Abstract
Acid-sensing pathways, which trigger mucosal defense mechanisms in response to luminal acid, involve the rapid afferent-mediated ‘capsaicin pathway’ and the sustained, ‘prostaglandin (PG) pathway’. Luminal acid quickly increases protective PG synthesis and release from epithelia, although the mechanism by which luminal acid induces PG synthesis is still mostly unknown. Acid exposure augments purinergic ATP-P2Y signaling by inhibition of intestinal alkaline phosphatase (IAP) activity. Since P2Y activation increases intracellular Ca2+, we further hypothesized that ATP-P2Y signals increase the generation of H2O2 derived from dual oxidase (Duox), a member of the NADPH oxidase family activated by Ca2+. Our recent studies suggest that acid exposure increases H2O2 output, followed by phospholipase A2 (PLA2) and cyclooxygenase (COX) activation, increasing PG synthesis. Released PGE2 augments protective HCO3− and mucus secretion via EP4 receptor activation. Thus, the PG pathway as a component of duodenal acid sensing consists of acid-related IAP inhibition, ATP-P2Y signals, Duox2-derived H2O2 production, PLA2 activation, PGE2 synthesis and EP4 receptor activation. The PG pathway is also involved in luminal bacterial sensing in the duodenum via activation of pattern recognition receptors, including Toll-like receptors (TLRs) and NOD2. The presence of acute mucosal responses to luminal bacteria suggests that the duodenum is important for host defenses and may reduce bacterial loading to the hindgut using H2O2, complementing gastric acidity and anti-bacterial bile acids.
Keywords: acid sensing, ATP-P2Y signal, dual oxidase, bacterial sensing
INTRODUCTION
Prostaglandins (PGs) are a key component of mucosal defense, essential for maintaining the integrity of gastrointestinal (GI) tract. Nonsteroidal anti-inflammatory drugs (NSAIDs), through PG synthesis inhibition, injure the GI mucosa. In this review, we will discuss how foregut chemosensors activate signaling pathways that enhance mucosal defense mechanisms via PG-related mechanisms.
The duodenal mucosa, regularly exposed to gastric acid, and endogenous and exogenous chemicals including nutrients, has a unique luminal chemosensing capacity that enables the mucosa to sense luminal chemicals followed by rapid mucosal responses that protect the mucosa from injury, releasing mediators and hormones that have local and systemic effects 1.
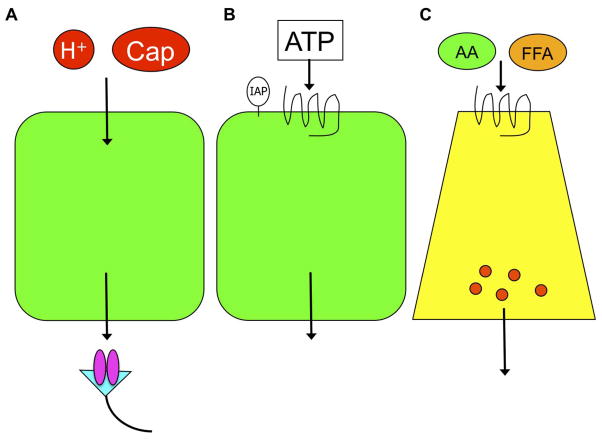
The duodenal mucosa possesses three chemosensing modes (Fig. 1): 1) Luminal chemicals traverse epithelial cells, activating chemosensors expressed on subepithelial afferent nerves (Fig. 1A). This pattern includes luminal CO2/H+ sensing and spice sensing. Luminal CO2 rather than H+ traverses apical membrane of villous cells, acidifies the cytoplasm due to carbonic anhydrase activity, followed by H+ extrusion through basolateral Na+/H+ exchanger-1 (NHE-1), which activates transient receptor potential vanilloid-1 (TRPV-1) expressed on capsaicin-sensitive afferent nerves 2, 3. Luminal capsaicin or the TRP channel ankylin-1 (TRPA1) agonist also shares this pathway 4, 5. 2) Luminal chemicals activate apical chemoreceptors, followed by mediator release from epithelial cells (Fig. 1B). Examples include luminal ATP-P2Y signaling or acid-induced PG release, stimulating protective HCO3− and mucus secretion 6, 7. 3) Luminal chemicals activate G-protein coupled receptors (GPCR) expressed on enteroendocrine cells, followed by mediator or hormone release (Fig. 1C). Examples include luminal nutrient sensing by enteroendocrine cells. We have reported that luminal umami substances such as L-glutamate and 5′-inosine monophosphate (IMP) activate taste receptors expressed on enteroendocrine L cells, which release the incretin glucagon-like peptide-1 (GLP-1) and intestinotrophic GLP-2, the latter stimulating duodenal HCO3− secretion 8, 9.
Fig. 1. Luminal chemosensing patterns in the duodenum.
The scheme shows three patterns of luminal chemosensing, with resulting local mucosal protective responses as well as remote effects via neuronal or hormonal pathway. Luminal acid (H+) or capsaicin (Cap) is transported through the epithelial layer and activates the receptors on afferent nerves (A). Luminal ATP with inhibition of hydrolysis by intestinal alkaline phosphatase (IAP), activates the apical P2Y receptor, exerting epithelial responses (B). Luminal nutrients such as amino acids (AA) or free fatty acids (FFA) activate the corresponding receptors on enteroendocrine cells, releasing gut hormones (C). Adapted from ref 1.
The acid-sensing pathway in duodenum, the most important mucosal defense pathway, mediates PGE2 release in response to luminal acid 10, 11. Released PGE2 then increases epithelial intracellular pH (pHi), HCO3− secretion and mucus output, all important mucosal defense factors to luminal acid 7, 12, 13. How luminal acid increases epithelial PGE2 synthesis, is however still uncertain. Furthermore, whether other luminal stimuli increase PGE2 synthesis and release is also unknown.
Here, we introduce our novel hypothesis that epithelial H2O2 production is related to duodenal acid-induced PGE2 synthesis, a mechanism that can also be extrapolated to luminal bacterial sensing. We will show how the PG pathway is essential for duodenal acid and bacterial sensing, augmenting mucosal and host defense mechanisms.
Duodenal mucosal defense factors
Duodenal defense factors include HCO3− and mucus secretion (pre-epithelial), pHi regulation with ion transporters and ecto- and cytosolic enzyme activities (epithelial), and blood flow regulated via afferent nerves and mediator releases (subepithelial). Rapid changes in these defense factors in response to topical application of luminal chemicals imply the presence of mucosal recognition of luminal chemicals via the pathways depicted in Fig. 1. We have assessed duodenal mucosal defense factors using microscopic mucosal imaging in vivo, enabling the measurement of mucosal defense factors such as mucosal blood flow, mucus secretion, and enterocyte pHi in response to luminal chemicals, in addition to measuring the rate of HCO3− secretion using a duodenal loop perfusion system. These approaches enable us to observe a rapid response to luminal compounds and identify the mechanisms using pharmacological or genetic tools.
Surface pH regulation via purinergic signaling
The second pattern of luminal chemosensing is brush border ecto-enzyme-related signals, including duodenal ATP-P2Y receptors and pH-dependent intestinal alkaline phosphatase (IAP) activity 14, 15 (Fig. 1B). Since the optimal pH of IAP is 8 – 9 and IAP activity is closely correlated to the HCO3− secretory rate 14, IAP may act as a surface pH sensor in the duodenum.
At neutral luminal pH, extracellular ATP, non-lytically released from the epithelial cells is rapidly degraded to adenosine (ADO), which is further degraded to inosine by adenosine deaminase. Once surface pH (pHs) is lowered by gastric acid, surface ATP concentrations increase due to the decreased degradation by IAP or the increased release of ATP, since IAP activity is reduced at acidic pH. Ecto-ATPases, as known as ectonucleoside triphosphate diphosphohydrolases (ENTPDs; CD39 family), and 5′-nucleotidase (5′-NT; CD73) are also involved in the degradation of ATP to ADO. Increased surface ATP concentration stimulates P2Y receptors expressed on the apical membrane of epithelial cells, increasing HCO3− secretion. Increased surface HCO3− concentration increases the pHs, increasing IAP activity, which degrades surface ATP, terminating ATP-P2Y signaling. Luminal ADO additionally increases HCO3− secretion via A2B receptors 16. These studies suggest that IAP acts as a pH sensor, regulating surface ATP concentrations according to its hydrolytic activity, serving a negative feedback loop. The mechanism of ATP-P2Y receptor signaling is implicated in other HCO3−-secreting epithelia such as bile ducts, oviduct and bone 17.
H2O2 production via ATP-P2Y signals in the duodenum
Our recent studies further identify the mechanism underlying ATP-P2Y signal-induced HCO3− secretion 18. We reported that luminal ATP-induced HCO3− secretion was inhibited by pretreatment with the cyclooxygenase (COX) inhibitor indomethacin (IND), suggesting that PG synthesis is a component of ATP-P2Y signaling. Indeed, luminal perfusion of ATP increased HCO3− secretion accompanied by increased mucosal PGE2 content, similar to the response to luminal acid 18. Since P2Y activation increases intracellular Ca2+ concentration 19, Ca2+-sensitive mechanisms may be involved in the ATP-induced PG synthesis.
The NADPH oxidase family includes neutrophil NADPH oxidase (Nox2), which generates superoxide anion, contributing to host defense, but also to tissue damage during inflammation 20. Among NADPH oxidase family members, dual oxidase (Duox) 1 and 2 are known as thyroid oxidases, which generate H2O2 essential for thyroid peroxidase to produce thyroid hormone 21, 22. Duox2 is also localized to the gastrointestinal tract, especially in the apical membrane of epithelial cells 23. Duox has an intracellular Ca2+ binding EF-hand motif, and is activated by intracellular Ca2+ 22. Activated Duox transports an electron from cytosolic NADPH to extracellular O2, generating extracellular H2O2 24. Thus, Duox2 is a candidate for downstream of ATP-P2Y signals in duodenal epithelial cells.
Our study showed that Duox2 was predominantly expressed in rat duodenal mucosa with its accessary protein DuoxA2 18, which localizes Duox2 to plasma membranes 25. Using the H2O2-sensitive fluorogenic compound Amplex® Red, we reported that luminal perfusion of ATP increased H2O2 output accompanied by increased HCO3− secretion 18. ATP-induced HCO3− secretion and H2O2 production were inhibited by co-perfusion of a P2Y receptor antagonist or a NADPH oxidase inhibitor, supporting the hypothesis that ATP-P2Y signals produce H2O2 via NADPH oxidase. Extracellular ATP increases H2O2 production in thyroid cells 26, and airway epithelial cells 27.
How does luminal H2O2 generated by ATP-P2Y signals induce PG synthesis? Previous in vitro studies demonstrate that extracellular H2O2 activates cytosolic phospholipase A2 (cPLA2), determined by radiolabeled arachidonic acid release 28–30, even in the intestinal cell line 31, suggesting that H2O2 activates epithelial PLA2, which generates the COX substrate arachidonic acid. A cPLA2 inhibitor reduced ATP-induced HCO3− secretion with no effect on H2O2 output. Furthermore, IND inhibited ATP-induced HCO3− secretion, whereas H2O2 output was increased. ATP-induced HCO3− secretion was then inhibited by co-perfusion with an EP4 receptor antagonist. These results suggest that H2O2 production by luminal ATP-P2Y signals precede PG synthesis, followed by HCO3− secretion due to EP4 receptor activation. Mucosal PGE2 contents were also increased by luminal perfusion of ATP, reduced by P2Y receptor antagonists, NADPH oxidase inhibitors, or cPLA2 inhibitors, further supporting our hypothesis that ATP-P2Y signal-induced H2O2 production increases PGE2 synthesis, augmenting HCO3− secretion. H2O2 increases electrogenic Cl− secretion via PGE2 synthesis in rat colon 32 and in primary inner medullary collecting duct cells 33, consistent with our results.
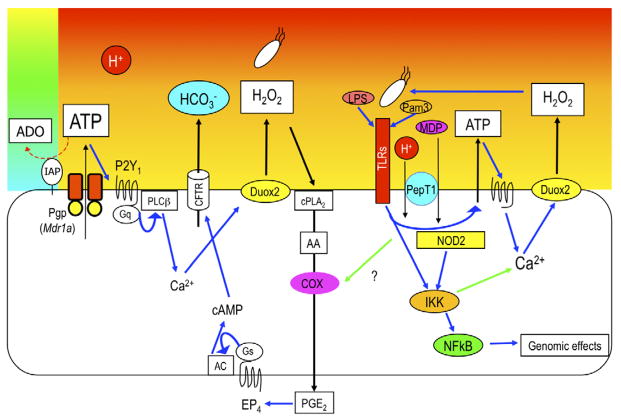
Luminal acid exposure increases HCO3− secretion accompanied by increased H2O2 output into the perfusate, inhibited by co-perfusion of P2Y receptor antagonists or NADPH oxidase inhibitors. Furthermore, acid-induced HCO3− secretion was reduced by inhibition of cPLA2 without affecting H2O2 output. Acid-augmented mucosal PGE2 content was also reduced by these inhibitors, suggesting that the duodenal mucosa exposed to luminal ATP or acid generates H2O2 and PGE2 via the same pathway. Therefore, acid exposure triggers ATP-P2Y signals, which activate Duox2 to generate extracellular H2O2, which activates epithelial cPLA2, which increases PGE2 synthesis via COX, followed by EP4 receptor activation, intracellular cAMP increase, and cystic fibrosis transmembrane conductance regulator (CFTR) activation, augmenting the rate of luminal HCO3− secretion (Fig. 2). This sequential pathway may explain the fundamental question of how luminal acid augments PG synthesis.
Fig. 2. Proposed mechanisms of luminal ATP-P2Y signaling during acid exposure.
Acid exposure increases luminal ATP concentration. ATP stimulates P2Y receptors on the apical membrane of epithelial cells, and then increases intracellular Ca2+ concentration. Ca2+ activates dual oxidase 2 (Duox2), which generates H2O2 in the lumen. H2O2 activates cytosolic phospholipase A2 (cPLA2), followed by cyclooxygenase (COX)-mediated prostaglandin E2 (PGE2) synthesis. PGE2 stimulates EP4 receptor, followed by cystic fibrosis transmembrane conductance regulator (CFTR) stimulation, increasing protective HCO3− secretion. On the other hand, toll-like receptor (TLR) ligand, Pam3 or lipopolysaccharide (LPS), and nucleotide-binding oligomerization domain 2 (NOD2) ligand muramyl dipeptide (MDP) coordinately increases H2O2 production with enhanced ATP release and IkB kinase (IKK) activation. This bacterial sensing pathway also increases PGE2 production and stimulates HCO3− secretion.
Duodenal epithelial cells possess high catalase activity 34–36, which may protect them from self-generated H2O2. Luminal exposure to H2O2 ≤ 0.3 mM dose-dependently increased HCO3− secretion without epithelial injury or increasing mucosal permeability 18, consistent with the effect of H2O2 on rat colonic Cl− secretion 32. In contrast, 0.5 mM H2O2 inhibits cAMP-induced or Ca2+-dependent Cl− secretion in colonic T84 cells37, 38. H2O2 also increases epithelial permeability and cellular toxicity at higher concentration (≥0.5 mM) 39, 40, suggesting that the effect of luminal H2O2 is dependent on whether its concentration is in the physiological or pathological ranges.
Since generation of H2O2 by Duox2 requires sufficient luminal O2, and since activation of HCO3− secretion consumes intracellular ATP, epithelial O2 consumption may be increased during acid exposure. We reported that post-prandial epithelial hypoxia was present in duodenal villous cells, induced by acid exposure, and inhibited by pretreatment with proton pump inhibitor (PPI) or oral catalase 41. Since duodenal hypoxia increases hypoxia-inducible factor-2α (HIF-2α) signaling, enhancing iron absorption 42, 43, and since PPI treatment decreased COX expression in the duodenal mucosa 41, acid exposure may maintain mucosal integrity by inducing villous hypoxia. This mechanism may be implicated in the clinical observation of PPI-induced iron deficiency 44, 45.
Duodenal bacterial sensing
The presence of H2O2 production in the duodenal lumen in response to luminal acid further suggests the presence of bacterial sensing, since H2O2 has anti-bacterial properties in and of itself or combined with other molecules 46, 47. Orally ingested commensal bacteria in the food or drink, or derived from oral flora, may be killed by gastric acid or bile acids in the duodenum 48, 49, possibly explaining why the duodenal lumen is relatively sterile compared with lower small intestinal lumen. Further data obtained from drosophila suggest that Duox may also affect the composition of intestinal microbiota. Drosophila intestine expresses Duox, which generates superoxide anion via Ca2+-sensitive NADPH oxidase activity 50. Knockdown of intestinal Duox in drosophila using siRNA increases mortality due to intestinal bacterial overgrowth 50, suggesting that Duox-mediated intestinal epithelial H2O2 production affects the composition of the luminal microbiome. These data form the basis of the hypothesis that the duodenal mucosa senses luminal bacteria to produce H2O2, which complements gastric acid and bile acids to curb the viability of foregut microbiota.
Bacterial components are recognized by pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) or nucleotide-binding oligomerization domain-containing proteins (NODs) 51, 52. TLRs and NODs, primarily studied in immune cells, are also expressed in intestinal epithelial cells 53, where they are expressed on the apical membrane of villous and Paneth cells 53, 54. These results suggest that the duodenal mucosa may recognize luminal bacteria, generating anti-bacterial H2O2 in response.
When evoking a mucosal response to bacterial components, we observed that TLR ligands or a NOD2 ligand alone had no effect, whereas the combination of a TLR with NOD2 ligands stimulated HCO3− secretion, accompanied by increased H2O2 output and mucosal PGE2 synthesis 55, akin to the mucosal response to luminal acid. Ligands for TLR and NOD2 synergistically increase inflammatory responses in murine macrophages 56, consistent with our results. Although a delayed (hours-days) inflammatory response to TLR or NOD2 activation is well described, presumably due to genomic activation, this is the first description in mammals of an acute epithelial response to luminal bacterial components, reinforcing the notion that PRR sensing mediates rapid anti-bacterial mucosal responses (Fig. 2). Extracellular ATP activates Duox1, mediating airway epithelial pro-inflammatory responses to bacterial stimuli 27, similar to our results.
Clinical relevance of duodenal H2O2 production
Mucosal H2O2 production via Nox1/Duox2 in response to bacterial exposure was reported in human duodenal biopsies 57, suggesting the anti-bacterial activity of duodenal Duox2. Compared with the lower intestine with its abundant flora, the duodenal lumen is ‘clean’ from bacterial residency. It is possible that, in addition to gastric acid and bile acids, toxic to microorganisms, duodenal H2O2 may participate in sterilizing the duodenal lumen. PPI-induced bacterial overgrowth, the presence of which is controversial 58–60, may be also affected by duodenal H2O2 production.
NSAID-induced enteropathy is associated with bacterial translocation 61. PPI pretreatment aggravates NSAID-induced enteropathy by inducing dysbiosis 62, suggesting that disruption of sterility in the foregut lumen by inhibition of PG synthesis combined with gastric acid suppression may also induce bacterial overload in the hindgut lumen, with resultant dysbiosis.
Enteric pathogens such as salmonella, campylobacter, or listeria possess catalase activity. Helicobacter pylori, a unique pathogen limited to the gastric mucosa, also possesses catalase and superoxide dismutase activity, as one explanation for its long-term survival in situ. Pathogenic bacteria can resist the deleterious effects of H2O2, gastric acid and bile acid, whereas some commensal bacteria and eukaryotes such as fungi and yeast are H2O2 sensitive 63, 64. Therefore, relative sterility of the duodenal lumen may be achieved by duodenal epithelial H2O2 production in addition to gastric acid and bile acid toxicity.
The duodenal mucosa also senses luminal nutrients via nutrient sensors in order to rapidly control gastric emptying, bile and pancreatic secretion, and intrinsic mucosal defenses through augmented ion secretion 1, 65. Since luminal bacteria may disrupt nutrient sensors by taking up nutrients or by their metabolites interfering with nutrient detection, Duox2-mediated H2O2 release may repel bacteria from the epithelial surface, enhancing nutrient chemosensing and nutrient-evoked mucosal responses 66. Luminal nutrients may also be important instigators of antibacterial foregut mucosal responses. Duodenal bacterial overload potentiates mucosal secretory responses*, further suggesting that the luminal bacterial environment affects the duodenal physiology.
In conclusion, acid-induced PG synthesis may be mediated by luminal ATP-P2Y signals, Duox2-mediated H2O2 production, and cPLA2 activation, followed by COX activation. Released PGE2 stimulates basolateral EP4 receptors, augmenting protective HCO3− secretion via CFTR activation. This pathway forms one of the most important regulatory schemes coordinating duodenal mucosal defense mechanisms in response to luminal acid. Furthermore, the PG pathway, including anti-bacterial H2O2 production is also an important component of foregut mucosal defenses. Therefore, the duodenal PG pathway not only protects the foregut from mucosal injury, but also contributes to host defenses to luminal dysbiosis.
Acknowledgments
Grant supports
We thank Drs. Masaaki Higashiyama, Izumi Kaji and David Strugatsky for their research contributions, and Bea Palileo for her assistance with manuscript preparation.
Supported by a research grant from Department of Veterans Affairs Merit Review Award (J. Kaunitz), and NIH-NIDDK R01 DK54221 (J. Kaunitz).
Footnotes
Akiba Y, Strugatsky D, Kaji I, Kaunitz JD, unpublished observation
CONFLICT OF INTEREST
None
References
- 1.Akiba Y, Kaunitz JD. Duodenal luminal chemosensing; Acid, ATP, and nutrients. Curr Pharm Des. 2013 doi: 10.2174/13816128113199990565. (in press) [DOI] [PubMed] [Google Scholar]
- 2.Akiba Y, Nakamura M, Nagata H, Kaunitz JD, Ishii H. Acid-sensing pathways in rat gastrointestinal mucosa. J Gastroenterol. 2002;37:133–8. doi: 10.1007/BF03326432. [DOI] [PubMed] [Google Scholar]
- 3.Kaunitz JD, Akiba Y. Acid-sensing protective mechanisms of duodenum. J Physiol Pharmacol. 2003;54:19–26. [PubMed] [Google Scholar]
- 4.Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acid-sensing pathways of rat duodenum. Am J Physiol Gastrointest Liver Physiol. 1999;277:G268–G74. doi: 10.1152/ajpgi.1999.277.2.G268. [DOI] [PubMed] [Google Scholar]
- 5.Akiba Y, Kaunitz JD. Capsiate, a non-pungent capsinoid, enhances mucosal defenses via activation of TRPV1 and TRPA1 in rat duodenum. Gastroenterology. 2011;140:S32. [Google Scholar]
- 6.Kaunitz JD, Akiba Y. Purinergic regulation of duodenal surface pH and ATP concentration: implications for mucosal defence, lipid uptake and cystic fibrosis. Acta Physiol (Oxf) 2011;201:109–16. doi: 10.1111/j.1748-1716.2010.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Sensory pathways and cyclooxygenase regulate mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2001;280:G470–G4. doi: 10.1152/ajpgi.2001.280.3.G470. [DOI] [PubMed] [Google Scholar]
- 8.Akiba Y, Watanabe C, Mizumori M, Kaunitz JD. Luminal L-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am J Physiol Gastrointest Liver Physiol. 2009;297:G781–G91. doi: 10.1152/ajpgi.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JH, Inoue T, Higashiyama M, et al. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther. 2011;339:464–73. doi: 10.1124/jpet.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flemström G, Garner A, Nylander O, Hurst BC, Heylings JR. Surface epithelial HCO3− transport by mammalian duodenum in vivo. Am J Physiol. 1982;243:G348–G58. doi: 10.1152/ajpgi.1982.243.5.G348. [DOI] [PubMed] [Google Scholar]
- 11.Sugamoto S, Kawauch S, Furukawa O, Mimaki TH, Takeuchi K. Role of endogenous nitric oxide and prostaglandin in duodenal bicarbonate response induced by mucosal acidification in rats. Dig Dis Sci. 2001;46:1208–16. doi: 10.1023/a:1010603026913. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi K, Kita K, Hayashi S, Aihara E. Regulatory mechanism of duodenal bicarbonate secretion: Roles of endogenous prostaglandins and nitric oxide. Pharmacol Ther. 2011;130:59–70. doi: 10.1016/j.pharmthera.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Akiba Y, Kaji I, Kaunitz JD. PGE2-induced mucus secretion is mediated via EP3 and EP4 receptors in parallel with bicarbonate secretion in rat duodenum. Gastroenterology. 2013;144:S340. [Google Scholar]
- 14.Akiba Y, Mizumori M, Guth PH, Engel E, Kaunitz JD. Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1223–G33. doi: 10.1152/ajpgi.00313.2007. [DOI] [PubMed] [Google Scholar]
- 15.Mizumori M, Ham M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Intestinal alkaline phosphatase regulates protective surface microclimate pH in rat duodenum. J Physiol. 2009;587:3651–63. doi: 10.1113/jphysiol.2009.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ham M, Mizumori M, Watanabe C, et al. Endogenous luminal surface adenosine signaling regulates duodenal bicarbonate secretion in rats. J Pharmacol Exp Ther. 2010;335:607–13. doi: 10.1124/jpet.110.171520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaunitz JD, Yamaguchi DT. TNAP, TrAP, ecto-purinergic signalling, and bone remodeling. J Cell Biochem. 2008;105:655–62. doi: 10.1002/jcb.21885. [DOI] [PubMed] [Google Scholar]
- 18.Higashiyama M, Akiba Y, Rudenkyy S, Guth PH, Engel E, Kaunitz JD. Dual oxidase: A novel antimicrobial duodenal defense mechanism. Gastroenterology. 2012;142:S489. [Google Scholar]
- 19.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284:F419–F32. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- 20.Rokutan K, Kawahara T, Kuwano Y, Tominaga K, Nishida K, Teshima-Kondo S. Nox enzymes and oxidative stress in the immunopathology of the gastrointestinal tract. Semin Immunopathol. 2008;30:315–27. doi: 10.1007/s00281-008-0124-5. [DOI] [PubMed] [Google Scholar]
- 21.Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem. 1999;274:37265–9. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 22.Ameziane-El-Hassani R, Morand S, Boucher JL, et al. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem. 2005;280:30046–54. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 23.El Hassani RA, Benfares N, Caillou B, et al. Dual oxidase 2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol. 2005;288:G933–G42. doi: 10.1152/ajpgi.00198.2004. [DOI] [PubMed] [Google Scholar]
- 24.Fischer H. Mechanisms and function of DUOX in epithelia of the lung. Antioxid Redox Signal. 2009;11:2453–65. doi: 10.1089/ars.2009.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem. 2006;281:18269–72. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura Y, Ohtaki S. Extracellular ATP-induced production of hydrogen peroxide in porcine thyroid cells. J Endocrinol. 1990;126:283–7. doi: 10.1677/joe.0.1260283. [DOI] [PubMed] [Google Scholar]
- 27.Boots AW, Hristova M, Kasahara DI, Haenen GR, Bast A, van der Vliet A. ATP-mediated activation of the NADPH oxidase DUOX1 mediates airway epithelial responses to bacterial stimuli. J Biol Chem. 2009;284:17858–67. doi: 10.1074/jbc.M809761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J, Chalimoniuk M, Shu Y, et al. Prostaglandin E2 production in astrocytes: regulation by cytokines, extracellular ATP, and oxidative agents. Prostaglandins Leukot Essent Fatty Acids. 2003;69:437–48. doi: 10.1016/j.plefa.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Balboa MA, Balsinde J. Involvement of calcium-independent phospholipase A2 in hydrogen peroxide-induced accumulation of free fatty acids in human U937 cells. J Biol Chem. 2002;277:40384–9. doi: 10.1074/jbc.M206155200. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, Na SI, Heo JS, et al. Arachidonic acid release by H2O2 mediated proliferation of mouse embryonic stem cells: involvement of Ca2+/PKC and MAPKs-induced EGFR transactivation. J Cell Biochem. 2009;106:787–97. doi: 10.1002/jcb.22013. [DOI] [PubMed] [Google Scholar]
- 31.Gustafson C, Lindahl M, Tagesson C. Hydrogen peroxide stimulates phospholipase A2-mediated arachidonic acid release in cultured intestinal epithelial cells (INT 407) Scand J Gastroenterol. 1991;26:237–47. doi: 10.3109/00365529109025037. [DOI] [PubMed] [Google Scholar]
- 32.Karayalcin SS, Sturbaum CW, Wachsman JT, Cha JH, Powell DW. Hydrogen peroxide stimulates rat colonic prostaglandin production and alters electrolyte transport. J Clin Invest. 1990;86:60–8. doi: 10.1172/JCI114715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soodvilai S, Jia Z, Yang T. Hydrogen peroxide stimulates chloride secretion in primary inner medullary collecting duct cells via mPGES-1-derived PGE2. Am J Physiol Renal Physiol. 2007;293:F1571–F6. doi: 10.1152/ajprenal.00132.2007. [DOI] [PubMed] [Google Scholar]
- 34.Connock M, Pover W. Catalase particles in the epithelial cells of the guinea-pig small intestine. Histochem J. 1970;2:371–80. doi: 10.1007/BF01004718. [DOI] [PubMed] [Google Scholar]
- 35.Novikoff PM, Novikoff AB. Peroxisomes in absorptive cells of mammalian small intestine. J Cell Biol. 1972;53:532–60. doi: 10.1083/jcb.53.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stiel D, Murray DJ, Peters TJ. Activities and subcellular localizations of enzymes implicated in gastroduodenal bicarbonate secretion. Am J Physiol. 1984;247:G133–G9. doi: 10.1152/ajpgi.1984.247.2.G133. [DOI] [PubMed] [Google Scholar]
- 37.DuVall MD, Guo Y, Matalon S. Hydrogen peroxide inhibits cAMP-induced Cl− secretion across colonic epithelial cells. Am J Physiol. 1998;275:C1313–C22. doi: 10.1152/ajpcell.1998.275.5.C1313. [DOI] [PubMed] [Google Scholar]
- 38.Chappell AE, Bunz M, Smoll E, et al. Hydrogen peroxide inhibits Ca2+-dependent chloride secretion across colonic epithelial cells via distinct kinase signaling pathways and ion transport proteins. FASEB J. 2008;22:2023–36. doi: 10.1096/fj.07-099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welsh MJ, Shasby DM, Husted RM. Oxidants increase paracellular permeability in a cultured epithelial cell line. J Clin Invest. 1985;76:1155–68. doi: 10.1172/JCI112071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hata Y, Kawabe T, Hiraishi H, Ota S, Terano A, Ivey KJ. Hydrogen peroxide-mediated cytotoxicity to cultured colonic epithelial cells. Life Sci. 1997;60:2221–30. doi: 10.1016/s0024-3205(97)00237-3. [DOI] [PubMed] [Google Scholar]
- 41.Akiba Y, Kaji I, Guth PH, Engel E, Kaunitz JD. Luminal acid-associated post-prandial epithelial hypoxia maintains the expression of iron absorptive DMT1 and DcytB, and cyclooxygenases. Gastroenterology. 2013;144:S10. [Google Scholar]
- 42.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9:152–64. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2α, but not HIF-1α, promotes iron absorption in mice. J Clin Invest. 2009;119:1159–66. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cook JD, Brown GM, Valberg LS. The effect of achylia gastrica on iron absorption. J Clin Invest. 1964;43:1185–91. doi: 10.1172/JCI105002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarzynski E, Puttarajappa C, Xie Y, Grover M, Laird-Fick H. Association between proton pump inhibitor use and anemia: a retrospective cohort study. Dig Dis Sci. 2011;56:2349–53. doi: 10.1007/s10620-011-1589-y. [DOI] [PubMed] [Google Scholar]
- 46.Clifford DP, Repine JE. Hydrogen peroxide mediated killing of bacteria. Mol Cell Biochem. 1982;49:143–9. doi: 10.1007/BF00231175. [DOI] [PubMed] [Google Scholar]
- 47.Thomas EL, Milligan TW, Joyner RE, Jefferson MM. Antibacterial activity of hydrogen peroxide and the lactoperoxidase-hydrogen peroxide-thiocyanate system against oral streptococci. Infect Immun. 1994;62:529–35. doi: 10.1128/iai.62.2.529-535.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giannella RA, Broitman SA, Zamcheck N. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut. 1972;13:251–6. doi: 10.1136/gut.13.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Percy-Robb IW, Collee JG. Bile acids: a pH dependent antibacterial system in the gut? Br Med J. 1972;3:813–5. doi: 10.1136/bmj.3.5830.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–50. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 51.Wells JM, Rossi O, Meijerink M, van BP. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci USA. 2011;108:4607–14. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rietdijk ST, Burwell T, Bertin J, Coyle AJ. Sensing intracellular pathogens-NOD-like receptors. Curr Opin Pharmacol. 2008;8:261–6. doi: 10.1016/j.coph.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Mantani Y, Kamezaki A, Udayanga KG, et al. Site differences of Toll-like receptor expression in the mucous epithelium of rat small intestine. Histol Histopathol. 2011;26:1295–303. doi: 10.14670/HH-26.1295. [DOI] [PubMed] [Google Scholar]
- 54.Ogura Y, Lala S, Xin W, et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut. 2003;52:1591–7. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akiba Y, Higashiyama M, Rudenkyy S, Guth PH, Engel E, Kaunitz JD. Novel nongenomic bacterial component sensing in duodenum via pattern recognition receptors. Gastroenterology. 2012;142:S491. [Google Scholar]
- 56.Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 57.Corcionivoschi N, Alvarez LA, Sharp TH, et al. Mucosal reactive oxygen species decrease virulence by disrupting Campylobacter jejuni phosphotyrosine signaling. Cell Host Microbe. 2012;12:47–59. doi: 10.1016/j.chom.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thorens J, Froehlich F, Schwizer W, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39:54–9. doi: 10.1136/gut.39.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fried M, Siegrist H, Frei R, et al. Duodenal bacterial overgrowth during treatment in outpatients with omeprazole. Gut. 1994;35:23–6. doi: 10.1136/gut.35.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ratuapli SK, Ellington TG, O’Neill MT, et al. Proton pump inhibitor therapy use does not predispose to small intestinal bacterial overgrowth. Am J Gastroenterol. 2012;107:730–5. doi: 10.1038/ajg.2012.4. [DOI] [PubMed] [Google Scholar]
- 61.Takeuchi K, Miyazawa T, Tanaka A, Kato S, Kunikata T. Pathogenic importance of intestinal hypermotility in NSAID-induced small intestinal damage in rats. Digestion. 2002;66:30–41. doi: 10.1159/000064419. [DOI] [PubMed] [Google Scholar]
- 62.Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–22. doi: 10.1053/j.gastro.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 63.Bonvillain RW, Painter RG, Ledet EM, Wang G. Comparisons of resistance of CF and non-CF pathogens to hydrogen peroxide and hypochlorous acid oxidants in vitro. BMC Microbiol. 2011;11:112. doi: 10.1186/1471-2180-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lenander-Lumikari M. Inhibition of Candida albicans by the Peroxidase/SCN−/H2O2 system. Oral Microbiol Immunol. 1992;7:315–20. doi: 10.1111/j.1399-302x.1992.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 65.Dockray GJ. Luminal sensing in the gut: an overview. J Physiol Pharmacol. 2003;54:9–17. [PubMed] [Google Scholar]
- 66.Allaoui A, Botteaux A, Dumont JE, Hoste C, de DX. Dual oxidases and hydrogen peroxide in a complex dialogue between host mucosae and bacteria. Trends Mol Med. 2009;15:571–9. doi: 10.1016/j.molmed.2009.10.003. [DOI] [PubMed] [Google Scholar]