Abstract
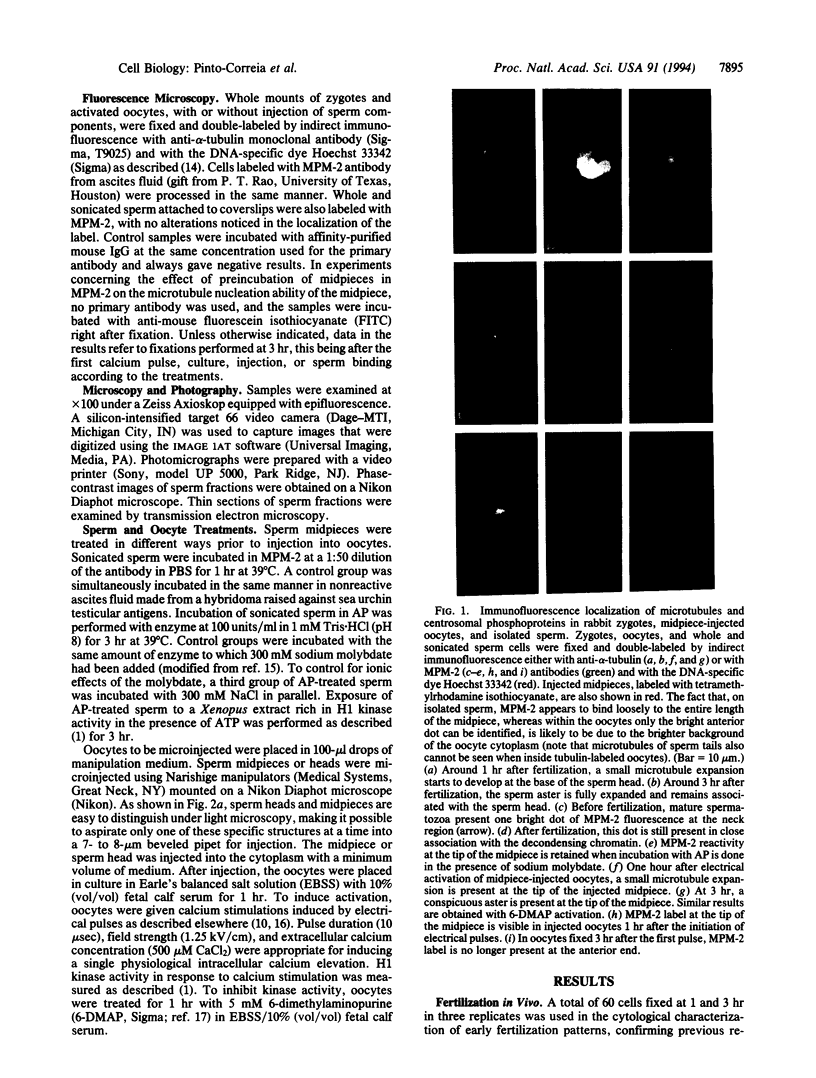
During fertilization in most mammals, the penetrating sperm organizes an aster of microtubules. We have investigated the mechanisms underlying this function of the sperm by a series of experiments based on microinjection of isolated sperm midpieces into unfertilized oocytes. These midpieces contain antigens recognized by the MPM-2 antibody. These antigens, which are absent from the rest of the tail fraction, correspond to three phosphorylated polypeptides of 77, 81, and 85 kDa. Dephosphorylation with alkaline phosphatase abolishes antigenicity on blots and in whole sperm. Reactivity to the antibody disappears between 1 and 3 hr after calcium stimulation of oocytes, following the decline in H1 kinase activity and coincident with aster formation. In unactivated oocytes, no aster forms and the antigen remains unchanged. MPM-2 treatment of midpieces prior to injection blocks their ability to form asters in oocytes activated by calcium stimulation. The epitope also disappears in 6-methyl-aminopurine-treated oocytes, implying that maintenance of the phosphorylated state requires kinase activity. A result that confirms this view is that sperm midpieces dephosphorylated by alkaline phosphatase can be rephosphorylated after injection into oocytes or by exposure in vitro to a Xenopus oocyte cytoplasmic fraction high in H1 kinase activity. We suggest that the microtubule nucleation activity of sperm midpieces after fertilization is triggered by the calcium-induced decrease in maturation promoting factor, which results in dephosphorylation of specific sperm centrosomal proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Centonze V. E., Borisy G. G. Nucleation of microtubules from mitotic centrosomes is modulated by a phosphorylated epitope. J Cell Sci. 1990 Mar;95(Pt 3):405–411. doi: 10.1242/jcs.95.3.405. [DOI] [PubMed] [Google Scholar]
- Collas P., Balise J. J., Robl J. M. Influence of cell cycle stage of the donor nucleus on development of nuclear transplant rabbit embryos. Biol Reprod. 1992 Mar;46(3):492–500. doi: 10.1095/biolreprod46.3.492. [DOI] [PubMed] [Google Scholar]
- Collas P., Pinto-Correia C., Ponce de Leon F. A., Robl J. M. Effect of donor cell cycle stage on chromatin and spindle morphology in nuclear transplant rabbit embryos. Biol Reprod. 1992 Mar;46(3):501–511. doi: 10.1095/biolreprod46.3.501. [DOI] [PubMed] [Google Scholar]
- Collas P., Sullivan E. J., Barnes F. L. Histone H1 kinase activity in bovine oocytes following calcium stimulation. Mol Reprod Dev. 1993 Feb;34(2):224–231. doi: 10.1002/mrd.1080340215. [DOI] [PubMed] [Google Scholar]
- Crozet N. Behavior of the sperm centriole during sheep oocyte fertilization. Eur J Cell Biol. 1990 Dec;53(2):326–332. [PubMed] [Google Scholar]
- Davis F. M., Tsao T. Y., Fowler S. K., Rao P. N. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. W., Phillips D. M. The fine structure and development of the neck region of the mammalian spermatozoon. Anat Rec. 1969 Oct;165(2):153–164. doi: 10.1002/ar.1091650204. [DOI] [PubMed] [Google Scholar]
- Fissore R. A., Robl J. M. Intracellular Ca2+ response of rabbit oocytes to electrical stimulation. Mol Reprod Dev. 1992 May;32(1):9–16. doi: 10.1002/mrd.1080320103. [DOI] [PubMed] [Google Scholar]
- Kuang J., Zhao J., Wright D. A., Saunders G. F., Rao P. N. Mitosis-specific monoclonal antibody MPM-2 inhibits Xenopus oocyte maturation and depletes maturation-promoting activity. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4982–4986. doi: 10.1073/pnas.86.13.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiak J. Z., Weber M., de Pennart H., Winston N. J., Maro B. The metaphase II arrest in mouse oocytes is controlled through microtubule-dependent destruction of cyclin B in the presence of CSF. EMBO J. 1993 Oct;12(10):3773–3778. doi: 10.1002/j.1460-2075.1993.tb06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R., Rao P. N., Borisy G. G. Immunocytochemical evidence for centrosomal phosphoproteins in mitotic sea urchin eggs. Cell Struct Funct. 1990 Feb;15(1):13–20. doi: 10.1247/csf.15.13. [DOI] [PubMed] [Google Scholar]
- Lewin B. Driving the cell cycle: M phase kinase, its partners, and substrates. Cell. 1990 Jun 1;61(5):743–752. doi: 10.1016/0092-8674(90)90181-d. [DOI] [PubMed] [Google Scholar]
- Maggi A., Schrader W. T., O'Malley B. W. Progesterone-binding sites of the chick oviduct receptor. Presence of a weaker ligand site which is destroyed by phosphatase treatment. J Biol Chem. 1984 Sep 10;259(17):10956–10966. [PubMed] [Google Scholar]
- Rime H., Neant I., Guerrier P., Ozon R. 6-Dimethylaminopurine (6-DMAP), a reversible inhibitor of the transition to metaphase during the first meiotic cell division of the mouse oocyte. Dev Biol. 1989 May;133(1):169–179. doi: 10.1016/0012-1606(89)90308-4. [DOI] [PubMed] [Google Scholar]
- Sathananthan A. H., Kola I., Osborne J., Trounson A., Ng S. C., Bongso A., Ratnam S. S. Centrioles in the beginning of human development. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4806–4810. doi: 10.1073/pnas.88.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten H., Schatten G., Mazia D., Balczon R., Simerly C. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc Natl Acad Sci U S A. 1986 Jan;83(1):105–109. doi: 10.1073/pnas.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice S. L., Robl J. M. Activation of mammalian oocytes by a factor obtained from rabbit sperm. Mol Reprod Dev. 1990 Mar;25(3):272–280. doi: 10.1002/mrd.1080250309. [DOI] [PubMed] [Google Scholar]
- Szollosi D., Calarco P., Donahue R. P. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci. 1972 Sep;11(2):521–541. doi: 10.1242/jcs.11.2.521. [DOI] [PubMed] [Google Scholar]
- Vandré D. D., Borisy G. G. Anaphase onset and dephosphorylation of mitotic phosphoproteins occur concomitantly. J Cell Sci. 1989 Oct;94(Pt 2):245–258. doi: 10.1242/jcs.94.2.245. [DOI] [PubMed] [Google Scholar]
- Vandré D. D., Burry R. W. Immunoelectron microscopic localization of phosphoproteins associated with the mitotic spindle. J Histochem Cytochem. 1992 Dec;40(12):1837–1847. doi: 10.1177/40.12.1453002. [DOI] [PubMed] [Google Scholar]
- Vandré D. D., Centonze V. E., Peloquin J., Tombes R. M., Borisy G. G. Proteins of the mammalian mitotic spindle: phosphorylation/dephosphorylation of MAP-4 during mitosis. J Cell Sci. 1991 Apr;98(Pt 4):577–588. doi: 10.1242/jcs.98.4.577. [DOI] [PubMed] [Google Scholar]
- Whitaker M., Patel R. Calcium and cell cycle control. Development. 1990 Apr;108(4):525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]
- Yllera-Fernández M. M., Crozet N., Ahmed-Ali M. Microtubule distribution during fertilization in the rabbit. Mol Reprod Dev. 1992 Jul;32(3):271–276. doi: 10.1002/mrd.1080320313. [DOI] [PubMed] [Google Scholar]