Abstract
Objectives:
Our objective was to investigate cross-sectional associations between odor identification ability and imaging biomarkers of neurodegeneration and amyloid deposition in clinically normal (CN) elderly individuals, specifically testing the hypothesis that there may be an interaction between amyloid deposition and neurodegeneration in predicting odor identification dysfunction.
Methods:
Data were collected on 215 CN participants from the Harvard Aging Brain Study. Measurements included the 40-item University of Pennsylvania Smell Identification Test and neuropsychological testing, hippocampal volume (HV) and entorhinal cortex (EC) thickness from MRI, and amyloid burden using Pittsburgh compound B (PiB) PET. A linear regression model with backward elimination (p < 0.05 retention) evaluated the cross-sectional association between the University of Pennsylvania Smell Identification Test and amyloid burden, HV, and EC thickness, assessing for effect modification by PiB status. Covariates included age, sex, premorbid intelligence, APOE ε4 carrier status, and Boston Naming Test.
Results:
In unadjusted univariate analyses, worse olfaction was associated with decreased HV (p < 0.001), thinner EC (p = 0.003), worse episodic memory (p = 0.03), and marginally associated with greater amyloid burden (binary PiB status, p = 0.06). In the multivariate model, thinner EC in PiB-positive individuals (interaction term) was associated with worse olfaction (p = 0.02).
Conclusions:
In CN elderly, worse odor identification was associated with markers of neurodegeneration. Furthermore, individuals with elevated cortical amyloid and thinner EC exhibited worse odor identification, elucidating the potential contribution of olfactory testing to detect preclinical AD in CN individuals.
Odor identification deficits are an early feature of Alzheimer disease (AD)1 and have been shown to predict progression from mild cognitive impairment (MCI) to AD dementia,2 particularly when combined with clinical assessments and imaging biomarkers.3 Deficits in odor identification begin early in the clinical course of AD, preceding more frank impairment in odor detection.4 Early in AD, neurofibrillary tangles (NFTs) are found in the olfactory bulb and entorhinal cortex (EC).5 Odor identification deficits in the elderly are associated with NFT pathology in the central olfactory system.6 In mice, overexpression of either a pathogenic isoform of the amyloid precursor protein, β-amyloid (Aβ)-42, or Aβ-40 is sufficient to cause olfactory deficits without amyloid deposition.7
Since the AD pathologic process likely begins more than a decade before emergence of symptoms,8,9 a simple, accurate, cost-effective screening tool for preclinical disease is desirable, particularly for treatment and prevention trial design. The 40-item University of Pennsylvania Smell Identification Test (UPSIT-40),10 a method to assess odor identification ability in humans, predicts progression from MCI to AD dementia.2,11 Studies have reported associations between worse performance on the UPSIT-40 and lower hippocampal volume (HV) in clinically normal (CN) elderly12 and increased cortical amyloid burden measured by Pittsburgh compound B (PiB)-PET across CN, MCI, and AD dementia individuals.13 Our study hones in on CN elderly at risk of preclinical AD with markers of neurodegeneration and amyloid.
The objective of this study was to elucidate the utility of olfactory testing in detecting preclinical AD by investigating cross-sectional associations in CN elderly individuals. We assessed the association between olfaction (UPSIT) and imaging biomarkers of AD, including amyloid burden (PiB status), EC thickness, and HV. Building on an emerging model of AD pathogenesis, we hypothesized that amyloid deposition may modify the effect of neurodegeneration, as measured by EC thickness, on odor identification ability.
METHODS
Participants.
Data were collected on 215 community-dwelling CN elderly in the Harvard Aging Brain Study, a longitudinal cohort study involving neuropsychological testing, imaging, and biomarker sampling.14,15 Participants had a Mini-Mental State Examination16 score of 27 to 30 (inclusive, allowing for lower scores down to 25 for individuals with low levels of education, using the Mungas adjustment17), a Clinical Dementia Rating18 global score of 0, no significant memory impairment (performed within 1 SD of age- and education-adjusted cutoff scores on the delayed recall portion of one Logical Memory story of the Wechsler Memory Scale–Revised,19 as per the Alzheimer's Disease Neuroimaging Initiative20), a Geriatric Depression Scale,21 long form ≤10, and a Modified Hachinski Ischemic Score22 ≤4.
Participants completed a baseline smell functioning questionnaire11 assessing medical factors that may have affected their ability to identify odors correctly (see e-Methods on the Neurology® Web site at Neurology.org).
Standard protocol approvals, registrations, and participant consents.
The study was approved by the institutional review board of Partners Healthcare Inc. Written informed consent was obtained from all participants before initiation of any study procedures in accordance with institutional review board guidelines.
Clinical assessments.
Participants underwent odor identification testing using the UPSIT-40 (range 0–40, higher score denotes better performance).23 Participants completed an extensive set of neuropsychological tests used to derive cognitive domain factors of executive function, episodic memory, and processing speed, as previously described (see e-Methods for more detail).14 An estimate of premorbid intelligence was assessed with the American National Adult Reading Test (AmNART)24 yielding a verbal IQ. Confrontation naming was assessed with the 30-item Boston Naming Test; this variable was included in the model, as done in previous studies,6 to control for the possibility that naming deficits may contribute to odor identification deficits.25
Structural MRI acquisition and processing.
MRI scanning was completed on a Siemens TIM Trio 3T System (Siemens AG, Erlangen, Germany) with a 12-channel head coil. Structural T1-weighted volumetric magnetization-prepared rapid-acquisition gradient echo scans were collected (repetition time/echo time/inversion time = 6,400/2.8/900 milliseconds, flip angle = 8°, 1 × 1 × 1.2 mm resolution). Region-of-interest labeling was performed using FreeSurfer v5.1 (http://surfer.nmr.mgh.harvard.edu). Ex vivo measurements of EC thickness were averaged across hemispheres to yield bilateral EC thickness values. HV was summed across hemispheres.
PiB-PET acquisition and processing.
PiB-PET scanning was completed on a Siemens ECAT EXACT HR + PET scanner. C11-PiB was synthesized using a previously published protocol.26–28 After injection of 8.5 to 15 mCi PiB, 60 minutes of dynamic data were acquired in 3-dimensional acquisition mode. The Logan graphical analysis method with cerebellar cortex as the reference tissue input function was used to evaluate specific PiB retention expressed as the distribution volume ratio (DVR).29 PiB DVR was calculated for an aggregate of cortical regions that typically have elevated PiB retention in AD dementia. Binary PiB status was assigned with a cutoff value of 1.20 DVR for the aggregate described above (DVR ≥ 1.20, PiB-positive; DVR < 1.20, PiB-negative); the cutoff was established using gaussian mixture modeling.15 Binary PiB status was used in the linear regression models given the typical nonnormal distribution of PiB DVR (rightward tail) in this sample of CN elderly and also to facilitate graphical interpretation of the effect of amyloid status on the association between measures of neurodegeneration and odor identification ability. For comparison, PiB DVR was used as a continuous variable in the models as well; it was included as an untransformed nonnormally distributed continuous variable.
Statistical analyses.
Analyses were performed using STATA v11.2 (StataCorp, College Station, TX). Univariate associations were explored between the predictors of interest and UPSIT-40. These were completed using nonparametric Spearman correlation and Wilcoxon rank sum tests given the nonnormal distributions of UPSIT-40 and EC thickness. They were also conducted by fitting sequential simple linear regression models with each predictor of interest as the independent variable to facilitate comparison to the multivariate regression models. A descriptive analysis was conducted to identify those participants who fell in the bottom 25th percentile of both olfaction and episodic contextual memory testing.
For the multivariate model, a backward elimination (p < 0.05 for retention) multiple linear regression model evaluated the cross-sectional association between UPSIT (dependent variable) and amyloid burden (PiB status), bilateral HV and EC thickness, and interaction terms between PiB and HV and PiB and EC thickness. Covariates included age, sex, AmNART IQ, Boston Naming Test, and APOE ε4 carrier status (carriers were designated as having at least one ε4 allele, while noncarriers had none). The sample of observations under consideration was limited to observations with nonmissing values for all the variables specified. AmNART was included in lieu of years of education as a proxy of cognitive reserve given a bias toward lower education among female participants in the study sample; both variables were not included together given that they are highly correlated. Main effects that were nonsignificant were included in the model if one of the interaction terms containing these main effects reached significance.
RESULTS
The initial sample included 287 participants; this was reduced to 215 (2 missing olfaction testing, 32 with active cold at time of olfaction testing, and 38 missing both MRI and PiB data).
Table 1 provides baseline demographic, clinical, and imaging data for the 215 participants. Seventy-one percent of participants were classified as PiB-negative, while 29.0% were classified as PiB-positive.
Table 1.
Baseline demographic, clinical, and imaging data for study participants
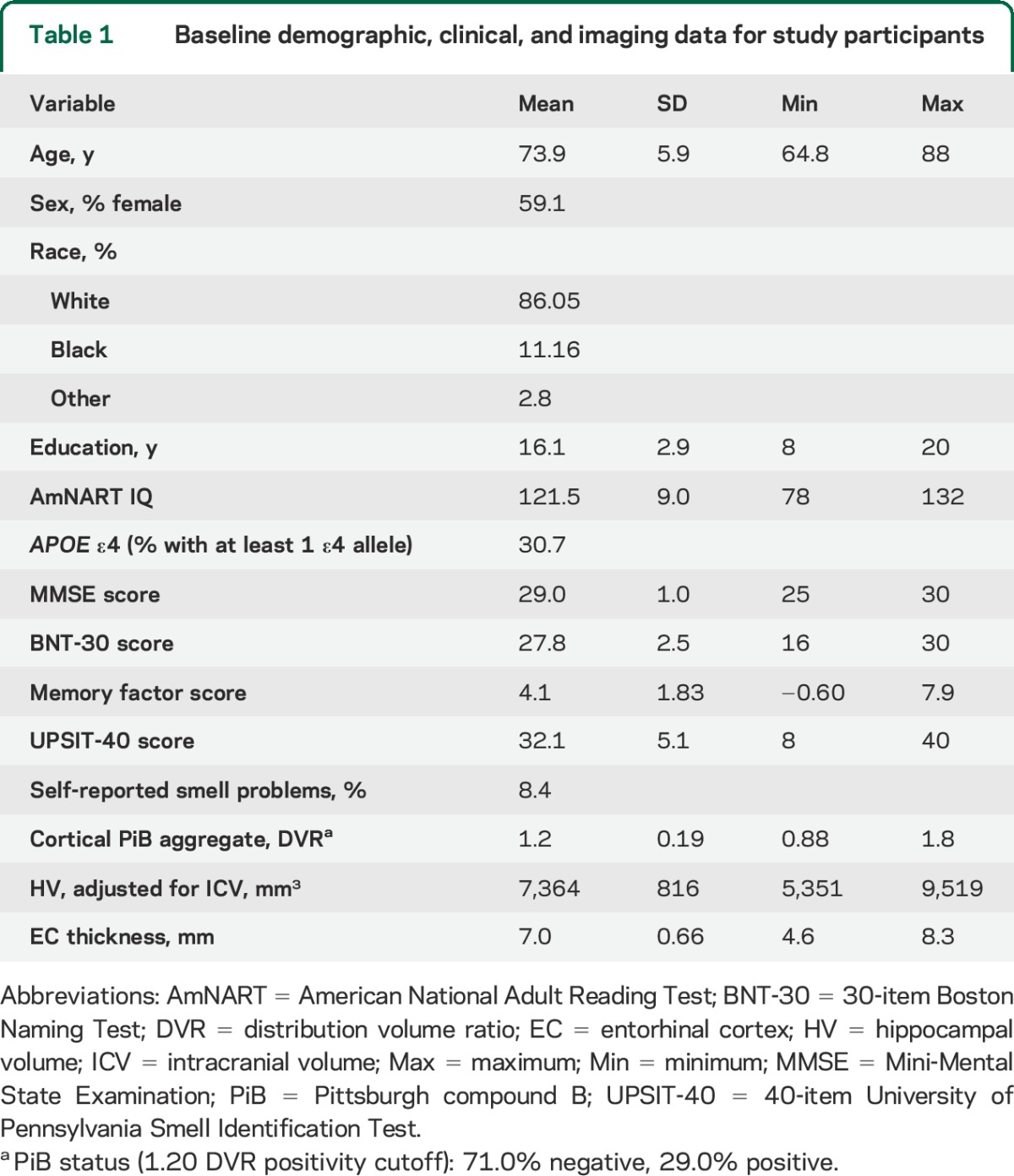
Figure 1 depicts the relative sizes and overlap between 2 groups defined by having UPSIT-40 or memory factor scores below the 25th percentile for the sample used in analyses (n = 212, given 3 participants missing episodic memory testing); 42.9% of participants fell into the lower quartile for either or both of these tests, while 10.4% fell into the lower quartile for both tests. There was a significant association between the presence of self-reported smell problems on the screening questionnaire and worse olfaction as measured by UPSIT (β = −3.6, p = 0.004).
Figure 1. UPSIT identifies a subset of the cohort with normal episodic memory performance that is putatively at risk.
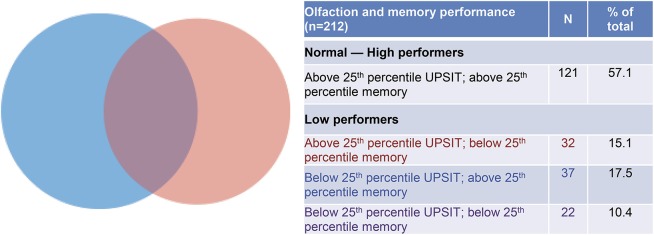
Venn diagram depicting overlap of groups defined by lower 25th percentile of episodic memory and odor identification testing (n = 91 in the low performer groups). Blue depicts low performers on UPSIT (n = 59), red depicts low performance on memory testing (n = 54), and purple depicts participants who had low performance on both tests (n = 22). Note the relatively small overlap between low-performing groups, and the fact that a slightly greater number of participants had low performance on the odor identification test compared with those who had low performance on the episodic memory tests. UPSIT = 40-item University of Pennsylvania Smell Identification Test.
Table 2 presents the results of sequential univariate regression models predicting UPSIT-40. In unadjusted analyses, worse olfaction was significantly associated with decreased HV (p < 0.001), EC thickness (p = 0.003), and episodic contextual memory function (p = 0.03). Worse olfaction was marginally associated with binary PiB status (p = 0.06) with PiB-positive participants having worse olfaction.
Table 2.
Results of unadjusted univariate models predicting UPSIT-40
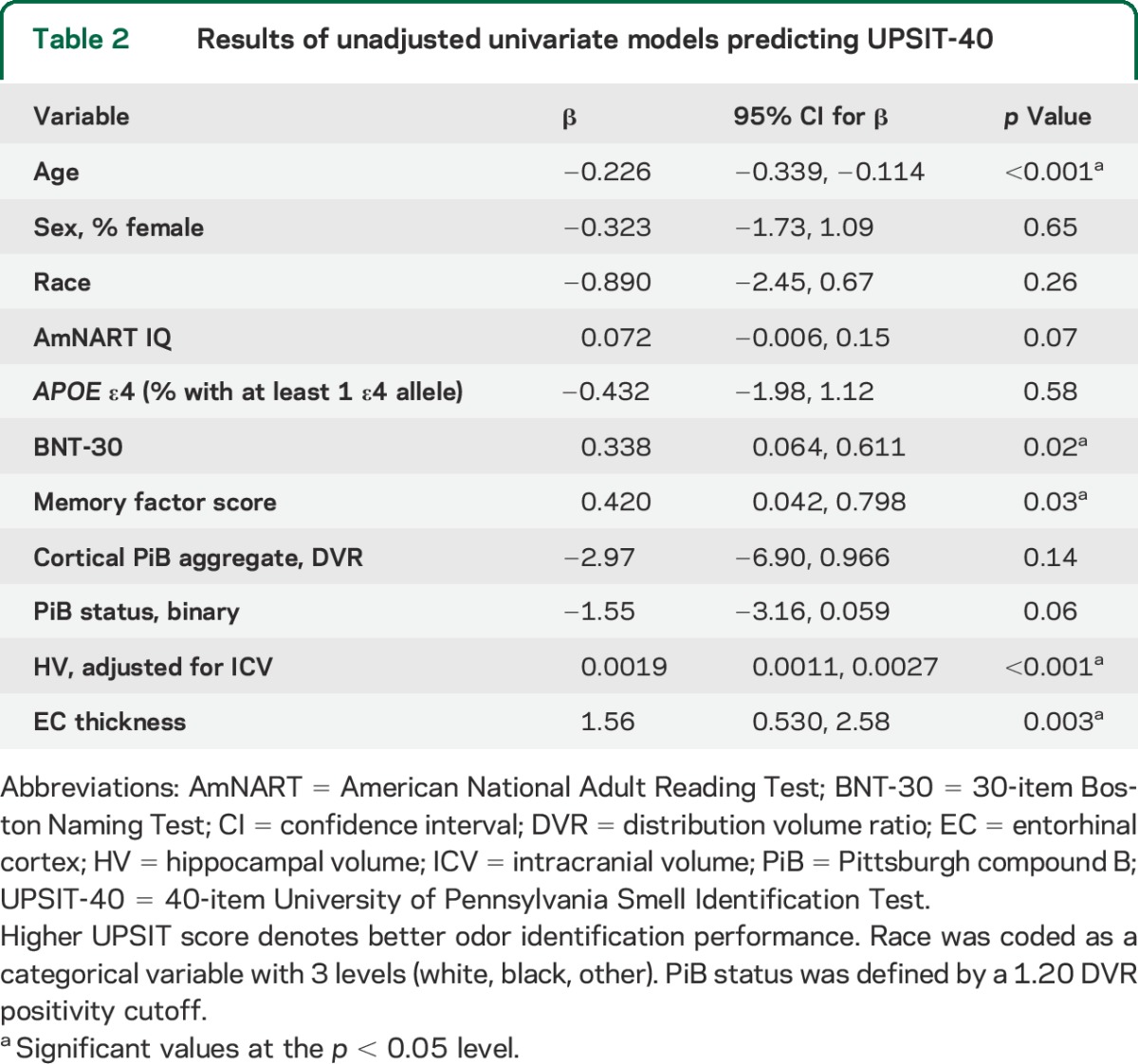
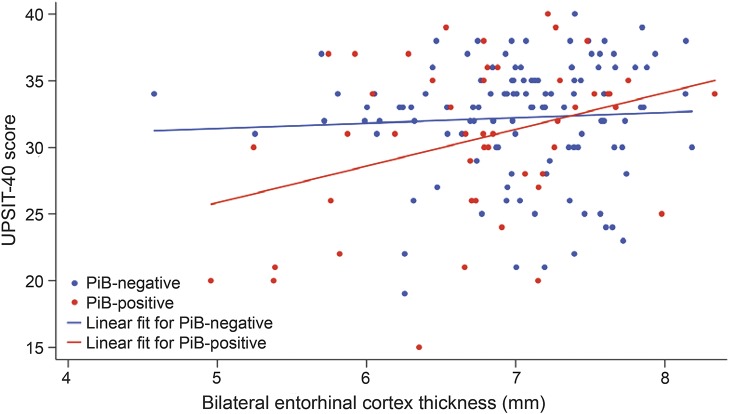
Table 3 presents the results of the multivariate model predicting UPSIT-40 scores (R2 = 0.17, overall model p < 0.001). Thinner EC in PiB-positive individuals (interaction term) was significantly associated with worse olfaction (p = 0.02, figure 2). There was a significant association between thinner EC and worse olfaction in PiB-positive individuals (β = 2.6, p = 0.004) but not in PiB-negative individuals (β = 0.038, p = 0.96). Greater age (p < 0.001) and lower AmNART IQ (p = 0.01) were also associated with worse olfaction. When the multivariate model was repeated using PiB as a continuous variable, the interaction term between PiB and EC thickness remained significant (p = 0.04).
Table 3.
Results of multivariate model predicting UPSIT-40
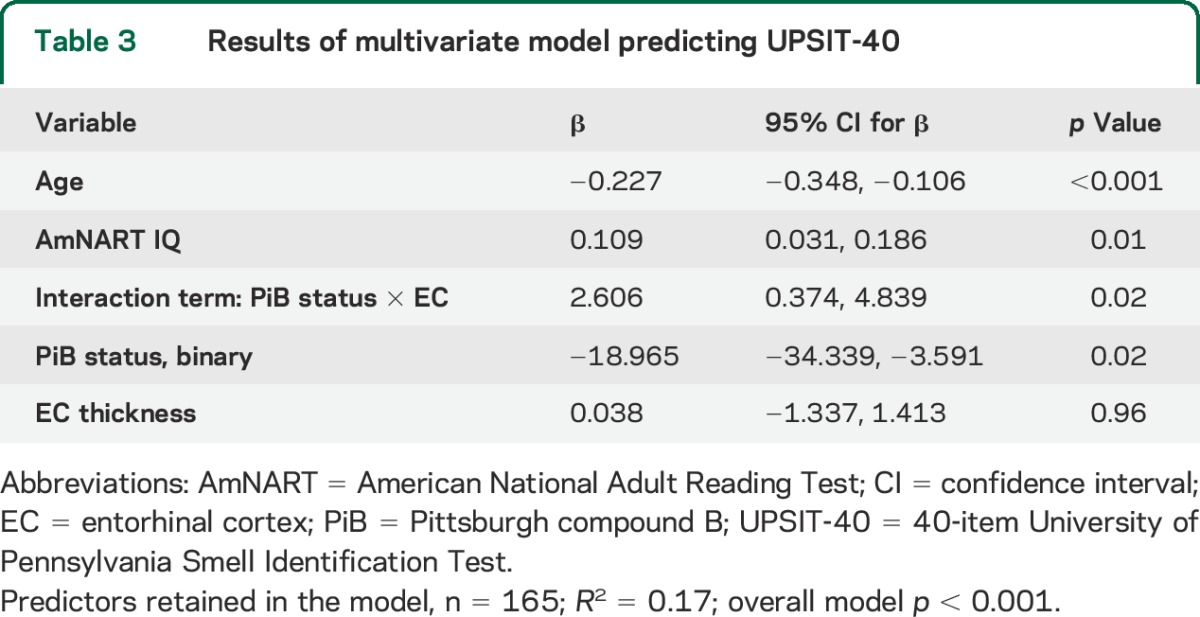
Figure 2. Depiction of the associations among UPSIT, EC thickness, and amyloid.
In the multivariate model with UPSIT-40 as the dependent variable (n = 165), in PiB-positive individuals, thinner EC was significantly associated with worse odor identification (interaction term, p = 0.02). The slope for the PiB-positive individuals was significant (β = 2.6, 95% confidence interval: 0.88, 4.4; p = 0.004), while the slope for PiB-negative individuals was not (β = 0.038, 95% confidence interval: −1.3, 1.4; p = 0.96). EC = entorhinal cortex; PiB = Pittsburgh compound B (PiB status 1.20 distribution volume ratio positivity cutoff); UPSIT = 40-item University of Pennsylvania Smell Identification Test.
DISCUSSION
In this sample of CN, community-dwelling, elderly individuals, there were significant cross-sectional associations between worse odor identification ability measured by the UPSIT and biomarkers of neurodegeneration, including HV and EC thickness. We report a significant association between worse olfaction and thinner EC in individuals with elevated cortical amyloid; this was not seen in amyloid-negative individuals. This association was adjusted for age, sex, premorbid intelligence, confrontation naming, and APOE ε4 carrier status.
Characterizing the pathologic substrates underlying olfactory changes in AD is an area of ongoing research. In an autopsy study involving participants who had undergone odor identification testing before death, after adjusting for age at death, sex, education, and time from smell testing until death, there was a significant association between lower odor identification and greater AD pathology.6 While significant across both amyloid and tau pathologic changes separately, this association was stronger for NFT pathology, particularly localized in the EC and CA1/subiculum of the hippocampus, than it was for amyloid plaque pathology. When amyloid and NFT pathologic changes were assessed together, the effect of NFTs remained significant, while the effect of amyloid became nonsignificant, suggesting a stronger association between tau pathology, compared with plaque burden, and odor identification. Of note, this analysis did not account for the degree of neurodegeneration across samples. Measures of regional tissue loss, presumably due to neurodegeneration, have been shown to correlate better with cognitive decline, particularly in older individuals,30 and have been shown to correlate significantly with odor identification deficits in participants without dementia.12
Previous cross-sectional studies demonstrated reduced odor identification abilities, measured by clinical tests such as the UPSIT, in individuals with an increased risk of developing dementia, such as those diagnosed with MCI.31 Driven by the scenario that anti-AD therapies will likely prove most effective in individuals before the onset of symptoms, there is increased interest in simple, low-cost, noninvasive tests, such as the USPIT, capable of identifying at-risk individuals. One study that utilized the Brief Smell Identification Test (a 12-item test derived from and shown to correlate with the 40-item UPSIT) included a small group of individuals who died without evidence of cognitive impairment. In this group, lower Brief Smell Identification Test score was associated with a higher level of AD pathology on autopsy (measured by a composite measure of cortical amyloid plaques and NFTs), after controlling for age, sex, education, time from olfactory testing to death, APOE ε4 carrier status, and episodic memory.32 This finding supports the notion that odor identification ability is linked with the pathologic manifestations of AD, even in asymptomatic individuals; however, it is important to note the small sample size (n = 34) and the advanced age of this sample (mean 85.2 years, SD = 6.4 years).
Few studies have utilized odor identification testing in conjunction with in vivo amyloid imaging. A cross-sectional study by Bahar-Fuchs et al.13 using PiB-PET showed an association between poor odor identification and greater global neocortical amyloid burden across a total of 63 participants including CN elderly, MCI, and AD dementia. However, within the MCI group alone, there was no significant difference in olfactory identification scores between PiB-positive and PiB-negative individuals, and there was also no significant correlation between olfactory identification scores and global or regional PiB uptake as a continuous measure. This led the authors to conclude that AD-related odor identification deficits are not directly related to fibrillar Aβ burden, and to ascribe olfactory deficits to other neuropathologic features such as NFTs, in line with the histopathologic study by Wilson et al.6 However, the analysis relied on a 6-item subset of the 10-item version of the UPSIT developed by Tabert et al.11 while the 40-item UPSIT was used in the present study. Moreover, the sample size of 24 participants diagnosed with MCI was considerably smaller than the present study (n = 215), likely hampering a subgroup analysis. The present study was better powered to detect such an association, even in a cohort of CN individuals, and to assess the interplay between neurodegeneration and fibrillar amyloid.
Since the correlation between episodic memory performance and odor identification ability in this sample was modest (Spearman ρ = 0.19, p = 0.005), it is possible that these tests are detecting 2 largely distinct but partially overlapping groups of at-risk individuals. Building on the notion that subtypes of AD may be classified by differing brain regions of initiation—such as the syndrome of posterior cortical atrophy, considered an atypical variant of AD in which visual disturbances and visuospatial deficits predominate the clinical presentation early on—it is possible that odor identification tests would be more sensitive to a form of AD that begins in the olfactory neural systems before progressing to a more typical phenotype seen clinically as amnestic MCI, followed by AD dementia. Along this line of reasoning, the relationship depicted in the Venn diagram in figure 1 has identified several key groups of individuals to be followed prospectively. The large number of participants who had low performance on odor identification may speak to nonspecific, age-related changes in olfactory ability, or it may suggest a cohort of individuals at risk of progressing to MCI and AD dementia, in keeping with emerging models of the association between sensory/motor changes and AD dementia.33
These findings support the hypothesis that thinner EC, in the setting of high amyloid burden, is associated with odor identification deficits in preclinical AD. This is in line with an emerging conceptual model of preclinical AD, which postulates an interaction between the presence of amyloid and presumed neurodegeneration associated with accelerated cognitive decline. Future studies aimed at elucidating the interplay between neurodegenerative changes and pathologic accumulation of amyloid may account for the fact that neurodegeneration is more closely associated with worse cognition than amyloidosis.15,34,35 It is important to note that aging is strongly associated with both odor identification deficits and the risk of developing AD dementia. Therefore, as the primary biological events of aging are elucidated, new hypotheses of the molecular and cellular underpinnings of the decline of odor identification and other cognitive functions will likely emerge.
There are several limitations to this study. First, the analyses are based on cross-sectional data, providing a snapshot of associations that are subject to potential confounding. However, the backward selection mechanism allowed adjustment for known confounders. With time, it may be possible to track the progression of some CN participants to MCI and eventually AD dementia; however, this relatively small cohort may be underpowered to do this, in which case the progression of cognitive decline over time could be examined. Previous large prospective cohort studies have established the association between decreased odor identification ability and increased risk of cognitive decline.36,37 A 2012 systematic review identified 2 prospective, longitudinal studies that examined the utility of odor identification testing as a predictor of the development of AD, defined more rigorously with clinical criteria.3,38,39 A recent analysis added substantively to this area, showing that baseline odor identification deficits were predictive of cognitive decline and transition to AD dementia in a large multiethnic population-based cohort of patients without dementia.2 Our study, when continued longitudinally, offers the opportunity to test this hypothesis at an even earlier stage, with participants who do not have memory concerns or cognitive deficits.
Second, the study sample was not necessarily representative of the general elderly population. Although there was a wide range of years of education, performance on a test estimating premorbid intelligence, socioeconomic status, and minority representation, the mean performance indicates a highly educated and intelligent sample. However, this sample is typical of biomarker studies and clinical trials in early AD, suggesting that these results could inform future clinical trial design. Moreover, participants with various nasal pathologies were included in the analyses since they did not differ significantly from the rest of the sample in terms of demographics and the dependent variables. Nevertheless, these results must be replicated in a more representative sample of the general population, with greater racial diversity, before determining the utility of odor identification testing and its association with relevant biomarkers.
Third, odor identification deficits are also a risk factor for developing Parkinson disease, and some individuals in the sample may in fact have preclinical Parkinson disease. Similarly, it is also possible that individuals with odor identification deficits could be at higher risk of developing dementia with Lewy bodies, rather than AD dementia.40 Finally, since the PiB ligand only binds to fibrillar amyloid, the inferences in this study cannot be extended to detecting the presence of soluble amyloid or to commenting on the presence of tau-related pathology. Future studies using traditional CSF measures of Aβ-42, total-tau, and phospho-tau, and potentially still early in development measures of oligomeric Aβ, will better address these questions. New tau PET tracers, once validated, will allow for the examination of the longitudinal association between accumulation of regional tau pathology and the development of olfactory deficits.
In this sample of CN elderly individuals, we report a significant cross-sectional association between thinner EC and worse scores on odor identification testing among individuals with elevated cortical fibrillar amyloid levels, a finding that is in line with an emerging model of AD pathogenesis.34 Future studies involving longitudinal data will potentially allow further investigation of the utility of odor identification testing in tracking the progression from CN to MCI to AD dementia. This research will further investigate the potential for odor identification tests such as the UPSIT to serve as cost-effective screening tools for preclinical AD, or at least as contributing factors in the diagnostic workup of this condition.
Supplementary Material
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- AmNART
American National Adult Reading Test
- CN
clinically normal
- DVR
distribution volume ratio
- EC
entorhinal cortex
- HV
hippocampal volume
- MCI
mild cognitive impairment
- NFT
neurofibrillary tangle
- PiB
Pittsburgh compound B
- UPSIT-40
40-item University of Pennsylvania Smell Identification Test
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Matthew E. Growdon: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis. Aaron P. Schultz: analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, statistical analysis. Alexander S. Dagley: analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, study supervision. Rebecca E. Amariglio: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval. Trey Hedden: study concept or design, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision. Dorene M. Rentz: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision. Keith A. Johnson: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and will give final approval, contribution of vital reagents/tools/patients, acquisition of data, statistical analysis, study supervision, obtaining funding. Reisa A. Sperling: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision, obtaining funding. Mark W. Albers: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, study supervision. Gad A. Marshall: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data.
STUDY FUNDING
This study was supported by the Harvard Aging Brain Study (P01 AGO36694, R01 AG037497), R01 AG027435, K23 AG033634, K24 AG035007, the Massachusetts Alzheimer's Disease Research Center (P50 AG005134), and DP2 OD006662.
DISCLOSURE
M. Growdon, A. Schultz, and A. Dagley report no disclosures relevant to the manuscript. R. Amariglio has received research salary support from Janssen Alzheimer Immunotherapy and Wyeth/Pfizer Pharmaceuticals. T. Hedden and D. Rentz report no disclosures relevant to the manuscript. K. Johnson has received research salary support from Avid Radiopharmaceuticals. R. Sperling has received research salary support from Bristol-Myers Squibb. M. Albers has received research salary support from Merck. G. Marshall has received research salary support from Janssen Alzheimer Immunotherapy, Wyeth/Pfizer Pharmaceuticals, Eisai Inc., and Eli Lilly and Company. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Doty RL, Reyes PF, Gregor T. Presence of both odor identification and detection deficits in Alzheimer's disease. Brain Res Bull 1987;18:597–600. [DOI] [PubMed] [Google Scholar]
- 2.Devanand DP, Lee S, Manly J, et al. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 2015;84:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devanand DP, Liu X, Tabert MH, et al. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer's disease. Biol Psychiatry 2008;64:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serby M, Larson P, Kalkstein D. The nature and course of olfactory deficits in Alzheimer's disease. Am J Psychiatry 1991;148:357–360. [DOI] [PubMed] [Google Scholar]
- 5.Kovács T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer's disease: olfactory bulb is involved in early Braak's stages. Neuroreport 2001;12:285–288. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer's disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry 2007;78:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao L, Schrank BR, Rodriguez S, et al. Aβ alters the connectivity of olfactory neurons in the absence of amyloid plaques in vivo. Nat Commun 2012;3:1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol 2013;12:357–367. [DOI] [PubMed] [Google Scholar]
- 9.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association Workgroups on Diagnostic Guidelines for Alzheimer's Disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984;32:489–502. [DOI] [PubMed] [Google Scholar]
- 11.Tabert MH, Liu X, Doty RL, et al. A 10-item smell identification scale related to risk for Alzheimer's disease. Ann Neurol 2005;58:155–160. [DOI] [PubMed] [Google Scholar]
- 12.Devanand DP, Tabert MH, Cuasay K, et al. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging 2010;31:1593–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahar-Fuchs A, Chételat G, Villemagne VL, et al. Olfactory deficits and amyloid-β burden in Alzheimer's disease, mild cognitive impairment, and healthy aging: a PiB PET study. J Alzheimers Dis 2010;22:1081–1087. [DOI] [PubMed] [Google Scholar]
- 14.Hedden T, Mormino EC, Amariglio RE, et al. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci 2012;32:16233–16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mormino EC, Betensky RA, Hedden T, et al. Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology 2014;82:1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 17.Mungas D, Marshall SC, Weldon M, Haan M, Reed BR. Age and education correction of Mini-Mental State Examination for English and Spanish-speaking elderly. Neurology 1996;46:700–706. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. WMS-R: Wechsler Memory Scale—Revised Manual. New York: Psychological Corp., Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 20.Weiner MW, Veitch DP, Aisen PS, et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement 2012;8:S1–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17:37–49. [DOI] [PubMed] [Google Scholar]
- 22.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol 1980;7:486–488. [DOI] [PubMed] [Google Scholar]
- 23.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 1984;94:176–178. [DOI] [PubMed] [Google Scholar]
- 24.Nelson HE, O'Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex 1978;14:234–244. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 26.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 2004;55:306–319. [DOI] [PubMed] [Google Scholar]
- 27.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology 2008;71:903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker JA, Hedden T, Carmasin J, et al. Amyloid-β associated cortical thinning in clinically normal elderly. Ann Neurol 2011;69:1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab 2005;25:1528–1547. [DOI] [PubMed] [Google Scholar]
- 30.Savva GM, Wharton SB, Ince PG, et al. Age, neuropathology, and dementia. N Engl J Med 2009;360:2302–2309. [DOI] [PubMed] [Google Scholar]
- 31.Albers MW, Tabert MH, Devanand DP. Olfactory dysfunction as a predictor of neurodegenerative disease. Curr Neurol Neurosci Rep 2006;6:379–386. [DOI] [PubMed] [Google Scholar]
- 32.Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer's disease. Ann NY Acad Sci 2009;1170:730–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement 2015;11:70–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knopman DS. β-Amyloidosis and neurodegeneration in Alzheimer disease: who's on first? Neurology 2014;82:1756–1757. [DOI] [PubMed] [Google Scholar]
- 35.Boyle PA, Wilson RS, Yu L, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol 2013;74:478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graves AB, Bowen JD, Rajaram L, et al. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology 1999;53:1480–1487. [DOI] [PubMed] [Google Scholar]
- 37.Schubert CR, Carmichael LL, Murphy C, Klein BE, Klein R, Cruickshanks KJ. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc 2008;56:1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun GH, Raji CA, Maceachern MP, Burke JF. Olfactory identification testing as a predictor of the development of Alzheimer's dementia: a systematic review. Laryngoscope 2012;122:1455–1462. [DOI] [PubMed] [Google Scholar]
- 39.Bahar-Fuchs A, Moss S, Rowe C, Savage G. Awareness of olfactory deficits in healthy aging, amnestic mild cognitive impairment and Alzheimer's disease. Int Psychogeriatr 2011;23:1097–1106. [DOI] [PubMed] [Google Scholar]
- 40.Williams SS, Williams J, Combrinck M, Christie S, Smith AD, McShane R. Olfactory impairment is more marked in patients with mild dementia with Lewy bodies than those with mild Alzheimer disease. J Neurol Neurosurg Psychiatry 2009;80:667–670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.