Abstract
Introduction
Revascularization treatment is rapidly becoming an accepted alternative for the management of endodontic pathology in immature permanent teeth with necrotic dental pulps. However, the success and timing of clinical resolution of symptoms and of radiographic outcomes of interest, such as continued hard tissue deposition within the root, are largely unknown.
Methods
In this prospective cohort study, 20 teeth were treated with a standardized revascularization treatment protocol, and monitored for clinical and radiographic changes for one year. Standardized radiographs were collected at regular intervals and radiographic changes were quantified.
Results
All 20 treated teeth survived during the 12 month follow up period and all 20 also met the clinical criteria for success at 12 months. As a group, the treated teeth demonstrated a statistically significant increase in radiographic width and length, and a decrease in apical diameter, although the changes in many cases were quite small such that the clinical significance is unclear. The within-case percent change in apical diameter after 3 months was 16% and had increased to 79% by 12 months, with 55% (11/20) showing complete apical closure. The within-case percent change in root length averaged less than 1% at 3 months and increased to 5% at 12 months. The within-case percent change in root thickness averaged 3% at 3 months and 21% at 12 months.
Conclusions
Although clinical success was highly predictable with this procedure, clinically meaningful radiographic root thickening and lengthening is less predictable at one year of follow up. Apical closure is the most consistent radiographic finding.
Introduction
Since the first case of revascularization of an immature permanent tooth with apical periodontitis and sinus tract was reported by Iwaya and co-authors in 2001 (1), many more case reports and case series of such treatments have been published (2). Unlike apexification therapy, thickening of the canal walls and continued root maturation is sometimes observed after revascularization/revitalization therapy (3, 4); therefore, the treatment procedure is currently widely accepted for the management of endodontic pathology when treating immature permanent necrotic teeth. Although there are multiple possible etiologies for pulp necrosis and/or apical periodontitis in immature permanent teeth, it appears traumatic injury is the most frequent contributory etiology leading to revascularization/revitalization therapy (2). Although several studies have attempted to predict the timing of periapical wound healing, increased thickening of the canal walls, and continued root lengthening of immature permanent necrotic teeth after revascularization/ endodontic treatment, the expected rate of these outcomes remain unknown (3–11).
Infection can damage the pulp, and if not controlled can lead to total pulpal necrosis and apical periodontitis, as well as root resorption and/or arrested root development of immature permanent teeth. Traumatic injury to the tooth also can cause pulpal necrosis and arrested root development, even when the coronal dentin and enamel remain intact, most likely by damaging the periapical vasculature and Hertwig’s epithelial root sheath (12, 13). It is not yet clear how trauma as an etiology for pulpal necrosis influences the outcomes of revascularization/revitalization therapy, especially continued root development. In this prospective study, the time to resolution of clinical symptoms, radiographic periapical healing, and radiographic changes in root dimension, were investigated in a cohort of patients receiving standardized revascularization/revitalization therapy.
Materials and Methods
Subjects
This study was approved by the Institutional Review Board of Alexandria University Faculty of Dentistry. A single operator (TS) completed all procedures at the Alexandria University Faculty Practice.
A total of 17 subjects with one or more immature permanent anterior teeth (n=20 cases), met the study criteria and were recruited as subjects into this prospective cohort study. No subjects dropped out of the study during the one year follow up period. The inclusion criteria for the study was: 1) Subject with one or more anterior teeth with a non-vital pulp and immature root; the exclusion criteria for the study were: 1) teeth with radiographic signs of internal or external resorption 2) teeth with a root fracture 3) alveolar fracture 4) presence of periodontal disease 5) subjects with chronic systemic illness 6) uncooperative subjects 7) subjects with poor oral hygiene. The subjects included in this study represent a convenience sample of the patients who presented to the University Faculty Practice during the time of study enrollment and met the inclusion/exclusion criteria.
Study Procedures
Baseline exams included clinical examinations with standard endodontic diagnostic procedures, clinical photographs, and imaging of teeth with periapical radiographs. Pulp sensibility testing was completed using both thermal and electric stimulation. Sensibility to cold was tested by application of a cotton pellet saturated with Endo Frost (Endo FrostTM, Roeko, Langenau, Germany). Sensibility to heat was tested by application of a heated gutta-percha stick applied to the tooth. Sensibility to electrical stimulation was evaluated using the Digitest Pulp Tester (Parkell Inc., Edgewood, New York, USA). For electrical stimulation testing, teeth were isolated with cotton rolls, and dried. Toothpaste was use a conduction medium and the probe was applied to the incisal 1/ 3 of the labial surface. Radiographs were taken of all teeth using custom-made bite registration and a paralleling device to standardize all radiographic images in the study. Radiographs were scanned and saved in the computer as JPEG format.
All 20 teeth were treated following a clinical protocol based on the publication by Banchs & Trope (14). At the first appointment, the tooth was isolated with a rubber dam. The crown of involved tooth and the surrounding rubber dam were disinfected by swabbing the area with 30% hydrogen peroxide, followed by Betadine (Tizaro Suppl Limited, London, UK), for one minute each. The canal was accessed with a carbide no. 4 round bur. The pulp chamber was irrigated with 2.5% sodium hypochlorite solution (Clorox, Nobelwax Factories for Chemicals, Egypt). The length of the canal was estimated using the pre-operative radiograph, and the canal was gently debrided with a K-file using push and pull motion in order to remove any necrotic pulp tissue. The canal was then irrigated with copious amounts of 2.5% sodium hypochlorite, followed by a sterile saline solution. The canal was dried with sterile paper points. A triple antibiotic paste was used that was made from tablets of antibiotics. After removing any coating, the tablets were crushed into a powder, combined in a 1:1:1 ratio, and mixed with sterile saline to form a paste like consistency. (ciprofloxacin: Ciprofloxazine 200 mg, European Egyptian Pharm. Ind., Alexandria, Egypt; metronidazole: Flagyl 500 mg, Amriya Pharm. Ind., Alexandria, Egypt; minocycline: Minocin 100 mg, Wyeth Pharmaceuticals, Ghagzhou, China.) The antibiotic paste was delivered into the apical third of the canal using a lentulo spiral. A sterile cotton pellet was placed into the canal below the cemento-enamel junction (CEJ) and the access cavity was sealed with intermediate restorative material (Dentsply DeTrey, Konstanz, Germany).
At the second appointment, two weeks after the first visit, if the clinical signs/symptoms persisted, the first appointment treatment procedures were repeated. If symptoms were resolved, the tooth was isolated with a rubber dam swabbed with disinfectant. The IRM and cotton pellet were removed from the access cavity. The paste was removed from the canal by irrigation with sterile saline solution, and the canal was dried with sterile paper points. A pre-curved K-file was introduced into the canal and extruded 2 mm past the apical foramen, in order to induce bleeding into the canal to the level of CEJ by rotating the file. A sterile cotton pellet was placed over the bleeding for 3–4 minutes to allow blood clot formation. Mineral trioxide aggregate (MTA) (Pro-Root MTA; Dentsply Tulsa Dental Specialties, Tulsa, OK, USA.) was mixed with sterile saline solution according to manufacturer’s instructions and placed over the blood clot, just below the level of the CEJ. The access cavity was restored with a light-cured composite resin (Ice composite, Southern Dental Industries, Australia.). The patient was followed up at 1, 3, 6, 9, and 12 months after completion of revascularization/revitalization therapy. Follow up visits assessed for any subjective patient report of pain or discomfort, the integrity of restoration, pulp sensibility testing, tenderness to palpation and percussion, mobility, sinus tract, swelling, periodontal pocket depth measurements, and acquisition of a periapical radiograph.
The clinical outcomes of survival and success were evaluated throughout the study period. Survival was defined as the tooth remaining present in the arch throughout the study period. Clinical success was defined as a tooth that survived and also showed no signs of endodontic pathology including tenderness to percussion or palpation, a swelling or sinus tract, or spontaneous pain.
Radiographic Analysis
The width of the apical foramen, thickness of the canal walls, and length of the root were measured from images obtained pre-operatively and at each follow-up study visit using the open source software, Image J (National Institutes of Health, Bethesda, MD, USA). The TurboReg plug-in (Biomedical Imaging Group, Swiss Federal Institute of Technology, Lausanne, VD, Switzerland) was used to minimize distortions in angulation between compared radiographs. The methodology used by Bose et al. (3) was modified and applied in the present study. Briefly, the image with the least visible distortion was chosen as the source image, while the other images were designated as target images. Three stable landmarks that were easily identifiable in the compared images were selected on the source and target images. Images were calibrated by using the “set scale” option in Image J to measure the distance between 2 stable reference points on the source image and that measurement was used to set the scale of the adjusted target image. This calibration process allowed for the measurement of radiographic changes on a millimeter scale.
After image standardization, root length, dentinal wall thickness at the apical third, and the apical foramen diameter were measured by using Image J. The root length was measured as a straight line from the CEJ or alveolar crest to the radiographic apex of the tooth. The dentinal wall thickness was measured at the level of the apical one third. The root canal width and the pulp space were measured at this level, and the remaining dentin thickness was calculated by subtracting the pulp space from the root canal width. The apical diameter was measured as a straight line across the radiographic apical foramen. Radiographs were also assessed for the presence or absence of a periapical radiolucency as well as the apical foramen being open or closed.
Statistical analysis
McNemar’s test was used to compare the proportion of cases with and without a periapical radiolucency present and with or without closure of the apical foramen at 3,6,9 and 12 months follow up, versus the baseline proportion of cases. Repeated measures ANOVA test was used to test for significant changes in root length and thickness of the canal walls (mm) at 3, 6, 9, and 12 months follow-up examinations versus baseline. P≤0.05 was considered statistically significant. Statistical analyses were performed using Stata 11.2 for Mac (StataCorp LP, College Station, TX, USA). Graphs were generated using GraphPad Prism 5 for Mac OS X (La Jolla, CA, USA).
Results
Baseline Study Population Characteristics
The characteristics of the study population are summarized in Table 1. The majority of the subjects were male (70%) and the average age was 11.3 years. All treated teeth were anterior teeth with a non-vital pulpal sensibility testing pre-operatively, and demonstrated either radiographic periapical pathology and/or periapical symptomatology. Most cases had radiographic evidence of a periapical lesion (85%) and half had an active swelling or sinus tract. The etiology of the endodontic pathology for all teeth was trauma, and most teeth had some degree of coronal fracture (Table 1).
Table 1.
Patient demographics and baseline characteristics
| Variable | Revascularization Cases N=20 |
|---|---|
| Male Sex – no. (%)* | 14 (70) |
| Female Sex- | 6 (30) |
| Age –years | 11.3 ± 1.9 |
| Anterior Tooth | 20 (100) |
| Maxillary | 18 (90) |
| Mandibular | 2 (10) |
| Etiology | |
| Trauma | 20 (100) |
| Enamel/dentin/pulp fx | 16 (80) |
| Enamel/dentin fx | 2 (10) |
| No loss of tooth structure | 2 (10) |
| Periapical Radiolucency Present | 17(85) |
| Pre-op pain | 12 (60) |
| Swelling or Sinus Tract | 10 (50) |
| Pre-op negative pulp sensibility | 20 (100) |
| Pulpal necrosis | 15 (75) |
| Previously initiated RCT | 5 (25) |
Continuous and ordinal variables presented as mean ± standard deviation. Frequency of all categorical variables presented as number (no.) and percentage (%).
Each tooth is treated as an individual case for analysis. However, some patients had multiple teeth included in the study.
Clinical Outcomes
All 20 revasc/revit cases were followed for a total of 12 months, with follow up exams taking place every 3 months. All 20 treated teeth survived during the 12 month follow up period and all met the study criteria for clinical success (tooth survival without clinical symptoms including tenderness to percussion or palpation, a swelling or sinus tract, or spontaneous pain) at 12 months. No teeth required additional endodontic treatment beyond the initial treatment. For all cases, any symptoms or sign of active infection was resolved by the 3 month follow up visit. None of the teeth regained responsiveness to pulpal sensibility tests (cold, heat, and electrical) during the one year follow up period.
Radiographic Outcomes
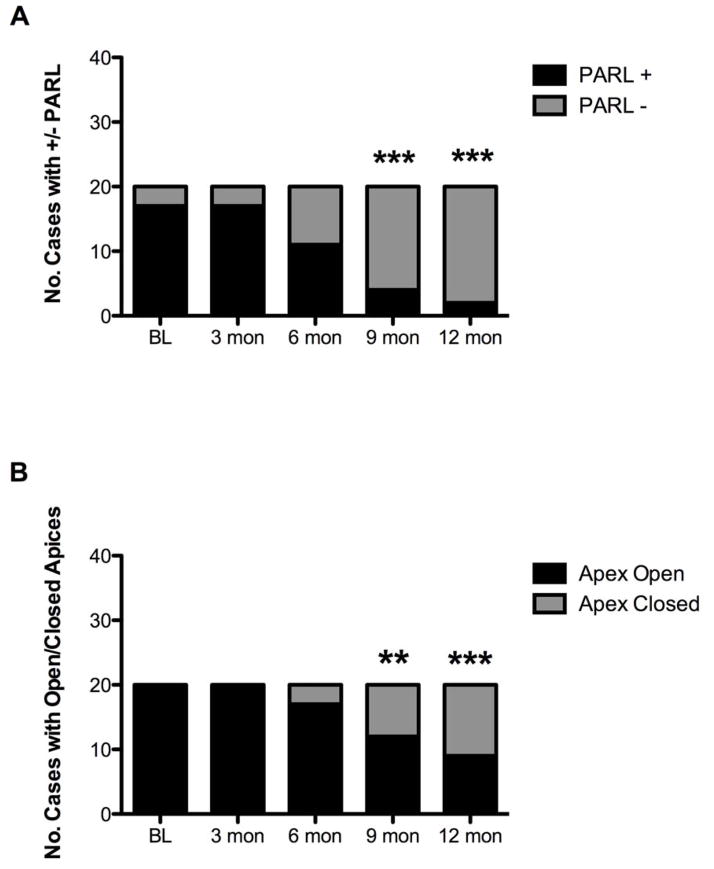
The proportion of cases with radiographic evidence of a PARL had significantly decreased by the 6 month follow up visit (Fig. 1). Ninety percent of cases (18/20) had complete resolution of radiographic periapical lesions at the 12 months follow-up visit. The remaining 2 cases still had evidence of a PARL but had no clinical symptoms, so they still met the clinical criteria for success in this study. The earliest time point where radiographic evidence for complete apical closure was observed was at 6 months post-treatment (3 cases), with the proportion of cases with apical closure differing significantly from baseline at the 9-month visit (Fig 1). Fifty-five percent of cases (11/20) had complete radiographic apical closure 12 months after treatment.
Figure 1.
Radiographic findings regarding the proportion of subjects with periapical radiolucencies and open apices at various time points of the study. A) The proportion of subjects with a periapical radiolucency decreased throughout the study period and became significantly different from baseline after 9 months (McNemar’s chi2 test, 6 months: p= 0.06; 9 and 12 month: p<0.0001). B) The proportion of subjects with an open apex began to decrease after 6 months, and became significantly different from baseline at 9 months (McNemar’s chi2 test, 9 months p<0.05; 12 months p<0.001).
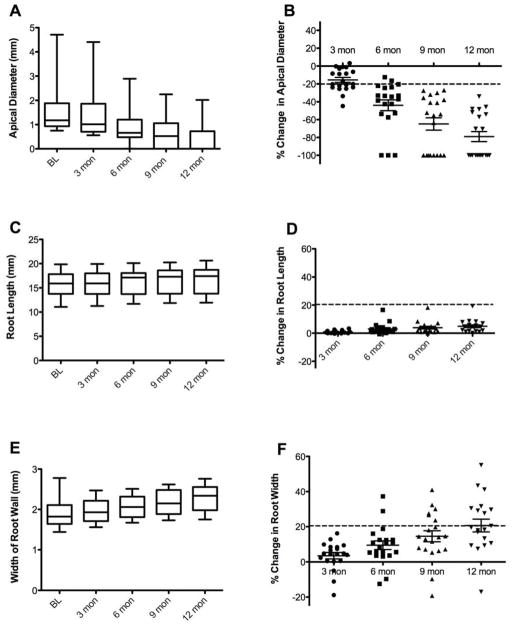
By quantifying the actual distance of the apical opening in millimeters at each follow-up time, we found that the average apical diameter of the population decreased throughout the follow-up period, and became significantly different from baseline at the 6 month time point, at which point the mean apical diameter had decreased by 0.65 mm (Fig 2A, one way repeated measures ANOVA p<0.0001, Dunnet’s multiple comparison test baseline vs 6 mon, p<0.001). Comparison of the amount of change in apical diameter within individual cases demonstrated that on average the apical diameter at 3 months had decreased by 16%, and by 12 months had decreased to 79% the size observed in the pre-operative radiographs. Many teeth had complete apical closure (Fig 2B). Assuming a 20% change as a cutoff for a clinically meaningful change in apical diameter, all 20 cases had achieved this measure by 9 months.
Figure 2.
Radiographic changes in apical diameter, root length, and root width over the study period. A) Box and whiskers plot of apical diameter (mm) measured at 3 month intervals. The band inside the box represents the median. The lower chamber of the box contains the 1st quartile and the upper chamber of the box contains the 3rd quartile. The upper and lower boundaries of the whiskers represent the minimum and maximum values of the population. The change was highly significant when analyzed using repeated measures ANOVA. B) Scatter plot of the calculated percent change from baseline in apical diameter for each case measured at each follow up visit. The error bars represent the mean +/− SEM. The horizontal line represents an arbitrary cutoff of 20% change representing a clinically significant finding. C) Box and whiskers plot of root length (mm) measured at 3 month intervals. The change was highly significant when analyzed using repeated measures ANOVA. D) Scatter plot of the calculated percent change from baseline in root length for each case measured at each follow up visit. E) Box and whisker plot of the width of root wall (mm) measured at 3 month intervals. The change was highly significant when analyzed using repeated measures ANOVA. F) Scatter plot of the calculated percent change from baseline in root width for each case measured at each follow up visit.
Significant changes from baseline in the average root length (mm) of the population (Fig 2C, one way repeated measures ANOVA p<0.0001, Dunnet’s multiple comparison test BL vs 6 mon, p< 0.001) and in the mean thickness of the canal walls (mm) (Fig 2E, one way repeated measures ANOVA p<0.0001, Dunnet’s multiple comparison test BL vs 6 mon p<0.01) were also observed after 6 months of follow up. The within-case percent change in root length averaged less than 1% at 3 months and increased to only 5% at 12 months (Fig 2D). The within-case percent change in root width averaged 3% at 3 months and 21% at 12 months. Assuming a 20% cutoff for a clinically meaningful radiographic change, it is interesting to note that no cases met this criteria for root length measurements, but 9 cases met this criteria for root width measurements at the 12 month time point. When considering the clinical significance of these findings, it is important to note that although many cases demonstrated a measurable increase in root width or length, on a case-by-case basis many of these changes would not be discernable by visual examination and would not have been detectible without using software to quantify the radiographic changes.
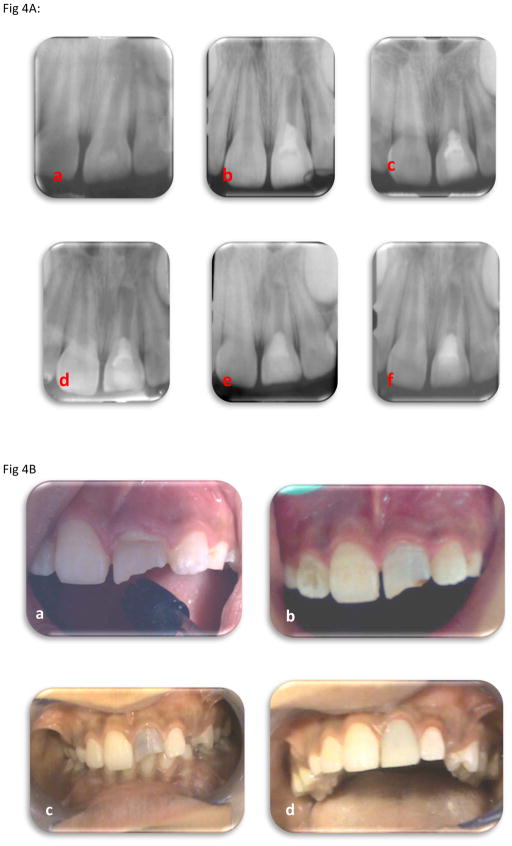
The final radiographic outcome assessed was hard-tissue bridge formation that occurred within the canal, and not at the apex. Three teeth showed hard tissue bridge formation in the coronal third and 2 teeth showed formation in the middle third of the root (Fig 3 and Fig 4).
Fig 3.
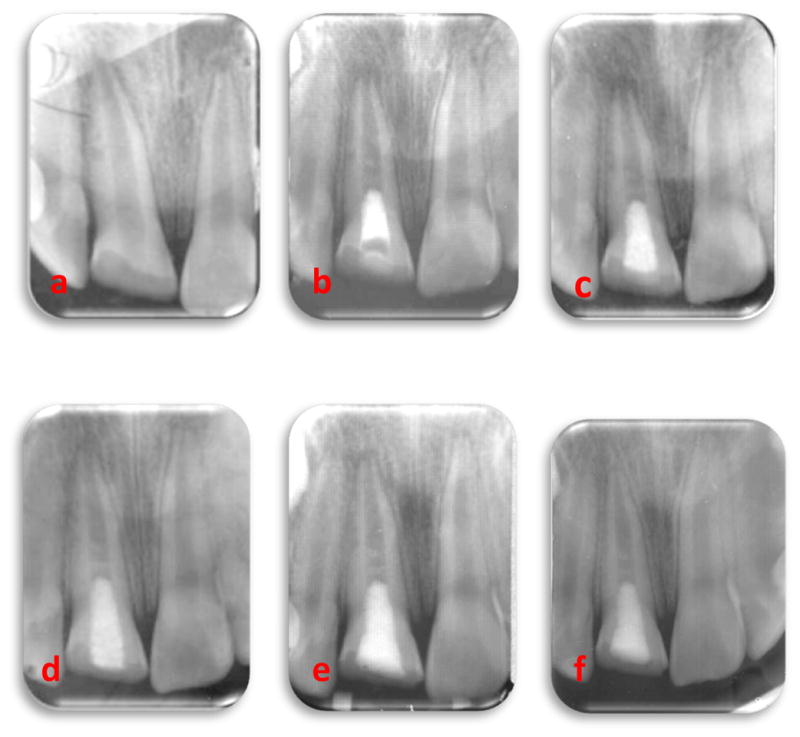
An immature, fratured #8 with open apex and large periradicular radiolucency in a 11 year old boy. Periapical radiographs included showing showing: (a) Periapical radiolucent lesion with open apex. (b) Following the placement of MTA (c) At 3 months follow-up, partial regression of periapical radiolucent lesion and partial closure of the root end (d) At 6 months follow-up, with marked reduction in periapical lesion with contiuned development of the root (e) At 9 months follow-up, nearly complete healing of periapical lesion with contiuned development of the root apex (f) At one year follow-up, complete maturation of the root apex with hard tissue bridge formation noted mid root.
Fig 4.
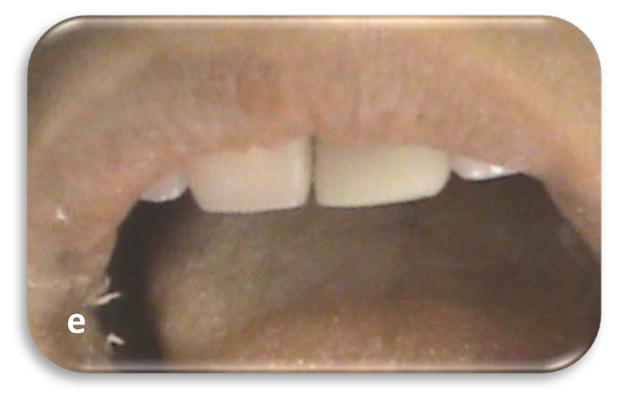
Fig.4A: A 12 year old boy presented with labial swelling and cervical pus discharge over #9. Intra-oral periapical radiograph showing: (a) Immature root with thin dentinal root walls associated with large periapical pathology. (b) Following the placement of MTA (c) At 3 months follow-up, regression of periapical radiolucent lesion and hard tissue formation at the apex; minimal thickening and lengthening of the root noted. (d) At 6 month's follow-up, with marked reduction in periapical lesion with some root thickening (e) At 9 month's follow-up, nearly complete healing of periapical lesion with continued development of the root apex. (f) At one year follow-up, mid-root calcific bridge formation noted.
Fig: 4 B: Clinical photographs showing:
(a) Pre-operative clinical photograph illustrates labial swelling and cervical pus discharge over #9. (b) Three-week post-operative photograph demonstrates complete healing of the swelling. (c) At 3 moth's follow-up, normal gingival contour was observed. Significant coronal staining, likely from the triple antibiotic paste was also noted. This was observed in many of the cases. (d&e) At one year follow-up with final treatment with composite resin veneer.
Discussion
This study provides unique insights into the temporal nature of clinical and radiographic outcomes of revasc/revit treatment. First, this was a prospective study with all treatments being completed by a single practitioner using a standardized protocol. Thus, case to case variability in treatment protocols was minimized. Also the radiographs were collected in a standardized manner at predetermined time points, using a bite registration and paralleling device, allowing for accurate quantification of radiographic changes. Collectively, the design of this study provided an optimal opportunity to assess temporal aspects of hard tissue deposition, resolutions of symptoms, and resolution of radiographic periapical pathology after revasc/revit treatment.
In the present study, 90% (18/20) of revascularized cases had complete resolution of periapical lesions at 12 months of follow-up and all cases were clinically successful, without signs or symptoms of active infection. Several clinical case series have been published using a revasc/revit procedures with follow up time ranging from 6 months- 3.5 years, which also report high levels of clinical success (78–100%) and resolution radiographic periapical pathology (80–100%) (10, 15–17). However, case series are not a useful study design for estimating rates of clinical success, as the inclusion of treatment failures is at the discretion of the authors. Rather, cohort studies are needed with well defined inclusion/exclusion criteria, and which take into account subjects lost during the follow up period. Two retrospective cohort studies including about 20 revasc/revit studies have also reported fairly high rates of clinical success (78 and 100%) and radiographic healing of periapical lesions subsequent to treatment (93 and100%) (4, 18). Taken together, regenerative endodontic therapy appears to have a high clinical success rate, in the initial 1–2 years after treatment completion. Studies with longer follow up periods are still needed to better estimate the long term success.
In the present study, complete apical closure occurred in 55% (11/20) of revasc/revit treated teeth, with all cases showing at least a 20% decrease in apical diameter by the one year follow up visit (Fig 2B). The average decrease in apical diameter within individual cases from the pre-operative radiograph to the one year follow up was 81%. This was the most robust and consistent radiographic change observed in the population treated in this study. Radiographic evidence of apical closure of revascularized teeth has been observed in other studies at various follow up times (10, 18–20), however, the frequency and time course of the occurrence of apical closure after revasc/revit treatment was not appreciated before this study.
The second most frequently observed radiographic finding in this study was thickening of the canal walls. Although all cases showed a measurable increase in canal thickness, in some cases this increase was quite small, such that it would not be detectable without quantitative assessment of the radiographic changes. In order to estimate the proportion of cases with a clinically meaningful change in root thickness, we applied a threshold of 20% change. Using this criteria, we found 9 cases (45%) met this criteria, with another 4 cases just missing the 20% cut-off at the 12 month follow up visit (Fig 2F). Although the remaining 5 cases did still have a measurable increase in root width, the clinical significance of this increase in these cases is questionable. It is possible that with further follow up time, the root walls will continue to thicken, strengthening the predictability of this radiographic outcome after revascularization. At 12 months, the average within-case percent change from the pre-operative radiograph was 20%. This is comparable to what was observed in other cohort studies including Jeeruphan et al., who reported an average 27% increase with an average of 21 months follow up, and Alobaid et al., who saw a 10% increase with an average of 15 months follow up (4, 18). Differences in within- case radiographic changes could be due to non-standardized radiographs being used in the retrospective cohort studies, which often can reduce the accuracy in measuring the actual radiographic change. Another potentially important factor the could influence root growth is the contributing etiology in the different study populations, including the frequency of severe trauma (avulsions, intrusions) as the contributing etiology for pulpal necrosis in the study population (the cases in the Alobaid study were primarily due to trauma including avulsions and luxation, while the Jeeruphan study was primarily a due to an etiology of dens evaginatus). As severe trauma has the potential to produce damage to Herwig’s epithelial root sheath and/or the apical papilla, traumatized teeth may be less likely to achieve clinically meaningful continued root development than treated immature teeth with pulpal necrosis due to caries or dens evaginatus.
Continued root lengthening was the least robust and consistent radiographic finding in this study. Although a statistically significant change in root length was observed in the study population over time, the clinical significance of the measured radiographic changes in root length are not clear. Again, using a 20% threshold for a clinically meaningful within-case change in radiographic root length, no cases met this criteria at 12 months, with only one case approaching the threshold (Fig 2D). Overall at 12- months, there was a 5% average increase in root length across all the cases. Jeeruphan et al., observed a 15% average change in root length, and Alobaid et al., observed a 5% change. Thus, all 3 cohort studies support the finding that a greater amount of root thickening occurs than root lengthening after revasc/revit treatment.
All revascularized/revitalized teeth in the present study did not respond to pulp sensibility tests (cold, heat, electric current) at the end of 12 months study. Based on histological studies of animal and human revascularized/revitalized teeth, thickening of the canal walls is mainly due to deposition of cementum-like tissue without the tubular structure observed in dentin (21–26). Even if there was a regeneration of sensory nerve fibers adjacent to the newly formed hard tissue in the canal, these sensory nerve fibers probably would not be activated by sensibility tests relying on hydrodynamic mechanisms, as there are no tubules present in the newly formed hard tissue. It is also important to point out that a positive response of revasc/revit tooth to an electric pulp test (15, 16, 19) does not necessarily imply that regeneration of pulp-like tissue has occurred. This is because most vital tissues are innervated, (including periodontal ligament and alveolar bone) as nerves play a critical role in immunologic defense mechanisms (via release of neuropeptides), healing, and protection.
In conclusion, the findings reported here support the idea that revascularization/revitalization treatment has a high short-term clinical success rate. Apical closure was the most predictable and robust radiographic finding, followed by thickening of the canal, and only modest changes in canal lengthening were observed during one year after treatment. The knowledge provided regarding the frequency and timing of radiographic hard tissue deposition, will help clinicians to know when they might expect to observe these outcomes in their patients.
Acknowledgments
This work was partially supported by funding from the National Institutes of Health (K23DE019461, JLG).
Footnotes
The authors deny any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17(4):185–187. doi: 10.1034/j.1600-9657.2001.017004185.x. [DOI] [PubMed] [Google Scholar]
- 2.Diogenes A, Henry MA, Teixeira FB, Hargreaves KM. An update on clinical regenerative endodontics. Endod Topics. 2013;28:2–23. [Google Scholar]
- 3.Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009;35(10):1343–1349. doi: 10.1016/j.joen.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Jeeruphan T, Jantarat J, Yanpiset K, Suwannapan L, Khewsawai P, Hargreaves KM. Mahidol study 1: comparison of radiographic and survival outcomes of immature teeth treated with either regenerative endodontic or apexification methods: a retrospective study. J Endod. 2012;38(10):1330–1336. doi: 10.1016/j.joen.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Jung IY, Lee SJ, Hargreaves KM. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod. 2008;34(7):876–887. doi: 10.1016/j.joen.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Chueh LH, Ho YC, Kuo TC, Lai WH, Chen YH, Chiang CP. Regenerative endodontic treatment for necrotic immature permanent teeth. J Endod. 2009;35(2):160–164. doi: 10.1016/j.joen.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB. Challenges in regenerative endodontics: a case series. J Endod. 2010;36(3):536–541. doi: 10.1016/j.joen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Jadhav G, Shah N, Logani A. Revascularization with and without platelet-rich plasma in nonvital, immature, anterior teeth: a pilot clinical study. J Endod. 2012;38(12):1581–1587. doi: 10.1016/j.joen.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Chen MY, Chen KL, Chen CA, Tayebaty F, Rosenberg PA, Lin LM. Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. Int Endod J. 2012;45(3):294–305. doi: 10.1111/j.1365-2591.2011.01978.x. [DOI] [PubMed] [Google Scholar]
- 10.Shah N, Logani A, Bhaskar U, Aggarwal V. Efficacy of revascularization to induce apexification/apexogensis in infected, nonvital, immature teeth: a pilot clinical study. J Endod. 2008;34(8):919–925. doi: 10.1016/j.joen.2008.05.001. Discussion 1157. [DOI] [PubMed] [Google Scholar]
- 11.Cehreli ZC, Isbitiren B, Sara S, Erbas G. Regenerative endodontic treatment (revascularization) of immature necrotic molars medicated with calcium hydroxide: a case series. J Endod. 2011;37(9):1327–1330. doi: 10.1016/j.joen.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 12.Andreasen JO, Borum MK, Andreasen FM. Replantation of 400 avulsed permanent incisors. 3. Factors related to root growth. Endod Dent Traumatol. 1995;11(2):69–75. doi: 10.1111/j.1600-9657.1995.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 13.Andreasen JO. Pulp and periodontal tissue repair - regeneration or tissue metaplasia after dental trauma. A review Dent Traumatol. 2012;28(1):19–24. doi: 10.1111/j.1600-9657.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- 14.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30(4):196–200. doi: 10.1097/00004770-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB. Challenges in regenerative endodontics: a case series. J Endod. 2010;36(3):536–541. doi: 10.1016/j.joen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Cehreli ZC, Isbitiren B, Sara S, Erbas G. Regenerative endodontic treatment (revascularization) of immature necrotic molars medicated with calcium hydroxide: a case series. J Endod. 2011;37(9):1327–1330. doi: 10.1016/j.joen.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 17.Chen MY, Chen KL, Chen CA, Tayebaty F, Rosenberg PA, Lin LM. Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. Int Endod J. 2012;45(3):294–305. doi: 10.1111/j.1365-2591.2011.01978.x. [DOI] [PubMed] [Google Scholar]
- 18.Alobaid AS, Cortes LM, Lo J, Nguyn TT, Abu-Melha AS, Albert J, et al. Radiographic and Clinical Outcomes of the Treatment of Immature Permanent Teeth by Revascularization or Apexification: A Pilot Retrospective Cohort Study. J Endod. 2014;40(8):1063–70. doi: 10.1016/j.joen.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding RY, Cheung GS, Chen J, Yin XZ, Wang QQ, Zhang CF. Pulp revascularization of immature teeth with apical periodontitis: a clinical study. J Endod. 2009;35(5):745–749. doi: 10.1016/j.joen.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 20.McTigue DJ, Subramanian K, Kumar A. Case series: management of immature permanent teeth with pulpal necrosis: a case series. Pediatr Dent. 2013;35(1):55–60. [PubMed] [Google Scholar]
- 21.Wang X, Thibodeau B, Trope M, Lin LM, Huang GT. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod. 2010;36(1):56–63. doi: 10.1016/j.joen.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 22.da Silva LA, Nelson-Filho P, da Silva RA, Flores DS, Heilborn C, Johnson JD, et al. Revascularization and periapical repair after endodontic treatment using apical negative pressure irrigation versus conventional irrigation plus triantibiotic intracanal dressing in dogs' teeth with apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(5):779–787. doi: 10.1016/j.tripleo.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi N, Nagaoka H, Yamauchi S, Teixeira FB, Miguez P, Yamauchi M. Immunohistological characterization of newly formed tissues after regenerative procedure in immature dog teeth. J Endod. 2011;37(12):1636–1641. doi: 10.1016/j.joen.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Martin G, Ricucci D, Gibbs JL, Lin LM. Histological findings of revascularized/revitalized immature permanent molar with apical periodontitis using platelet-rich plasma. J Endod. 2013;39(1):138–144. doi: 10.1016/j.joen.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu E, Ricucci D, Albert J, Alobaid AS, Gibbs JL, Huang GT-J, et al. Clinical, Radiographic, and Histological Observation of a Human Immature Permanent Tooth with Chronic Apical Abscess after Revitalization Treatment. Journal of Endodontics. 2013;39(8):1078–83. doi: 10.1016/j.joen.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 26.Becerra P, Ricucci D, Loghin S, Gibbs JL, Lin LM. Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. J Endod. 2013;40(1):133–139. doi: 10.1016/j.joen.2013.07.017. [DOI] [PubMed] [Google Scholar]