihs; Jocelyn Roduit; Daniela Rogler; Gerhard Rogler; Jean-Benoît Rossel; Markus Sagmeister; Gaby Saner; Bernhard Sauter; Mikael Sawatzki; Michael Scharl; Sylvie Scharl; Nora Schaub; Martin Schelling; Susanne Schibli; Hugo Schlauri; Daniela Schmid; Sybille Schmid; Jean-François Schnegg; Alain Schoepfer; Christiane Sokollik; Frank Seibold; Gian-Marco Semadeni; Mariam Seirafi; David Semela; Arne Senning; Marc Sidler; Johannes Spalinger; Holger Spangenberger; Philippe Stadler; Volker Stenz; Michael Steuerwald; Alex Straumann; Michael Sulz; Alexandra Suter; Michela Tempia-Caliera; Joël Thorens; Sarah Tiedemann; Radu Tutuian; Ueli Peter; Stephan Vavricka; Francesco Viani; Roland Von Känel; Alain Vonlaufen; Dominique Vouillamoz; Rachel Vulliamy; Helene Werner; Paul Wiesel; Reiner Wiest; Tina Wylie; Jonas Zeitz; Dorothee Zimmermann.
Footnotes
Supported by research grants from the Swiss National Science Foundation (33CS30-148422 to Swiss IBD Cohort Study group, 32003B_135664/1 to A.M.S., 310030-120312 to G.R., and 320000-114009/3 and 32473B_135694/1 to S.R.V.), unrestricted research grants to A. M. Schoepfer from MSD Merck Sharp & Dohme AG Switzerland, Tillotts Pharma AG Switzerland, and a grant from the Zurich Center for Integrative Human Physiology of the University of Zurich (to G.R. and S.R.V.).
The authors have no conflicts of interest to disclose.
A list of members of the SIBDCS group is listed in the Acknowledgments.
This is an investigator-initiated study; pharmaceutical companies played no role in study design, acquisition, analysis, interpretation or presentation of the data.
REFERENCES
- 1.Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet. 2012;380:1606–1619. [DOI] [PubMed] [Google Scholar]
- 2.Sandborn WJ, Hanauer SB. Systematic review: the pharmacokinetic profiles of oral mesalazine formulations and mesalazine pro-drugs used in the management of ulcerative colitis. Aliment Pharmacol Ther. 2003;17:29–42. [DOI] [PubMed] [Google Scholar]
- 3.Desreumaux P, Ghosh S. Review article: mode of action and delivery of 5-aminosalicylic acid—new evidence. Aliment Pharmacol Ther. 2006;24(suppl 1):2–9. [DOI] [PubMed] [Google Scholar]
- 4.Loftus EV, Jr, Kane SV, Bjorkman D. Systematic review: short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2004;19:179–189. [DOI] [PubMed] [Google Scholar]
- 5.Ho GT, Ghiam P, Drummond H, et al. The efficacy of corticosteroid therapy in inflammatory bowel disease: analysis of a 5-year UK inception cohort. Aliment Pharmacol Ther. 2006;24:319–330. [DOI] [PubMed] [Google Scholar]
- 6.Rutgeerts P, Sandborn WJ, Feagan B, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;233:2462–2473. [DOI] [PubMed] [Google Scholar]
- 7.Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporine in ulcerative colitis: a five year experience. Am J Gastroenterol. 1999;94:1587–1592. [DOI] [PubMed] [Google Scholar]
- 8.Sjöberg D, Holmström T, Larsson M, et al. Incidence and natural history of ulcerative colitis in the Uppsala Region of Sweden 2005–2009—results from the IBD Cohort of the Uppsala Region (ICURE). J Crohns Colitis. 2013;7:e351–e357. [DOI] [PubMed] [Google Scholar]
- 9.Burisch J, Jess T, Martinato M, et al. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322–337. [DOI] [PubMed] [Google Scholar]
- 10.Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. [DOI] [PubMed] [Google Scholar]
- 11.Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut. 2014;63:423–432. [DOI] [PubMed] [Google Scholar]
- 12.Pittet V, Juillerat P, Mottet C, et al. Cohort profile: the Swiss Inflammatory Bowel Disease Cohort Study (SIBDCS). Int J Epidemiol. 2009;38:922–931. [DOI] [PubMed] [Google Scholar]
- 13.Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–1845. [DOI] [PubMed] [Google Scholar]
- 14.R Core Team. R. A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. ISBN 3-900051-07-0, URL. Available at: http://www.R-project.org/. Accessed March 3, 2014. [Google Scholar]
- 15.Turnbull BW. Nonparametric estimation of a survivorship function with doubly censored data. J Am Stat Assoc. 1974;69:169–173. [Google Scholar]
- 16.Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030. [DOI] [PubMed] [Google Scholar]
- 17.Faubion WA, Loftus EV, Harmsen SW, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. [DOI] [PubMed] [Google Scholar]
- 18.Lakatos L, Kiss LS, David G, et al. Incidence, disease phenotype at diagnosis, and early disease course in inflammatory bowel diseases in Western Hungary, 2002–2006. Inflamm Bowel Dis. 2011;17:2558–2565. [DOI] [PubMed] [Google Scholar]
- 19.Ramadas AV, Gunesh S, Thomas GAO, et al. Natural history of Crohn's disease in a population-based cohort from Cardiff (1986–2003): a study of changes in medical treatment and surgical resection rates. Gut. 2010;59:1200–1206. [DOI] [PubMed] [Google Scholar]
- 20.Munkholm P, Langholz E, Davidsen M, et al. Frequency of glucocorticoid resistance and dependency in Crohn's disease. Gut. 1994;35:360–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langholz E, Munkholm P, Davidsen M, et al. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994;107:3–11. [DOI] [PubMed] [Google Scholar]
- 22.Henriksen M, Jahnsen J, Lygren I, et al. Ulcerative colitis and clinical course: results of a 5-year population-based follow-up study (the IBSEN study). Inflamm Bowel Dis. 2006;12:543–550. [DOI] [PubMed] [Google Scholar]
- 23.Hoie O, Wolters FL, Riis L, et al. Low colectomy rates in ulcerative colitis in an unselected European cohort followed for 10 years. Gastroenterology. 2007;132:507–515. [DOI] [PubMed] [Google Scholar]
- 24.Solberg IG, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009;44:431–440. [DOI] [PubMed] [Google Scholar]