Abstract
It is hypothesized that a high dietary n-6:n-3 (e.g. 10-20:1) is partly responsible for the rise in obesity and related health ailments. However, no tightly controlled studies utilizing high-fat diets (HFDs) differing in the n-6:n-3 have tested this hypothesis. The aim of the study was to determine the role the dietary n-6:n-3 plays in Non-Alcoholic Fatty-Liver Disease (NAFLD) and colitis development. We hypothesized that reducing the dietary n-6:n-3 would hinder the development of NAFLD and colitis. Male C57BL/6J mice were fed HFDs, differing in the n-6:n-3 (1:1, 5:1, 10:1, 20:1), for 20 weeks. GC/MS was used to analyze the hepatic phospholipid arachidonic acid:eicosapentaenoic acid (AA:EPA) and AA:docosahexaenoic acid (AA:DHA). Hepatic metabolism, inflammatory signaling, macrophage polarization, gene expression of inflammatory mediators, oxidative and ER stress, and oxidative capacity were assessed as well as colonic inflammatory signaling, and gene expression of inflammatory mediators, and tight-junction proteins. Although reducing the dietary n-6:n-3 lowered the hepatic phospholipid AA:EPA and AA:DHA in a dose-dependent manner and mildly influenced inflammatory signaling, it did not significantly attenuate NAFLD development. Further, the onset of NAFLD was not paired to colitis development nor changes in tight-junction protein gene expression. In conclusion, reducing the dietary n-6:n-3 did not attenuate NAFLD progression nor is it likely that colitis, or gut permeability, play a role in NAFLD initiation in this model.
Keywords: Omega-6:omega-3, Non-Alcoholic Fatty-Liver Disease, high-fat diet, α linolenic acid, colitis, murine model
1. INTRODUCTION
Non-Alcoholic Fatty-Liver Disease (NAFLD) is quickly becoming a major health concern as it is the third most common reason for liver transplantation and is believed to be prevalent in 30% of the United States’ general population [1]. Even more alarming is the fact that the disease presents itself in 70–80% of diabetic and obese patients and is the principal cause of hepatological clinical referrals in the United States [2, 3]. NAFLD encompasses a condition, which manifests itself in the form of hepatic steatosis, develops into non-alcoholic steatohepatitis (NASH), and can ultimately result in fibrosis, cirrhosis, and end-stage liver disease [4]. Although cellular perturbations, including dysfunctional lipid metabolism, hepatic oxidative and endoplasmic reticulum (ER) stress, and inflammatory signaling play a role in initiating and fueling NAFLD progression, the molecular mechanisms responsible for NAFLD development are not completely understood. It has been hypothesized that NAFLD development may largely be explained by changes in gut function as increasing evidence points to the existence of a “gut-liver axis” in which obesity can lead to colitis, gut-barrier dysfunction, subsequent bacterial leakage and the promotion of NAFLD [5, 6]. Nonetheless, regardless of the underlying cause of NAFLD, it is clear that diet composition does play a role in its promotion and advancement as increased consumption of simple sugars, certain lipid species, as well as a low dietary polyunsaturated fatty-acid:saturated fatty-acid (PUFA:SFA) has been shown to influence NAFLD development [7, 8].
One such aspect of diet suggested to influence NAFLD development, and is typical of the present-day Western diet, is the increased consumption of n-6 fatty acids (FAs) relative to n-3 FAs. This may result in a higher ratio of pro-inflammatory-promoting, n-6 FAs (arachidonic acid (AA; C20:4)) to anti-inflammatory, n-3 FAs (eicosapentaenoic acid (EPA; C20:5) and docosahexaenoic acid (DHA; C22:6)) in hepatic phospholipids [9]. In fact, it has been found that individuals with NAFLD exhibit a higher hepatic phospholipid n-6:n-3 than non-NAFLD individuals [10]. Furthermore, reducing the dietary n-6:n-3 by increasing the consumption of n-3 FAs, the long-chain polyunsaturated FAs, EPA and DHA, in particular, has been shown to be a promising therapeutic treatment for the attenuation of NAFLD development through their ability to minimize endoplasmic reticulum (ER) and oxidative stress, down-regulate genes involved in lipogenesis (sterol regulatory element-binding protein-1 (Srebp-1) and carbohydrate-responsive element-binding protein (Chrebp)) while up-regulating those linked to lipid oxidation (e.g. peroxisome-proliferated receptor alpha (PPARα)) [9, 11–13]. Nevertheless, there have been no tightly controlled studies that have examined the influence of the manipulating the dietary n-6:n-3 utilizing a variety of HFDs on NAFLD development. We have previously shown, using the same exact mice from this current investigation, that reducing the dietary n-6:n-3 utilizing the essential n-3 FA, α-linolenic acid (ALA; C18:3), is not a sufficient therapy for attenuating high-fat-diet-induced obesity nor related detrimental metabolic and adipose tissue inflammatory outcomes [14]. Despite these unpromising results, we were interested to see how reducing the n-6:n-3 may have influenced NAFLD.
The purpose of this study was to determine if lowering the dietary n-6:n-3 (20:1, 10:1, 5:1, and 1:1) using the most commonly consumed n-6 and n-3 FAs in the Western diet [15] (essential n-6 and n-3 FAs (linoleic (LA; C18:2) and ALA, respectively)), could attenuate the development of NAFLD and colitis within a HFD-induced obese mouse model. We hypothesized that reducing the dietary n-6:n-3 would delay the development of NAFLD and colitis. In order to test this hypothesis, the liver of each mouse was examined histologically for lipid accumulation as well as for several of the underlying mechanisms believed to be responsible for NAFLD progression, including modulations to hepatic metabolism, inflammatory signaling, macrophage polarization, gene expression of inflammatory mediators, oxidative and ER stress, and oxidative capacity. Given the hypothesized link between gut function and NAFLD, mouse colons were examined histologically in addition to inflammatory signaling analysis, and gene expression of inflammatory mediators and tight-junction proteins.
2. METHODS & MATERIALS
Animals
Male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were cared for in the animal facility at the University of South Carolina. They were housed, 5/cage, maintained on a 12:12-h light-dark cycle in a low stress environment (22°C, 50% humidity, low noise) and given food and water ad libitum. All methods were in accordance with the American Association for Laboratory Animal Science, and the Institutional Animal Care and Usage Committee of the University of South Carolina approved all experiments
Diets
At four weeks of age, mice were randomly assigned to 1 of 5 treatment diets (n=10/group): a control diet (AIN-76A Mod) and four HFDs (1:1, 5:1, 10:1, and 20:1) (BioServ, Frenchtown, NJ) (Table 1). Each of the HFDs was comprised of 47%, 40% and 13% of total calories from carbohydrate, fat, and protein, respectively, with saturated fat making up 12% of total calories. The control diet contained 68.7%, 12.2%, and 19.1% of total calories from carbohydrate, fat, and protein, respectively, with saturated fat making up 1.7% of total calories. Mice were fed their respective diets for a total of 20 weeks. The percentage of calories provided by each of the three macronutrients and the ratio of monounsaturated FAs (MUFAs) to PUFAs (MUFA:PUFA) were identical for the HFDs and were designed to be similar to the standard American diet [16, 17]. According to National Health and Nutrition Examination Survey (NHANES) data, the standard American diet is comprised of approximately 34%, 15%, and 51% of total kcals from fat, protein, and carbohydrates, 11–12% of total kcals from saturated fat, and a MUFA:PUFA of 1.5:1 [18]. While the total fat content of the diets utilized in this study is higher than reported by NHANEs (40% vs. 34%), given the documented underreporting in this data set with respect to fat intake [19–23], we believe that our diets are within the realm of a standard American diet and vastly different from the typically used “original” HFDs for diet-induced obesity (20%, 60%, and 20% of total calories from carbohydrate, fat, and protein, respectively). Additionally, up to seven different sources of fat were utilized so that the total consumption of n-3 PUFAs in the diet fell within a clinically-appropriate dose (4.7%, 1.6%, .86%, and .46% of total calories from n-3 PUFAs for the 1:1, 5:1, 10:1, and 20:1 diets, respectively; it has been reported that the typical American’s diet is composed of up to .2–.7% of total calories from n-3 PUFAs [15]), and the only FA ratio which changed in the diet was the n-6:n-3 [17]. In order to manipulate the n-6:n-3, both the absolute values of the n-6 and n-3 FAs were changed in order to produce each diet’s respective n-6:n-3. This was done so that the percentage of total calories from PUFAs and the MUFA:PUFA did not change among the HFDs. None of the diets contained any EPA or DHA, as this study was designed to examine the potential benefits of reducing the dietary n-6:n-3 while only utilizing the most abundantly consumed n-6 and n-3 FAs in the Western diet independent of the minimally consumed EPA and DHA FAs (<0.1-0.2 grams/day consumption in the United States) [15]. The control diet (AIN-76A Mod) was used in order to match the MUFA:PUFA and n-6:n-3 of the 20:1 HFD.
Table 1.
Diet composition of treatment diets
| AIN-76A | 1:1 | 5:1 | 10:1 | 20:1 | |
|---|---|---|---|---|---|
| Modified | HFD | HFD | HFD | HFD | |
| Ingredient (g/kg) | |||||
| Casein | 200 | 165 | 165 | 165 | 165 |
| DL Methionine | 3 | 3 | 3 | 3 | 3 |
| Lard | 0 | 100 | 100 | 100 | 100 |
| Coconut Oil | 0 | 10.2 | 7.3 | 6.8 | 7.4 |
| Corn Oil | 15.6 | .9 | .5 | 22.5 | 50.1 |
| Soybean Oil | 3.6 | 1 | 41.1 | 28.1 | 3.8 |
| Olive Oil | 30.9 | 45 | 40 | 43.7 | 41.7 |
| Flaxseed Oil | - | 38.5 | 4.9 | 1 | - |
| Canola Oil | - | 7.4 | 9.2 | 1 | - |
| Corn Starch | 80 | 50 | 50 | 50 | 50 |
| Maltodextrin | 100 | 100 | 100 | 100 | 100 |
| Sucrose | 469.5 | 381.9 | 381.9 | 381.9 | 381.9 |
| Cellulose | 50 | 50 | 50 | 50 | 50 |
| Vitamin Mix (AIN-76A) | 10 | 10 | 10 | 10 | 10 |
| Mineral Mix (AIN-76A) | 35 | 35 | 35 | 35 | 35 |
| Choline Bitartrate | 2 | 2 | 2 | 2 | 2 |
| Energy (kcal/g) | 3.77 | 4.572 | 4.572 | 4.572 | 4.572 |
| Energy (% kcal) | |||||
| Carbohydrate | 68.7 | 47 | 47 | 47 | 47 |
| Fat | 12.2 | 40 | 40 | 40 | 40 |
| Protein | 19.1 | 13 | 13 | 13 | 13 |
| Fatty Acid Profile (g/kg) | |||||
| Caprylic Acid (C8:0) | 0 | 0.8 | 0.5 | 0.5 | .6 |
| Capric Acid (C10:0) | 0 | 0.7 | 0.5 | 0.5 | .5 |
| Lauric Acid (C12:0) | 0 | 4.8 | 3.5 | 3.2 | 3.5 |
| Myristic Acid (C14:0) | .004 | 3.1 | 2.5 | 2.4 | 2.6 |
| Palmitic Acid (C16:0) | 5.5 | 32.2 | 33.9 | 34.7 | 34.8 |
| Palmitoleic Acid (C16:1) | .4 | 3.3 | 3.2 | 3.3 | 3.3 |
| Stearic Acid (C18:0) | 1.05 | 16.2 | 16.7 | 16.3 | 15.6 |
| Oleic Acid (C18:1) | 27.1 | 85.9 | 86.1 | 86 | 85.9 |
| Linoleic Acid (C18:2) | 13.2 | 22.6 | 37.9 | 41.3 | 43.2 |
| α-Linolenic Acid (C18:3) | .66 | 22.6 | 7.6 | 4.13 | 2.2 |
| % of Total Calories from SFAs | 1.7% | 12% | 12% | 12% | 12% |
| % of Total Calories from MCFAs (C6:0-C12:0) |
- | 1.3% | .9% | .9% | 1% |
| % of Total Calories from LCSFAs (C14:0-C20:0) |
1.7% | 10.7% | 11.1% | 11.1% | 11% |
| % of Total Calories from USFAs | 10.5% | 28% | 28% | 28% | 28% |
| % of Total Calories from MUFAs | 7% | 18.6% | 18.6% | 18.6% | 18.6% |
| % of Total Calories from PUFAs | 3.5% | 9.4% | 9.4% | 9.4% | 9.4% |
| % of Total Calories from n-3 FAs | .16% | 4.7% | 1.6% | .86% | .46% |
| % of Total Calories from n-6 FAs | 3.2 % | 4.7% | 7.8% | 8.6% | 9% |
| Cholesterol (mg/kg) | 0 | 95 | 95 | 95 | 95 |
| Ratio: MUFA:PUFA | 2:1 | 2:1 | 2:1 | 2:1 | 2:1 |
| Ratio: n-6:n-3 FA | 20:1 | 1:1 | 5:1 | 10:1 | 20:1 |
SFAs, Saturated Fatty-Acids; MCSFAs, Medium-Chain Saturated Fatty Acids; LCSFAs, Long-Chain Saturated Fatty Acids; USFAs, Unsaturated Fatty Acids; MUFAs, Monounsaturated Fatty Acids; PUFAs, Polyunsaturated Fatty Acids.
Tissue collection
At 24 weeks of age, mice were euthanized for tissue collection via isoflurane inhalation. The liver of each mouse was removed, weighed, and immediately snap-frozen in liquid nitrogen and stored at −80°C until analysis. For colon processing, colons were excised from each mouse and flushed with phosphate-buffered saline. Subsequently, a small section of the distal portion of the colon was cut and fixed in 10% formalin. The remaining portion of the colon was opened longitudinally, and was successively dissected in half, lengthwise (one half each for western blot and RT-PCR analyses).
Oil red O staining and hepatic lipid accumulation
Hematoxylin and eosin (H&E) and oil red O staining, as well as quantification of hepatic lipid content were performed as previously described [24].
Western and slot blots
Liver and colon samples were homogenized in mueller buffer containing a cocktail protease inhibitor (Sigma Aldrich, St. Louis, MO) [25]. Total protein concentrations were determined by the Bradford method. For the slot blot technique, proteins were transferred to a polyvinylidene difluoride (PVDF) membrane utilizing a slot bot microfiltration apparatus (Bio-Rad, Hercules, CA). For the traditional western blot technique, proteins were fractioned onto Criterion precast gels (Bio-Rad, Hercules, CA) and were subsequently transferred to a PVDF membrane overnight. Membranes was stained with a Ponceau S solution in order to verify equal protein loading and transfer efficiency. Western blots were performed as previously described [25] using primary antibodies for phosphorylated (Thr183/Tyr185) and total c-Jun N-terminal kinase (JNK), phosphorylated (Thr202/Tyr204) and total extracellular signal-regulated kinase (ERK) ½, phosphorylated (Thr180/Tyr182) and total p38 mitogen-activated protein kinase (p38), phosphorylated (Ser536) and total nuclear factor kappa B p65 (NFκB p65), phosphorylated (Tyr705) and total signal transducer and activator of transcription 3 (STAT3), binding immunoglobulin protein (BiP), phosphorylated (Ser51) and total eukaryotic initiation factor 2 alpha (EIF2α), total inositol-requiring enzyme 1 alpha (IRE1α), nitrotyrosine, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and CCAAT-enhancer-binding protein homologous protein (CHOP) (Cell Signaling, Danvers, MA), phosphorylated (Ser724) IRE1α and activating transcription factor 6 p50 (ATF6 p50) (Novus Biologicals, Littleton, CO), the spliced form of x-box binding protein 1 (XBP1s) (Santa Cruz Biotechnology, Santa Cruz, CA), 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) (Alpha Diagnostics, San Antonio, TX), and five monoclonal antibodies against each of the five mitochondrial oxidative phosphorylation complexes (MitoProfile Total OXPHOS Antibody Cocktail (Abcam, Cambridge, England)).
Gene expression
Quantification of murine hepatic gene expression for metabolic markers (fatty-acid synthase (Fasn), acetyl-CoA carboxylase 1 (Acac1), Srebp-1, Chrebp, peroxisome proliferator-activated receptor gamma (Ppar-γ), Ppar-α, as well as macrophage (F4/80, Cd11b, Cd206) and inflammatory mediators (monocyte chemoattractant protein-1 (Mcp-1), tumor necrosis factor-alpha, (Tnf-α), interleukin-6 (Il-6), toll-like receptor-2 (Tlr-2), toll-like receptor-4 (Tlr-4), IL-10, transforming growth factor-beta (Tgf-β) and g protein-coupled receptor-120 (Gpr-120)) as well as murine colon gene expression of inflammatory mediators (Mcp-1, Tnf- α, Tlr-2, Tlr-4) and tight-junction proteins, tight-junction protein 1 (Tjp1) and occludin (Applied Biosystems, Foster City, CA) was performed as previously described [26]. Briefly, RNA was extracted using TRIzol reagent (Life Technologies, GIBCO-BRL, Carlsbad, CA) and chloroform procedures. Quantitative RT-PCR analysis was carried out as per the manufacturer’s instructions (Applied Biosystems, Foster City, CA) using TaqMan Gene Expression Assays [26].
AA:EPA and AA:DHA in hepatic phospholipids
Analysis AA:EPA and AA:DHA in hepatic phospholipids was performed as previously described utilizing GC-MS [14]. Liver samples were homogenized in a 2:1 (v/v) chloroform:methanol solution containing 100 mg/liter of butylated hydroxyl toluene in order to minimize autoxidation of PUFAs. Lipids were subsequently isolated using the folch extraction method [27]. The solution containing the lipids was dried under nitrogen gas and re-solubilized in chloroform. The lipid solution was then added to silica Sep-Pak cartridges (Waters Associated, Milford, MA). Neutral lipids, glycolipids, and phospholipids were eluted with chloroform, acetone, and methanol, respectively [28]. The phospholipid fraction was dried under nitrogen gas prior to the addition of methyl acetate in order to form fatty-acid methyl esters. After a 50°C incubation overnight, the phospholipid solution was dried under nitrogen gas, re-solubilized in chloroform, and was injected into the GC-MS in order to determine PUFA hepatic phospholipid composition.
GC-MS analysis was performed on a HP-5890 gas chromatograph interfaced to a VG-70S magnetic sector mass spectrometer. The column used was a 30 meter by 0.25mm ID Restek FameWax (Bellefonte, PA). The oven temperature was programmed from 70°C to 200°C at 10°C/min and then to 250°C at 4°C/min where it was held for 8 min. The mass spectrometer was scanned from 60 to 390 Da. Electron ionization was used at 70 eV and the GC peaks were manually integrated from the total ion chromatogram. The retention times were compared to a marine oil FAME MIX standard (Restek).
Statistical analyses
All data were analyzed using commercial software (SigmaStat, SPSS, Chicago, IL). All murine data were analyzed using a one-way ANOVA, with a Student-Newman-Keuls test for all post-hoc analyses. Data are presented as means (±SEM). Statistical significance was set with an alpha value of P≤0.05.
3. RESULTS
Animal phenotype data
Animal body weights, fat pad weights, body composition, and adipose tissue inflammatory and metabolic outcomes for the mice utilized in this study are presented in our previous publication [14]. In general, all HFDs, led to similar levels of adiposity, insulin resistance, and adipose tissue inflammation [14]. There was no difference in food intake (grams) among the HFD groups.
The dietary n-6:n-3 influences aspects of hepatic metabolism and has no effect on hepatic steatosis despite diet-specific changes to the hepatic phospholipid AA:EPA and AA:DHA
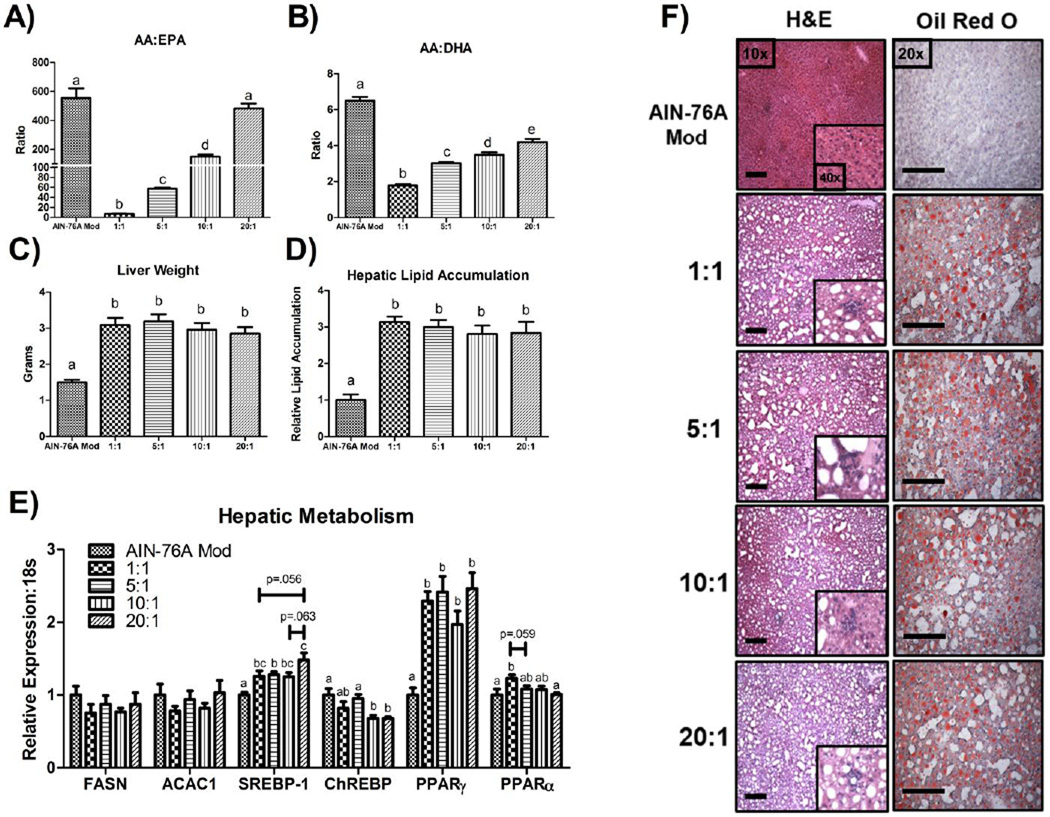
The AA:EPA and AA:DHA in hepatic phospholipids was influenced by the dietary n-6:n-3 in a ratio-dependent manner. All HFDs resulted in a significantly different AA:EPA and AA:DHA (P≤.05), with the 1:1 group resulting in the lowest AA:EPA (≈ 7:1 ± 0.2) and AA:DHA (≈ 1:8 ± 0.9) followed by the 5:1 (AA:EPA, ≈ 57:1 ± 2.3; AA:DHA, ≈ 3.0 ± 0.1) 10:1 (AA:EPA, ≈ 150:1 ± 13.5; AA:DHA, ≈ 3.5 ± 0.1), and 20:1 (AA:EPA, ≈ 482:1 ± 35.0; AA:DHA, ≈ 4:1 ± 0.2) groups. The control group displayed a significantly higher hepatic phospholipid AA:EPA (≈ 554:1 ± 67.0) and AA:DHA (≈ 6.5 ± 0.2) compared to all other groups, except for a similar AA:EPA as the 20:1 HFD (Figure 1A and 1B) (P≤.05) (representative chromatograms are presented in Supplemental Figure 1). Despite these differences in phospholipid composition, all HFDs led to similar degree of hepatomegaly (Figure 1C) and hepatic steatosis (Figure 1D, 1F).
Figure 1.
Impact of dietary n-6:n-3 on murine NAFLD development. (A) AA:EPA, and (B) AA:DHA in hepatic phospholipids, (C) liver weight, (D) hepatic lipid accumulation, (E) hepatic mRNA expression of metabolism-related genes, as well as (F) representative hepatic H&E (10x & 40x) and oil red O images (20x) displaying steatosis and inflammatory infiltrate (n=10/group). Scale bars represent 200 µm. Treatment groups included a control diet (AIN-76A Mod) and four HFDs differing in the n-6:n-3 (1:1, 5:1, 10:1, and 20:1) consumed for a 20-week period. Diets not sharing a common letter differ significantly from one another (P≤.05). Data are presented as means (±SEM).
Regarding hepatic metabolism, no difference was found in the mRNA expression of the lipogenesis markers, Fasn and Acac1, across the diets (Figure 1E). On the other hand, differences in gene expression of two key transcription factors known to regulate lipogenesis, Srebp-1 and Chrebp, was found to be altered depending on the diet consumed. Srebp-1 expression was found to be higher (ranging from ≈ +30–40%) in all HFDs compared to the control diet (P≤.05), and when comparing among the HFD groups, Srebp-1 mRNA content was significantly, or trending to be, greater in the 20:1 HFD compared to the 1:1 (≈ +18%) (P<.1), 5:1 (≈ +15%) (P≤.05), and 10:1 (≈ +16%) (P<.1) HFDs (Figure 1E). Alternatively, with respect to Chrebp, both consumption of the 10:1 and 20:1 HFDs resulted in significantly lower mRNA expression relative to the control (≈ −32%) and 5:1 (≈ −28%) diets (Figure 1E) (P≤.05). Of the two PPARs analyzed, Pparγ expression was found to be higher in all HFD groups (ranging from ≈ +100–150%) compared to the control diet (Figure 1E) (P≤.05), whereas, Pparα expression was significantly greater in the 1:1 HFD compared to the control (≈ +23%) and 20:1 (≈ +22%) diets (P≤.05), and trending to be elevated compared to the 5:1 (≈ +14%) HFD (Figure 1E) (P<.1).
Some, but not all, markers of hepatic inflammation are influenced by the dietary n-6:n-3
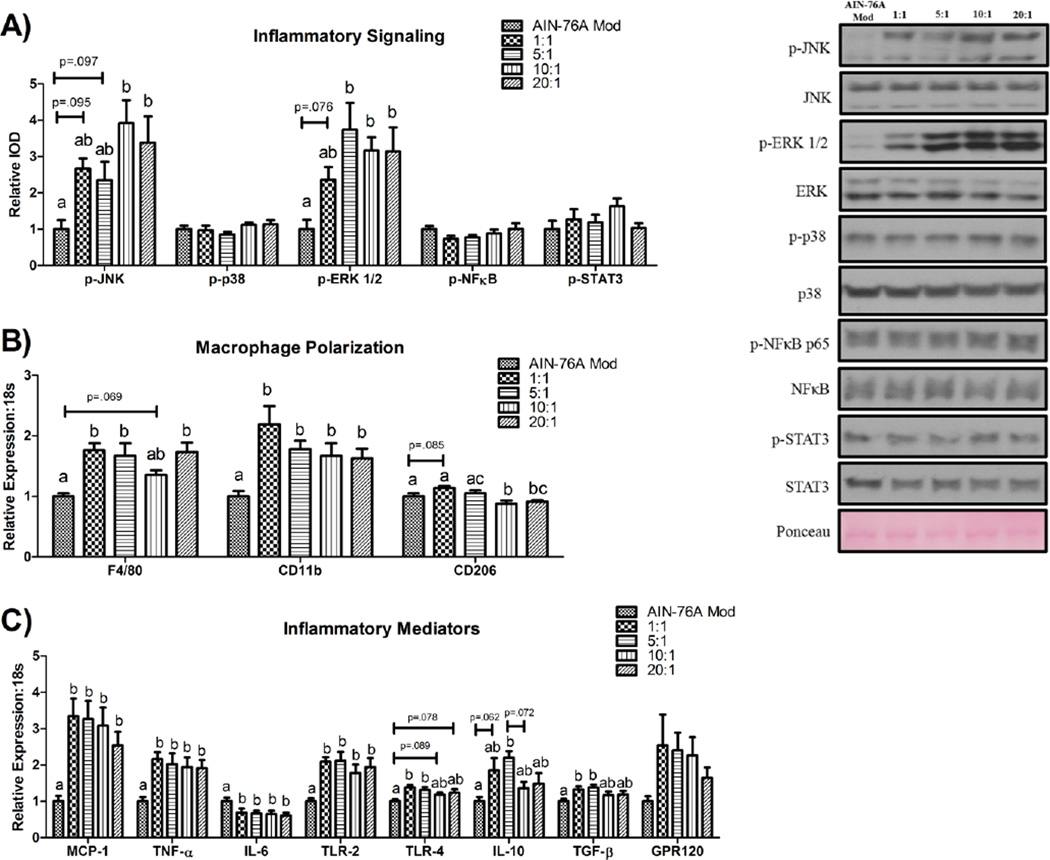
Phosphorylation of proteins associated with inflammatory signaling is presented in Figure 2A. HFD-consumption resulted in greater JNK (Thr183/Tyr185) (ranging from ≈ +140–240%) and ERK ½ phosphorylation (Thr202/Tyr204) (ranging from ≈ +140–210%) compared to the control diet. Although there was at least a trend (P<.1) for all HFDs to result in higher phosphorylation of these inflammatory mediators versus the control diet, this was only statistically significant (P≤.05) for the 10:1 (≈ +290%) and 20:1 (≈ +240%) HFDs in the case of p-JNK (versus the control diet), and the 5:1 (≈ +270%), 10:1 (≈ +220%), and 20:1 (≈ +210%) HFDs in the case of p-ERK ½ (versus the control diet). No differences were detected in the hepatic protein content of phosphorylated p38, NFκB (Ser536), or STAT3 (Tyr705) among any of the dietary treatments.
Figure 2.
Representative hepatic western blots for markers of (A) inflammatory signaling, as well as gene expression of (B) macrophage and (C) inflammatory markers (n=10/group). Treatment groups included a control diet (AIN-76A Mod) and four HFDs differing in the n-6:n-3 (1:1, 5:1, 10:1, and 20:1) consumed for a 20-week period. All western blots were run under the same experimental conditions. Diets not sharing a common letter differ significantly from one another (P≤.05). Data are presented as means (±SEM).
We assessed macrophage polarization by examining the gene expression of several macrophage markers (F4/80: a general macrophage marker, Cd11b: a pro-inflammatory, M1, macrophage marker, [29], and Cd206: an anti-inflammatory, M2, macrophage marker) [30] (Figure 2B). Consumption of all HFDs either trended to (P<.1), or significantly resulted in higher mRNA expression of F4/80 (≈ +40–70%) and Cd11b (≈ +60–120%) (P≤.05). Alternatively, gene expression of Cd206 was significantly lower in the 10:1 and 20:1 HFDs relative to the control (≈ −12% and −9% for the 10:1 and 20:1 HFDs, respectively) and 1:1 (≈ −23% and −21% for the 10:1 and 20:1 HFDs, respectively) diets and relative to the 5:1 HFD (≈ −17%) in the case of the 10:1 HFD (P≤.05).
Markers of hepatic inflammation are presented in Figure 2C. Consumption of all HFDs led to increased gene expression of the pro-inflammatory markers Mcp-1 (ranging from ≈ +150–230%), Tnf-α (ranging from ≈ +90–120%), and Tlr2 (ranging from ≈ +80–110%) relative to the control diet (P≤.05). A similar pattern was observed with respect to Tlr4 gene expression; it was either trending to be (10:1 and 20:1 diets; ≈ +20% for both diets) (P<.1), or was significantly higher (1:1 and 5:1 diets; ≈ +40% and +30%, respectively) in the HFD-fed mice compared to the control-diet-fed mice (P≤.05). Conversely, Il-6 gene expression was found to be lower (ranging from ≈ −30–40%) in all HFDs compared to the control diet (P≤.05). Regarding the mRNA expression of the anti-inflammatory markers, IL-10 and Tgf-β, both the consumption of the 1:1 (≈ +30% for Tgf-β) and 5:1 (≈ +120% and +40% for IL-10 and Tgf-β, respectively) HFDs either trended to, (P<.1 for IL-10 expression of the 1:1 HFD vs. control diet; ≈ +90%) or did significantly result in a greater expression of these genes relative to the control diet (P≤.05). There was also a trend for the 5:1 HFD to exhibit a higher Il-10 expression compared to the 10:1 HFD (≈ +61%) (P<.1). No significant difference in Gpr120 mRNA expression was found across the diets.
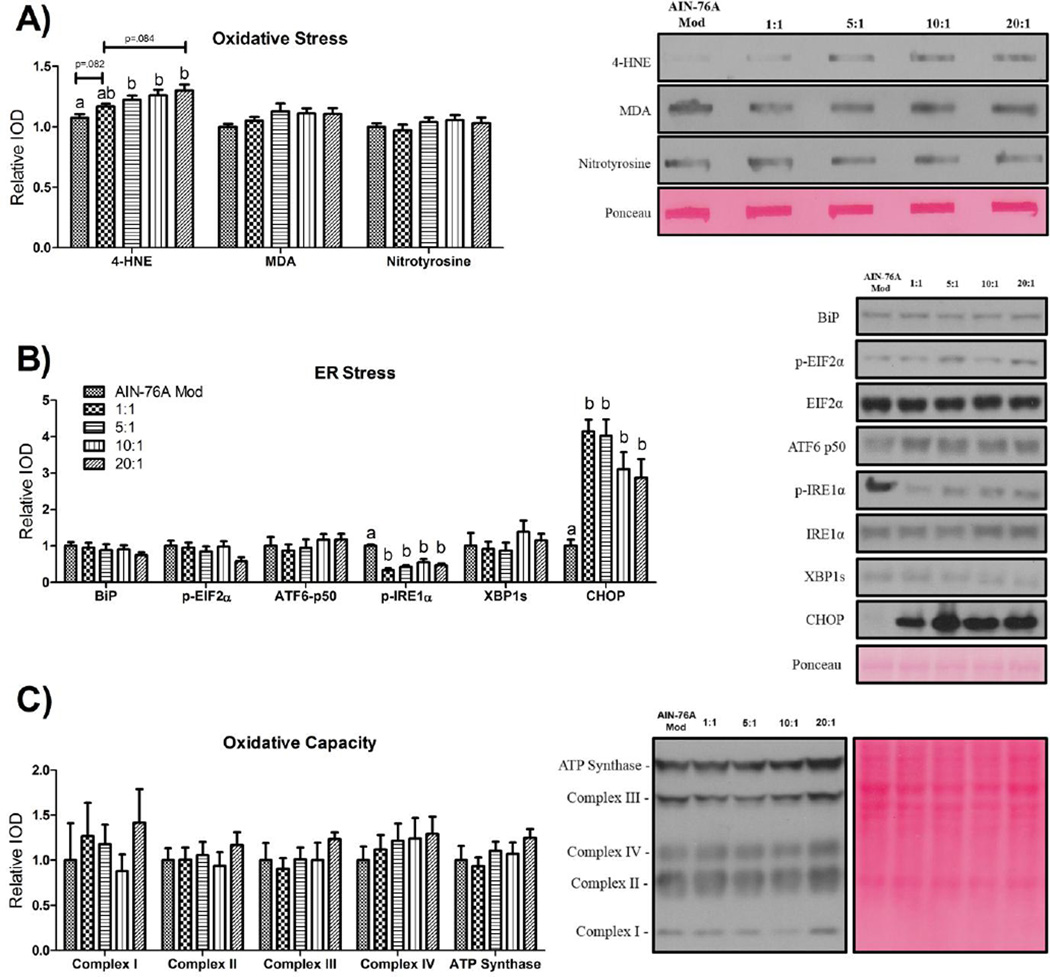
Oxidative stress is not significantly impacted by the dietary n-6:n-3, whereas high-fat feeding, independent of the dietary n-6:n-3, influences markers of ER Stress but not oxidative capacity
Analyses of hepatic oxidative and ER stress, as well as oxidative capacity, are presented in Figure 3. Oxidative stress was examined by looking for evidence of lipid peroxidation (4-HNE and MDA) and protein nitrosylation. There was no difference in the levels of nitrosylated proteins or MDA among any of the diets (Figure 3A). Content of 4-HNE, on the other hand, was elevated in a non-statistically significant step-wise manner paralleling the increase in the dietary n-6:n-3; a trend (P<.01) did exist, however, for the 20:1 HFD-fed mice to exhibit a greater content (≈ +11%) of 4-HNE than the 1:1 HFD-fed mice. All HFDs, save for the 1:1 HFD, which trended (P<.1) towards an increase (≈ +20%), exhibited higher (ranging from ≈ +20–30%) 4-HNE content compared to control-fed mice (P≤.05).
Figure 3.
Representative hepatic western blots for markers of (A) oxidative stress, (B) ER stress, and (C) oxidative capacity (n=10/group). All western blots were run under the same experimental conditions. Treatment groups included a control diet (AIN-76A Mod) and four HFDs differing in the n-6:n-3 (1:1, 5:1, 10:1, and 20:1) consumed for a 20-week period. Diets not sharing a common letter differ significantly from one another (P≤.05). Data are presented as means (±SEM).
Of the ER stress markers examined, only phosphorylated levels of IRE1α and CHOP were found to be significantly different among groups; consumption of all HFDs led to decreased (ranging from ≈ −44–67%) and increased (ranging from ≈ +190–320%) content, respectively, of these proteins relative to the control diet (Figure 3B) (P≤.05).
Oxidative capacity was unchanged by HFD consumption (Figure 3C).
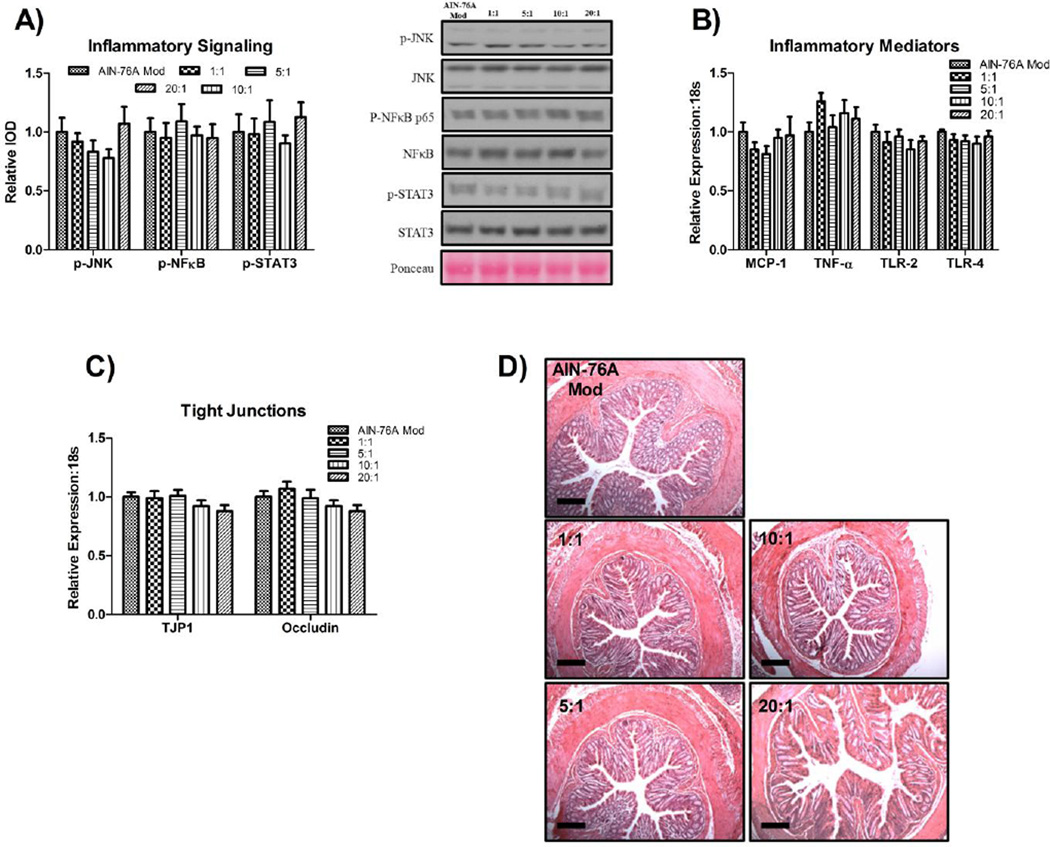
High-fat feeding does not lead to colon perturbations prior to NAFLD development
HFD-consumption did not influence inflammatory signaling (Figure 4A), colitis development (Figure 4B, 4D), or mRNA expression of tight junction proteins (Tjp1 and occludin) (Figure 4C).
Figure 4.
Representative colonic western blots for markers of (A) inflammatory signaling, gene expression of (B) inflammatory and (C) tight-junction markers, as well as (D) representative colonic H&E images (20x) (n=10/group). All western blots were run under the same experimental conditions. Scale bars represent 200 µm. Treatment groups included a control diet (AIN-76A Mod) and four HFDs differing in the n-6:n-3 (1:1, 5:1, 10:1, and 20:1) consumed for a 20-week period. Diets not sharing a common letter differ significantly from one another (P≤.05). Data are presented as means (±SEM).
4. DISCUSSION
Currently, there are limited treatment options for NAFLD [31]. Those that seem most promising fall under the umbrella of lifestyle changes, which include modifications to diet. An important aspect of diet, which has continued to gain publicity, is the impact that the dietary n-6:n-3 has on disease development, including obesity and NAFLD [9, 32]. It is hypothesized that a high dietary n-6:n-3 (e.g. 10–20:1), as is seen in the modern-day Western diet, is partly responsible for the rise in obesity and related health ailments [16]. We recently showed that reducing the n-6:n-3 with ALA does not sufficiently attenuate obesity, adipose tissue inflammation, or type II diabetes development [14]. Nonetheless, utilizing the livers of the same mice from our previous study [14], we were interested to see if reducing the dietary n-6:n-3 could attenuate NAFLD development.
One of the primary molecular mechanisms by which n-3 and 6 FAs are thought to mediate cellular processes is through incorporation of their long-chain forms (EPA and DHA in the case of the n-3 FAs and AA in the case of the n-6 FAs) into the phospholipid bilayer of the cellular membrane, ultimately leading to the synthesis of inflammatory eicosanoids [33]. Because n-3 and n-6 FAs have opposing inflammatory properties (they serve as precursors for anti- and pro-inflammatory mediators, respectively), the dietary n-6:n-3 is of importance as an excess consumption of n-6 FAs relative to n-3 FAs would theoretically lead to greater incorporation of AA and a decreased inclusion of EPA and DHA into the cellular membrane promoting more of a pro-inflammatory environment compared to a diet with a lower n-6:n-3. In order to investigate this hypothesis, we utilized a murine model and employed four different HFD’s differing solely in the n-6:n-3 (20:1, 10:1, 5:1, 1:1). We found that reducing the dietary n-6:n-3 did, in fact, lower the hepatic phospholipid n-6:n-3 in a ratio-dependent manner. However, despite this, all HFDs led to a similar degree of NAFLD development suggesting that the hepatic phospholipid AA:EPA and AA:DHA may not be a substantial driving force of NAFLD development.
Because n-3 FAs have been shown to modulate hepatic lipid metabolism (n-3 FAs have been shown to be anti-lipogenic [9]), we first investigated hepatic lipid accumulation and genes involved in FA synthesis and oxidation. When comparing among the HFDs, the 20:1 HFD modestly increased the mean expression of the lipogenic gene, Srebp-1, but also led to the lowest mean expression of another well-known lipogenic transcription factor, Chrebp. The mRNA content of Pparα, a protein thought to promote hepatic FA oxidation upon activation [9] (though this is currently under debate [34]), was found to be significantly increased in the liver of the 1:1 HFD-fed mice relative to the 20:1 HFD-fed mice. However, despite these differences in the expression of these lipid metabolism mediators among the HFD groups, all HFDs led to a similar degree of hepatic steatosis.
We next assessed the degree to which manipulating the dietary n-6:n-3 could modulate inflammatory processes associated with NAFLD progression. Irrespective of the dietary n-6:n-3, all HFDs exhibited evidence of elevated hepatic inflammation. In general, reducing the dietary n-6:n-3 was unable to illicit any statistically significant changes to the majority of the inflammatory markers measured. Interestingly, all HFDs downregulated the gene expression of IL-6 compared to the control diet. IL-6 is a puzzling cytokine as, although it is often considered a classical pro-inflammatory cytokine, it also possesses anti-inflammatory properties via its ability to promote M2 macrophage activation [35]. We also examined the hepatic gene expression of Gpr120, as n-3 FAs have recently been shown to regulate inflammatory processes through its stimulation [36]. Although there appeared to be a ratio-response effect among the HFDs to regulate Gpr120 gene expression, due to the large variability of Gpr120 mRNA induction within each group, this was not found to be statistically significant.
Oxidative stress is thought to play a significant role in the progression of hepatic steatosis into NASH [37]; it can manifest itself in several different forms including lipid peroxidation and nitrosylation [38]. Our analysis of oxidative stress included two markers of lipid peroxidation, 4-HNE and MDA, as well as a marker for nitrosylation, 3-nitrotyrosine. While there was no effect for any of the HFDs to augment oxidative stress in the form of MDA or 3-nitrotyrosine, although not statistically significant, we did see an apparent ratio-dependent effect on 4-HNE content. This observation was not surprising given that 4-HNE adducts are most commonly derived from n-6 FAs, which were of lowest content in the diet of the 1:1 mice followed by the diets of the 5:1, 10:1, and 20:1 mice, respectively [39].
The ER stress response is primarily coordinated by three ER-localized proteins: ATF6, PERK (protein kinase RNA-like endoplasmic reticulum kinase), and IRE1α, and the molecular chaperone protein, BiP - all of which are activated following a build-up of mis- and/or unfolded proteins [40]. If homeostasis is not brought back to the ER and chronic stress persists, CHOP is upregulated, leading to cell death. In addition to measuring the protein content of BiP, we assessed the activation of each of the three ER-stress pathways as well as the pro-apoptotic protein, CHOP. Given that p-IRE1α was found to be downregulated whereas CHOP was significantly increased in each of the HFDs after 20 weeks of feeding, it is our hypothesis that hepatic ER stress manifested itself at a much earlier time point than at 20 weeks as we have previously found an increase in p-EIF2α and ATF6-p50 in the liver of mice fed a similar diet as the 20:1 HFD for 16 weeks [24]. Thus, after 20 weeks of HFD-feeding, chronic ER stress may have persisted to a state in which it could not be resolved, resulting in CHOP upregulation and the initiation of apoptosis. With this being said, much of what scientists have learned about ER stress comes from in vitro studies. It is clear that in vivo time-course studies are necessary to better understand the different phases of the ER stress pathology. In a separate but similar note, it was unexpected to see that activation of IRE1α was significantly downregulated with the progression of NAFLD, as was observed by others examining ER stress as it relates to NAFLD [41]. It is generally believed that IRE-1α phosphorylation is increased at the onset of ER stress in order to facilitate the splicing of XBP-1 in order to help with ER stress resolution [42]. Because we did not see any change in the XBP-1s, we did not expect to see a change in the levels of p-IRE1α. It is likely that IRE1α regulates other processes independent of XBP-1 splicing and ER stress, as suggested by others [43].
We also investigated how the dietary n-6:n-3 may have affected oxidative capacity, as oxidative capacity is known to be compromised with NAFLD progression. However, there was no effect for any of the HFDs to negatively impact oxidative capacity, suggesting that decrements in oxidative capacity are secondary to other cellular perturbations (i.e. inflammation) in NAFLD.
Recent evidence has implicated gut integrity in the development of NAFLD [5]; it has been hypothesized that when gut integrity is compromised, bacteria may leak into the portal vein and initiate/augment hepatic inflammation [5]. Previous research has shown that HFD consumption can lead to colitis and gut-barrier permeability [6]. Therefore, we examined how HFD feeding and manipulation of the dietary n-6:n-3 may have impacted colitis development and tight junction protein gene expression. Surprisingly, we did not find any evidence for HFD feeding to promote colitis or influence tight junction protein mRNA expression. These findings lead us to believe that colitis development and increased gut-barrier permeability do not necessarily precede NAFLD development. Our group utilizes a purified HFD composed of up to seven sources of fat and designed to be similar in macronutrient content to the typical American diet (MUFA:PUFA of 2:1, 12% and 40% of calories from saturated and total fat, respectively) whereas others tend to use diets of significantly more total (60% of calories from fat) and saturated fat (>20% of total calories) with one main source of fat (typically lard) [6], which may explain the inconsistencies across studies.
Given the findings of this study, a question that begs to be asked is why did we not see any dramatic effects of attenuating NAFLD progression by reducing the dietary n-6:n-3 when others have shown that supplementing with n-3 FAs is a powerful therapy for NAFLD [9]? We reduced the n-6:n-3 utilizing the essential n-3 FA, ALA, which has to compete with LA for the enzymes needed to synthesize EPA and DHA [16]. Others who have shown n-3 FAs to benefit NAFLD have primarily utilized EPA and/or DHA, which have the capability to be directly stored in phospholipids and may serve as more potent agonists of certain transcription factors, including those that promote FA oxidation and limit liver fat accumulation, than the essential n-3. As a result of our manipulation of the dietary n-6:n-3, we were able to lower the hepatic phospholipid AA:EPA and AA:DHA in a ratio-dependent manner, with the AA:EPA and AA:DHA reaching the lowest (7:1 and 6.5:1, respectively) as a result of consuming the 1:1 HFD. However, others who have supplemented with fish oil, largely comprised of EPA and DHA, have produced a diet with a dietary n-6:n-3 as low as 1:0.76 eliciting a hepatic phospholipid AA:EPA and AA:DHA of 1:0.87 and 1:0.5, respectively – much lower ratios than what our 1:1 diet generated [44]. Thus, it may be that in order to see dramatic benefits with n-3 supplementation the long-chain n-3 FAs would need to be consumed over ALA, and may need to be consumed at such a quantity to invoke a hepatic phospholipid n-6:n-3 closer to 1:1 as further supported by Riediger et. al. [45]. With this being said, it is uncertain if the reduction in the hepatic phospholipid n-6:n-3 to extremely low levels (e.g. 1:1) is actually the driving force behind the potential beneficial effects of a lower dietary omega-6:omega-3 in studies utilizing EPA and DHA-rich diets or simply a bystander effect. It may be that the anti-lipogenic and GPR120-mediated anti-inflammatory properties are the key mediators of the beneficial effects of fish-oil-supplemented diets and not the lower cellular phospholipid n-6:n-3.
In summary, the onset of NAFLD was not paired to colitis development nor changes in tight-junction protein gene expression. Reducing the dietary n-6:n-3 with ALA, although it lowered the hepatic phospholipid AA:EPA and AA:DHA in a dose-dependent manner and mildly influenced inflammatory signaling, it did not significantly attenuate NAFLD development, thus rejecting our original hypothesis. Further, the consumption of the control diet resulted in the highest hepatic AA:EPA and AA:DHA – more so than any other HFD (except compared to the AA:EPA of 20:1 HFD) – it did not result in the development of NAFLD. This suggests that the dietary n-6:n-3 likely does not play a significant role in NAFLD initiation except under extreme conditions in which n-3 FAs are drastically depleted from the diet [46]. It is more probable that an imbalance between energy expenditure and energy intake resulting in a chronic energy surplus leading to hepatic lipid accumulation is largely responsible for the instigation of NAFLD. It should be noted, however, that this study was performed in a single model, utilizing only C57BL/6J male mice consuming hypercaloric HFDs where only one time-point was analyzed. Thus, it is not clear if our findings can be generalized to other experimental models. Although we have come a long way in understanding the potential therapies and molecular pathways influenced by n-3 FAs, it is clear from this study and from those of others that more research is necessary in order to further elucidate the mechanisms by which n-3 FAs invoke their potentially therapeutic properties, as well as the appropriate balance of dietary n-6:n-3, and perhaps most importantly, the most responsive dose, form, and species needed to produce such effects.
Supplementary Material
Highlights.
Reducing the dietary omega-6:omega-3 does not necessarily attenuate NAFLD progression
It is unlikely that a high dietary omega-6:omega-3 nor colitis play a role in NAFLD initiation
ACKNOWLEDGEMENTS
The authors would like to thank Kei Lam for her technical support and Dr. Jaime Lecker (Bio-Serv) for her help with diet creation.
FUNDING
This work was supported by grants from the National Institutes of Health (National Cancer Institute R21CA167058 and R21CA175636 to E.A.M.; National Center for Complementary and Alternative Medicine K01AT007824 to E.A.M.) and the University of South Carolina (Advanced Support Programs for Innovative Research Excellence to E.A.M.).
ABBREVIATIONS
- 4-HNE
4-Hydroxy-2-Nonenal
- AA
Arachidonic acid
- ACAC1
Acetyl-CoA carboxylase 1
- ALA
Alpha-linolenic acid
- ATF6
Activating transcription factor 6
- BiP
Binding immunoglobulin protein
- CHOP
CCAAT-enhancer-binding protein homologous protein
- CHREBP
Carbohydrate-responsive element-binding protein
- DHA
Docosahexaenoic acid
- EIF2α
Eukaryotic initiation factor 2 alpha
- EPA
Eicosapentaenoic acid
- ER
Endoplasmic reticulum
- ERK
Extracellular signal-regulated kinase
- FAs
Fatty acids
- FASN
Fatty-acid synthase
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GPRs
G-protein-coupled receptors
- H&E
Hematoxylin and Eosin
- HFD
High-fat diet
- IRE1α
Inositol requiring enzyme 1 alpha
- IL
Interleukin
- JNK
c-Jun N-terminal kinase
- LA
Linoleic acid
- LCSFAS
Long-chain saturated fatty acids
- MAPK
Mitogen-activated protein kinase
- MCFAs
Medium-chain fatty acids
- MCP-1
Monocyte chemotactic protein-1
- MDA
Malondialdehyde
- MUFA
Monounsaturated fatty acid
- NAFLD
Non-Alcoholic Fatty-Liver Disease
- NASH
Non-Alcoholic Steatohepatitis
- NFκB
Nuclear factor kappa-B
- p38
p38 mitogen-activated protein kinase
- PPAR
Peroxisome proliferator-activated receptor
- PVDF
Polyvinylidene difluoride
- PUFA
Polyunsaturated fatty acid
- SF
Saturated fat
- SFAs
Saturated fatty acids
- SREBP-1
Sterol regulatory element-binding protein-1
- STAT3
Signal transducer and activator of transcription 3
- TGF-β
Transforming growth factor-beta
- TJP1
Tight-junction protein 1
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor-alpha
- UPR
Unfolded-protein response
- USFAs
Unsaturated fatty acids
- XBP-1s
X-box binding protein 1 spliced
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
RTE, KTV, JLM, TLC, MDW, EAM do not have any conflicts of interest.
REFERENCES
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of nonalcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res. 2012 doi: 10.1016/j.plipres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology. 2014;147:754–764. doi: 10.1053/j.gastro.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 5.Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology. 2014;59:328–339. doi: 10.1002/hep.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asrih M, Jornayvaz FR. Diets and nonalcoholic fatty liver disease: the good and the bad. Clin Nutr. 2014;33:186–190. doi: 10.1016/j.clnu.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Ronis MJ, Baumgardner JN, Sharma N, Vantrease J, Ferguson M, Tong Y, et al. Medium chain triglycerides dose-dependently prevent liver pathology in a rat model of non-alcoholic fatty liver disease. Exp Biol Med (Maywood) 2013;238:151–162. doi: 10.1258/ebm.2012.012303. [DOI] [PubMed] [Google Scholar]
- 9.Scorletti E, Byrne CD. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr. 2013;33:231–248. doi: 10.1146/annurev-nutr-071812-161230. [DOI] [PubMed] [Google Scholar]
- 10.Elizondo A, Araya J, Rodrigo R, Poniachik J, Csendes A, Maluenda F, et al. Polyunsaturated fatty acid pattern in liver and erythrocyte phospholipids from obese patients. Obesity (Silver Spring) 2007;15:24–31. doi: 10.1038/oby.2007.518. [DOI] [PubMed] [Google Scholar]
- 11.Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944–951. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Di Minno MN, Russolillo A, Lupoli R, Ambrosino P, Di Minno A, Tarantino G. Omega-3 fatty acids for the treatment of non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18:5839–5847. doi: 10.3748/wjg.v18.i41.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valenzuela R, Espinosa A, Gonzalez-Manan D, D’Espessailles A, Fernandez V, Videla LA, et al. N-3 long-chain polyunsaturated fatty acid supplementation significantly reduces liver oxidative stress in high fat induced steatosis. PLoS One. 2012;7:e46400. doi: 10.1371/journal.pone.0046400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enos RT, Velazquez KT, McClellan JL, Cranford TL, Walla MD, Murphy EA. Reducing the dietary omega-6:omega-3 utilizing alpha-linolenic acid; not a sufficient therapy for attenuating high-fat-diet-induced obesity development nor related detrimental metabolic and adipose tissue inflammatory outcomes. PLoS One. 2014;9:e94897. doi: 10.1371/journal.pone.0094897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim W, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids--physiological relevance of dose. Prostaglandins Leukot Essent Fatty Acids. 2010;82:155–158. doi: 10.1016/j.plefa.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 17.Grotto D, Zied E. The Standard American Diet and its relationship to the health status of Americans. Nutr Clin Pract. 2010;25:603–612. doi: 10.1177/0884533610386234. [DOI] [PubMed] [Google Scholar]
- 18.Center for Disease Control and Prevention. National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services; pp. 2011–2012. [Google Scholar]
- 19.Archer E, Hand GA, Blair SN. Validity of U.S. nutritional surveillance:National Health and Nutrition Examination Survey caloric energy intake data, 1971–2010. PLoS One. 2013;8:e76632. doi: 10.1371/journal.pone.0076632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goris AHP, Westerterp-Plantenga MS, Westerterp KR. Undereating and underrecording of habitual food intake in obese men: selective underreporting of fat intake. Am J Clin Nutr. 2000;71:130–134. doi: 10.1093/ajcn/71.1.130. [DOI] [PubMed] [Google Scholar]
- 21.Scagliusi FB, Polacow VO, Artioli GG, Benatti FB, Lancha AH., Jr Selective underreporting of energy intake in women: magnitude, determinants, and effect of training. J Am Diet Assoc. 2003;103:1306–1313. doi: 10.1016/s0002-8223(03)01074-5. [DOI] [PubMed] [Google Scholar]
- 22.Lissner L, Heitmann BL, Bengtsson C. Population studies of diet and obesity. Br J Nutr. 2000;83(Suppl 1):S21–S24. doi: 10.1017/s000711450000091x. [DOI] [PubMed] [Google Scholar]
- 23.Heitmann BL, Lissner L, Osler M. Do we eat less fat, or just report so? Int J Obes Relat Metab Disord. 2000;24:435–442. doi: 10.1038/sj.ijo.0801176. [DOI] [PubMed] [Google Scholar]
- 24.Enos RT, Velazquez KT, Murphy EA. Insight into the impact of dietary saturated fat on tissue-specific cellular processes underlying obesity-related diseases. J Nutr Biochem. 2014;25:600–612. doi: 10.1016/j.jnutbio.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velazquez KT, Enos RT, Narsale AA, Puppa MJ, Davis JM, Murphy EA, et al. Quercetin Supplementation Attenuates the Progression of Cancer Cachexia in ApcMin/+ Mice. J Nutr. 2014 doi: 10.3945/jn.113.188367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enos RT, Davis JM, Velazquez KT, McClellan JL, Day SD, Carnevale KA, et al. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters. J Lipid Res. 2013;54:152–163. doi: 10.1194/jlr.M030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 28.Saunders RD, Horrocks LA. Simultaneous extraction and preparation for high-performance liquid chromatography of prostaglandins and phospholipids. Anal Biochem. 1984;143:71–75. doi: 10.1016/0003-2697(84)90559-1. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima H, Ogawa Y, Shono S, Kinoshita M, Nakashima M, Sato A, et al. Activation of CD11b+ Kupffer cells/macrophages as a common cause for exacerbation of TNF/Fas-ligand-dependent hepatitis in hypercholesterolemic mice. PLoS One. 2013;8:e49339. doi: 10.1371/journal.pone.0049339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith K. Liver disease: Kupffer cells regulate the progression of ALD and NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:503. doi: 10.1038/nrgastro.2013.140. [DOI] [PubMed] [Google Scholar]
- 31.Corrado RL, Torres DM, Harrison SA. Review of treatment options for nonalcoholic fatty liver disease. Med Clin North Am. 2014;98:55–72. doi: 10.1016/j.mcna.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Simopoulos AP. Importance of the omega-6/omega-3 balance in health and disease: evolutionary aspects of diet. World Rev Nutr Diet. 2011;102:10–21. doi: 10.1159/000327785. [DOI] [PubMed] [Google Scholar]
- 33.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 34.Yan F, Wang Q, Xu C, Cao M, Zhou X, Wang T, et al. Peroxisome Proliferator-Activated Receptor alpha Activation Induces Hepatic Steatosis, Suggesting an Adverse Effect. PLoS One. 2014;9:e99245. doi: 10.1371/journal.pone.0099245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Covarrubias AJ, Horng T. IL-6 strikes a balance in metabolic inflammation. Cell Metab. 2014;19:898–899. doi: 10.1016/j.cmet.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH) Int J Mol Sci. 2014;14:20704–20728. doi: 10.3390/ijms141020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ucar F, Sezer S, Erdogan S, Akyol S, Armutcu F, Akyol O. The relationship between oxidative stress and nonalcoholic fatty liver disease: Its effects on the development of nonalcoholic steatohepatitis. Redox Rep. 2013;18:127–133. doi: 10.1179/1351000213Y.0000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009;44:625–633. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagliassotti MJ. Endoplasmic reticulum stress in nonalcoholic fatty liver disease. Annu Rev Nutr. 2012;32:17–33. doi: 10.1146/annurev-nutr-071811-150644. [DOI] [PubMed] [Google Scholar]
- 41.Zhao M, Zang B, Cheng M, Ma Y, Yang Y, Yang N. Differential responses of hepatic endoplasmic reticulum stress and inflammation in diet-induced obese rats with high-fat diet rich in lard oil or soybean oil. PLoS One. 2013;8:e78620. doi: 10.1371/journal.pone.0078620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chambers JE, Marciniak SJ. Protein misfolding and ER stress. Am J Physiol Cell Physiol. 2014 doi: 10.1152/ajpcell.00183.2014. [DOI] [PubMed] [Google Scholar]
- 43.Coelho DS, Domingos PM. Physiological roles of regulated Ire1 dependent decay. Front Genet. 2014;5:76. doi: 10.3389/fgene.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tillander V, Bjorndal B, Burri L, Bohov P, Skorve J, Berge RK, et al. Fish oil and krill oil supplementations differentially regulate lipid catabolic and synthetic pathways in mice. Nutr Metab (Lond) 2014;11:20. doi: 10.1186/1743-7075-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riediger ND, Othman R, Fitz E, Pierce GN, Suh M, Moghadasian MH. Low n-6:n-3 fatty acid ratio, with fish- or flaxseed oil, in a high fat diet improves plasma lipids and beneficially alters tissue fatty acid composition in mice. Eur J Nutr. 2008;47:153–160. doi: 10.1007/s00394-008-0709-8. [DOI] [PubMed] [Google Scholar]
- 46.Pachikian BD, Essaghir A, Demoulin JB, Neyrinck AM, Catry E, De Backer FC, et al. Hepatic n-3 polyunsaturated fatty acid depletion promotes steatosis and insulin resistance in mice: genomic analysis of cellular targets. PLoS One. 2011;6:e23365. doi: 10.1371/journal.pone.0023365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.