Abstract
Purpose
Arthritis and bone trauma are often accompanied by bone marrow edema (BME). BME is challenging to detect in CT due to the overlaying trabecular structure but can be visualized using dual-energy (DE) techniques to discriminate water and fat. We investigate the feasibility of DE imaging of BME on a dedicated flat-panel detector (FPD) extremities cone-beam CT (CBCT) with a unique x-ray tube with three longitudinally mounted sources.
Methods
Simulations involved a digital BME knee phantom imaged with a 60 kVp low-energy beam (LE) and 105 kVp high-energy beam (HE) (+0.25 mm Ag filter). Experiments were also performed on a test-bench with a Varian 4030CB FPD using the same beam energies as the simulation study. A three-source configuration was implemented with x-ray sources distributed along the longitudinal axis and DE CBCT acquisition in which the superior and inferior sources operate at HE (and collect half of the projection angles each) and the central source operates at LE. Three-source DE CBCT was compared to a double-scan, single-source orbit. Experiments were performed with a wrist phantom containing a 50 mg/ml densitometry insert submerged in alcohol (simulating fat) with drilled trabeculae down to ~1 mm to emulate the trabecular matrix. Reconstruction-based three-material decomposition of fat, soft tissue, and bone was performed.
Results
For a low-dose scan (36 mAs in the HE and LE data), DE CBCT achieved combined accuracy of ~0.80 for a pattern of BME spherical lesions ranging 2.5 – 10 mm diameter in the knee phantom. The accuracy increased to ~0.90 for a 360 mAs scan. Excellent DE discrimination of the base materials was achieved in the experiments. Approximately 80% of the alcohol (fat) voxels in the trabecular phantom was properly identified both for single and 3-source acquisitions, indicating the ability to detect edemous tissue (water-equivalent plastic in the body of the densitometry insert) from the fat inside the trabecular matrix (emulating normal trabecular bone with significant fraction of yellow marrow).
Conclusion
Detection of BME and quantification of water and fat content were achieved in extremities DE CBCT with a longitudinal configuration of sources providing DE imaging in a single gantry rotation. The findings support the development of DE imaging capability for CBCT of the extremities in areas conventionally in the domain of MRI.
Keywords: cone-beam CT, extremities imaging, dual-energy imaging, bone marrow edema, bone mineral density
INTRODUCTION
Bone marrow edema (BME) is a biomarker of arthritis (e.g., as a component in the rheumatoid arthritis MRI score, RAMRIS), occult fractures and other bone trauma, and bone infarcts and metastases. BME forms lesions of decreased fat (yellow marrow) content and increased fluid (soft-tissue-like) content in cancellous bone. The capability for evaluation of bone marrow lesions would be of particular value for recently introduced dedicated flat-panel detector (FPD) cone-beam CT (CBCT) systems for musculoskeletal imaging [1,2], such as the system undergoing clinical evaluation in our institution and shown in Fig. 1 (A)–(B). Owing to the low radiation dose, simplified patient workflow, and high spatial resolution [e.g. excellent visualization of bone erosions in Fig. 1 (C)], extremities CBCT provides an attractive platform for longitudinal imaging of arthritis and fracture healing, where monitoring of bone marrow edema would be especially valuable.
Figure 1.
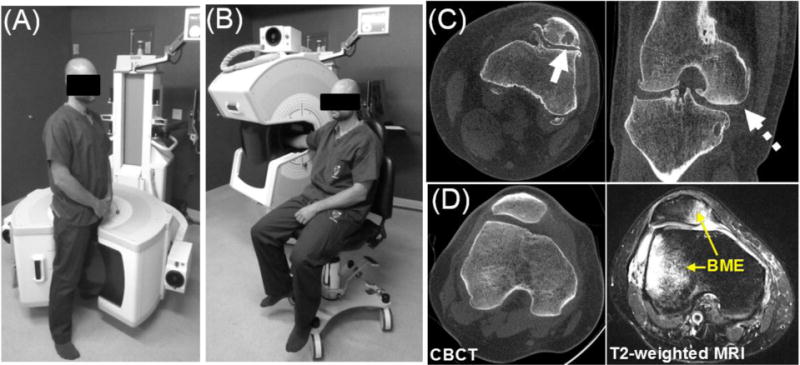
(A–B) The 2nd generation prototype for extremity CBCT imaging. (C) The system provides high spatial resolution enabling detailed visualization of bone erosions (often associated with arthritis, solid arrow) and optionally employs an innovative 3-source x-ray tube for reduction of cone-beam artifacts (e.g., reduced artifacts at the tibial plateau, dashed arrow). (D) Images of a patient with BME in extremities CBCT and in MRI.
Conventionally diagnosed in MRI, BME is challenging to detect in x-ray CT due to low subject contrast and partial-volume effect from the surrounding trabecular matrix [Fig. 1 (D)]. Recently, dual-energy (DE) CT was evaluated for BME with encouraging results [3] using a Virtual Non-Calcium technique similar to the Virtual Non-Contrast imaging known from other DE CT applications. We report the translation of this methodology to dedicated extremities CBCT (Fig. 1). Compared to the previously investigated DE CBCT of iodine enhancement in the joints [4], DE evaluation of BME involves a more challenging task of discrimination of endogenous materials – i.e., fat, soft-tissue, and bone (calcium). The performance of DE CBCT in this scenario was investigated in realistic simulation studies with anatomical phantoms and in test-bench experiments. The 2nd generation CBCT prototype of Fig. 1 was equipped with an innovative configuration of three sources arranged in a vertical (longitudinal) configuration for extended field-of-view and reduction of cone-beam artifacts [Fig. 1 (B)]. We experimentally evaluated this source configuration for single-rotation DE imaging without the need for a filter wheel by operating the three sources at different energy and filtration.
METHODS
2.1 Simulation Studies
Initial investigation of BME detection involved a computer simulation study with a digital BME knee phantom to assess baseline DE CBCT performance in a realistic anatomical context. The phantom was developed from a high-resolution (0.125 mm isotropic voxels) CBCT image of a knee. The reconstruction was segmented into soft tissue, fat, and bone (Fig. 2), and volume fraction of each material was assigned to the voxels. In particular, the voxels within the cortical boundaries of patella, tibia, and femur were represented as a mixture of fat and cortical bone (with no soft tissue allowed) emulating the composition of trabecular bone; the volume fractions of the base materials were determined from the attenuation values. Subsequently, regions of edema were added by replacing the fat in a region of trabecular bone with soft tissue, as indicated in the soft tissue image of Fig. 2. In addition to the diffuse BME lesions in the femur and patella, a contrast-detail soft-tissue pattern consisting of 4 spheres of 2.5 – 10 mm diameter separated by 3.75 mm was also included in the femur. Polyenergetic projections with realistic FPD response (assuming ~0.5 mm CsI:Tl) were computed using x-ray spectra and material attenuation data provided in the Spektr package [5]. The high-energy (HE) beam was 105 kVp (+0.2 mm Cu, +0.25 mm Ag), and the low energy beam was 60 kVp (+0.2 mm Cu), selected to fall within the power limits of the extremities CBCT x-ray source. The bare-beam photon fluence for the HE and LE beams was varied from 104–105 x-rays/pixel. Based on the estimates obtained from Spektr, a fluence of 104 x-rays/pixel corresponds to ~0.14 mAs exposure for the LE beam and ~0.08 mAs exposure for the HE beam. Voxel size was 0.125 mm, and detector pixel size was 0.388 mm. 360 projections were simulated at 1° increments. The geometry of the scanner in Fig. 1 was used: source-axis distance (SAD) of ~440 mm and source-detector-distance of SDD ~560 mm. The focus of this study was baseline performance of DE CBCT in BME imaging, so only a double-scan, single-source acquisition was considered. Simulated projections were reconstructed with FBP with a Hann apodizer onto 0.25 mm voxels, providing a realistic approximation of partial volume averaging effects and the low subject contrast of endogenous tissues of interest in BME.
Figure 2.
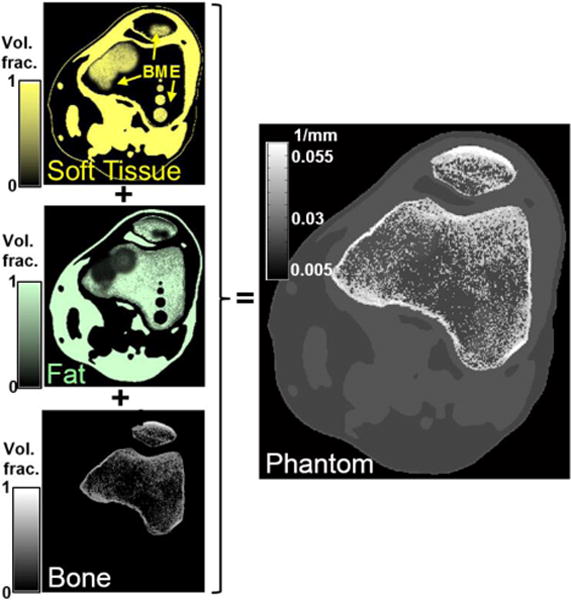
Digital BME phantom. Volume fractions of base materials (yellow: soft tissue, green: fat, white: cortical bone) were obtained from a CBCT volume. Trabecular bone was represented as a mixture of bone and fat. BME was simulated by replacing fat with soft-tissue.
2.3 Test Bench Investigations and Multi-Source DE CBCT
Experimental studies involved a test-bench [Fig. 3 (A)] configured to replicate the geometry of the extremities CBCT system, with the same SAD and SDD as in the simulation study and the same LE and HE x-ray spectra (60 kVp and 105 kVp + 0.25 mm Ag). The HE data was acquired at 0.2 mAs/frame, and the LE data at 0.5 mAs/frame. The FPD was a PaxScan 4030CB (Varian Imaging Products, Palo Alto, CA) with a ~0.5 mm CsI:Tl operated with 2×2 pixel binning (0.388 mm pixel pitch). Projections were acquired at 1° increments with a 10:1 antiscatter grid (Jungwon Precision Industrial, South Korea). In addition to a single source, double-scan trajectory (360 frames in the LE and HE data), a single-scan configuration with 3 longitudinally arranged sources was implemented [Fig. 3 (B)]. The proposed scanning protocol involves LE data acquired with the source located in the central axial plane (source positon “2” in Fig. 3) and collecting full rotation at 1° steps. The inferior and superior sources (positions “1” and “3” in Fig. 3) were offset by +/− 6 cm from the axial plane and collected the HE data, firing alternately in between the LE exposures at 1° steps, as shown in Fig. 3 (B). During the complete scan, 180 HE frames were acquired with each of the peripheral sources. The total dose in the HE scan was the same as for the HE data in a single source, doublescan scenario. The 3-source system was emulated by vertical translation of the x-ray tube for the acquisition of each of the LE and HE datasets. Reconstructions were performed with FBP with a Ramp filter on a 0.25 mm voxel grid. The HE volume of the three-source data was obtained by averaging the HE1 and HE3 reconstructions. Equal weighting of the two analytically reconstructed volumes is assumed to be appropriate in the regions adjacent to the central slice because of the symmetric arrangement of the sources (and their ray paths) about the central plane. Ongoing work involves application of model-based iterative reconstruction to jointly reconstruct the data from HE1 and HE3 by means of a forward model combining the projections measured by both sources. Experimental studies involved a “wrist” phantom (~9 cm × 5 cm) with two bone densitometry inserts (75 mg/mL and 150 mg/mL calcium hydroxyapatite CaHA) and ~3 cm diameter vial of alcohol (simulating fat) with a submerged bone mimicking insert (50 mg/mL calcium carbonate CaCO3) containing a pattern of 1 – 4 mm cavities filled with the surrounding alcohol to emulate the mixture and bone and fat in the trabecular matrix [Fig. 3(C)].
Figure 3.
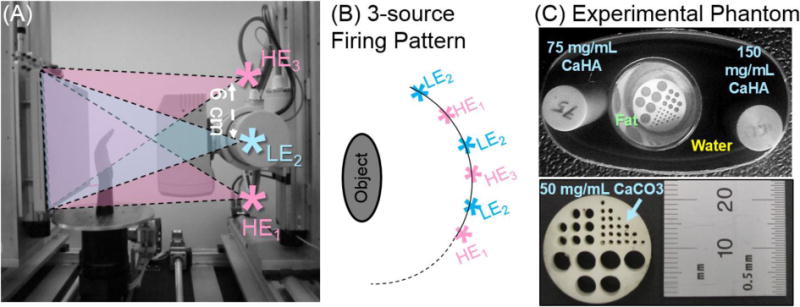
(A) Experimental CBCT bench emulating the 3-source CBCT system, with the HE data acquired as a combination of exposures with the source in the inferior and superior positions (HE1 and HE3), and the LE data collected with the source in the central axial position (LE2). (B) Example firing pattern of the three sources. (C) The 3-material phantom, with a bone cavity matrix submerged in alcohol (simulating fat) to emulate the trabecular bone.
2.1 Dual-Energy Decomposition, Calibration, and Performance Evaluation
Three-material DE decomposition was obtained from the pairs of high and low energy reconstructions by solving the well known system of linear DE equations for volume fractions of soft tissue, fat, and bone in each voxel combined with the constraint requiring the fractions of the three base materials to add to unity. A linear least squares method with a positivity constraint was employed. The attenuation of base materials in the simulation study were estimated from separate reconstructions of a cylindrical calibration phantom. In the experimental study, the attenuation of fat (alcohol) and soft-tissue (water) were computed from regions-of-interest (ROIs) placed in the LE and HE reconstructions (similar to what can be done in patients using known regions of fat and soft-tissue). The LE and HE attenuation coefficients of (pure) Calcium were also computed from reconstruction ROIs by examining the slope of the line defined by measured attenuation of water and the 150 mg/mL bone densitometry insert. Future studies will investigate calibration integrated in the scanner gantry as shown earlier for CBCT bone densitometry [6].
The quality of DE decomposition in the simulation study was evaluated using metrics of statistical decision theory (binary hypothesis tests). Elliptical ROIs were defined around each of the circular regions in the BME contrast-detail pattern. The ROIs contained equal number of voxels with a soft tissue fraction >50% (positive population) and voxels containing no soft-tissues. The true positive (TP) voxels where those that belonged to the positive population and were estimated by DE as having >30% fraction of soft-tissue. (Note that this fraction can potentially serve as a decision threshold in ROC-type studies.) True negatives (TN) corresponded to voxels with <30% soft-tissue fractions that indeed belonged to the negative population. Overall performance of the decomposition is reported as accuracy [Acc=(Sensitivity+Specificity)/2] computed jointly over all ROIs in the contrast-detail BME pattern (Acccomb).
RESULTS AND BREAKTHROUGH WORK
Fig. 4 (A) illustrates a composite FBP reconstruction (equally weighted sum of the LE and HE volumes) of the BME phantom, demonstrating the difficulty in detecting BME from the surrounding trabecular context. In Fig. 4 (B), soft-tissue volume fractions obtained with dual energy decomposition from pairs of LE and HE images computed with a variable FBP cutoff (equal for LE and HE) are overlaid on the composite image. The large, diffuse BME lesion on the left of the femur is readily identifiable in the DE decomposition for both dose levels (top and bottom rows), especially at lower cutoff values. The contrast-detail BME pattern visibility is hampered at very low cutoff frequencies by the reduction of the apparent soft-tissue fraction due to blur (reduced TP fraction) and for high cutoff frequencies due to noise (reduced TP fraction and increasing the fraction of false positives). As shown in Fig. 4 (C), optimal filtration at the FBP kernel cutoff of 0.26 mm−1 yielded accuracy of ~80% at the lower imaging dose (~36 mAs for both beams, solid black line), where the two largest contrast-detail inserts are distinguishable [Fig. 4 (B), top row]. At the ~10× increased dose (magenta dashed line), the optimal FBP cutoff increases to 0.89 mm−1 yielding Acccomb=0.89. The corresponding decomposition [bottom row in Fig. 4 (B)] clearly identifies the inserts down to the smallest diameter of 2.5 mm.
Figure 4.
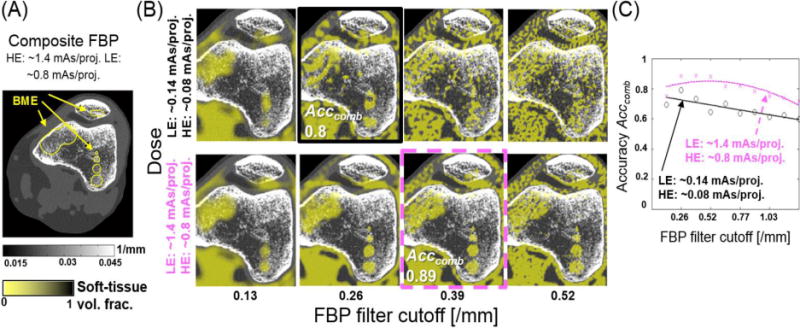
(A) Composite image of the BME phantom (sum of equally weighted low and high energy reconstructions). Note the difficulty in identifying bone marrow edema in single energy CBCT. (B) Dual Energy estimate of the soft tissue fraction superimposed on the composite image for two levels of dose and various settings of reconstruction filter. BME is readily identifiable in the DE decomposition as areas of soft tissue within trabecular bone. (C) Combined accuracy of the bone marrow edema contrast-detail pattern (4 soft-tissue spheres in the right femoral condyle).
Fig. 5 summarizes the results of the experimental study. On the left, a composite volume obtained from the LE and HE reconstructions acquired with the three axial source configuration in the alternating firing pattern of Fig. 3 is compared to the results of DE decomposition of the same projection data. The soft tissue (water) image does not show significant enhancement in the regions filled with alcohol (fat), but correctly indicates the presence of water in the bone densitometry inserts, where soft-tissue-like materials indeed form the bulk of the volume. Conversely, no fat is found in the densitometry rods, in agreement with their chemical composition. The alcohol-filled vial is correctly identified. The Ca image shows enhancement mainly in the densitometry inserts and reflects the difference in CaHA concentration in the two densitometry standards.
Figure 5.
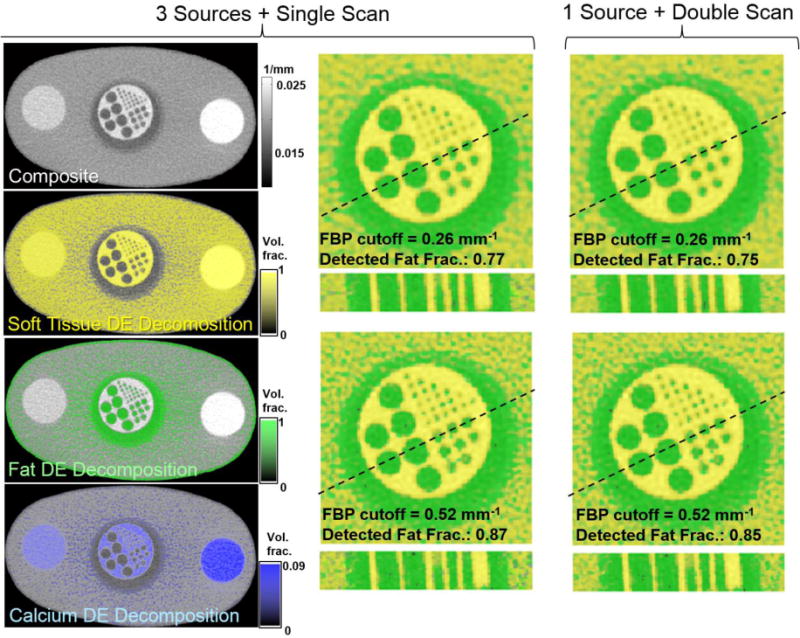
Experimental results. Left: composite image and DE material maps acquired using the 3-source configuration. Excellent discrimination of calcium, water (soft-tissue), and alcohol (fat) is achieved. Magnified view of the bone cavity insert submerged in alcohol is shown with overlays of fat (green) and soft-tissue (yellow) DE maps. The insert is compared for the 3-source and single source acquisitions (center and right) and for two values of the FBP cutoff. An example 6 mm thick oblique trans-axial cut is shown beneath each axial image.
Magnified view of the alcohol-filled vial (an axial slice and an oblique ~6 mm-thick trans-axial cut) is shown for two values of the reconstruction filter and for both the 3-source DE acquisition and for the more conventional single-source, double scan DE acquisition. Quality of the decomposition is assessed as the ratio of the total volume of voxels identified as fat inside the bone cavity insert to the volume expected for the design of the insert. There is almost no perceivable difference between the 3-source and single-source DE decompositions, with both acquisition patterns yielding a ~75% detected fat fraction at the cutoff of 0.26 mm1 (where blur reduces decomposition performance for the 1 mm inserts) and ~85% detected fat fraction at the cutoff of 0.52 mm−1.
CONCLUSIONS
The results indicate that DE CBCT imaging could allow detection of bone marrow edema in trabecular bone. A novel three-source configuration was investigated to permit single scan acquisition of the dual energy data without loss in decomposition quality compared to a double-scan, single-source acquisition. The longitudinal three-source configuration extends the field of view, reduces cone-beam artifacts, and permits faster DE CBCT image acquisition while maintaining the ability to form an equivalent composite single-energy CBCT image.
The proposed DE CBCT imaging method allowed quantification of the volume fractions of the three major components of bone – i.e. yellow marrow (fat), cortical bone, and red marrow (soft-tissue-like). This suggests potential applications beyond detection of BME, including assessment of bone composition and bone mineral density in osteoporosis and arthritis. Dual-energy CBCT discrimination of endogenous materials will support the application of extremities CBCT as a powerful diagnostic tool for quantitative evaluation of bone and joint health.
Acknowledgments
This research was conducted with support from the National Institutes of Health R01-CA-112163, R21-AR-062293, and R01-EB-018896-01 and academic-industry research collaboration with Carestream Health (Rochester NY).
Work supported by NIH Grant No. 2R01-CA-112163, R01-EB-018896-01, R21-AR-062293, and Carestream Health.
References
- 1.Carrino JA, Muhit AA, Zbijewski W, Thawait GK, Stayman JW, Packard N, Senn R, Yang D, Foos DH, Yorkston J, Siewerdsen JH. Dedicated Cone-Beam CT System for Extremity Imaging. Radiology. 2014;270(3):816–24. doi: 10.1148/radiol.13130225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuominen EK, Kankare J, Koskinen SK, Mattila KT. Weight-bearing CT imaging of the lower extremity. AJR Am J Roentgenol. 2013;200(1):146–148. doi: 10.2214/AJR.12.8481. [DOI] [PubMed] [Google Scholar]
- 3.Pache G, Krauss B, Strohm P, Saueressig U, Blanke P, Bulla S, Schäfer O, Helwig P, Kotter E, Langer M, Baumann T. Dual-energy CT virtual noncalcium technique: detecting posttraumatic bone marrow lesions–feasibility study. Radiology. 2010;256(2):617–24. doi: 10.1148/radiol.10091230. [DOI] [PubMed] [Google Scholar]
- 4.Zbijewski W, Gang GJ, Xu J, Wang AS, Stayman JW, Taguchi K, Carrino JA, Siewerdsen JH. Dual-energy cone-beam CT with a flat-panel detector: effect of reconstruction algorithm on material classification. Med Phys. 2014;41(2):021908. doi: 10.1118/1.4863598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siewerdsen JH, Waese AM, Moseley DJ, Richard S, Jaffray DA. Spektr: a computational tool for x-ray spectral analysis and imaging system optimization. Med Phys. 2004;31(11):3057–67. doi: 10.1118/1.1758350. [DOI] [PubMed] [Google Scholar]
- 6.Muhit AA, Arora S, Ogawa M, Ding Y, Zbijewski W, Stayman JW, Thawait G, Packard N, Senn R, Yang D, Yorkston J, Bingham CO, 3rd, Means K, Carrino JA, Siewerdsen JH. Peripheral quantitative CT (pQCT) using a dedicated extremity cone-beam CT scanner. Proc Soc Photo Opt Instrum Eng. 2013;8672:867203. doi: 10.1117/12.2006939. [DOI] [PMC free article] [PubMed] [Google Scholar]


