Abstract
OBJECTIVES
Results of animal studies suggest that maternal immune activation during pregnancy causes deficiencies in fetal neurodevelopment. Infectious disease is the most common path to maternal immune activation during pregnancy. The goal of this study was to determine the occurrence of common infections, febrile episodes, and use of antibiotics reported by the mother during pregnancy and the risk for autism spectrum disorder (ASD) and infantile autism in the offspring.
METHODS
We used a population-based cohort consisting of 96 736 children aged 8 to 14 years and born from 1997 to 2003 in Denmark. Information on infection, febrile episodes, and use of antibiotics was self-reported through telephone interviews during pregnancy and early postpartum. Diagnoses of ASD and infantile autism were retrieved from the Danish Psychiatric Central Register; 976 children (1%) from the cohort were diagnosed with ASD.
RESULTS
Overall, we found little evidence that various types of mild common infectious diseases or febrile episodes during pregnancy were associated with ASD/infantile autism. However, our data suggest that maternal influenza infection was associated with a twofold increased risk of infantile autism, prolonged episodes of fever caused a threefold increased risk of infantile autism, and use of various antibiotics during pregnancy were potential risk factors for ASD/infantile autism.
CONCLUSIONS
Our results do not suggest that mild infections, febrile episodes, or use of antibiotics during pregnancy are strong risk factors for ASD/infantile autism. The results may be due to multiple testing; the few positive findings are potential chance findings.
Keywords: antibiotics, autism, autistic disorder, fever, infection, pregnancy
Autism spectrum disorder (ASD) is a serious neurodevelopmental disorder characterized by impairments in the following areas: (1) social skills; (2) communication skills; and/or (3) stereotyped interests/repetitive behavior.1 Infantile autism is a subgroup within ASD and is defined by the presence of abnormal functioning in all 3 areas of autism symptoms as well as symptoms being discernible before 3 years of age.
Results of animal studies suggest that maternal immune activation during pregnancy is associated with deviations in brain development.2–4 Infectious disease is the most common path to maternal immune activation during pregnancy. Several previous studies have investigated infection during pregnancy as a part of a larger assessment of various prenatal factors.5 These studies did not look at specific infections per se but pooled all infections into 1 exposure group and did not differentiate between infections occurring within different trimesters. A meta-analysis of 4 such multivariate case-control studies found an almost twofold increased risk of ASD after any infection during pregnancy. A more recent population-based Swedish study used inpatient hospital register data and found no association between any prenatal infection and ASD.6 In addition, in a previous analysis of Danish hospital-based register data,7 we found no overall association between any prenatal infection and ASD in the child.
To our knowledge, no previous study has investigated the association between self-reported common infections and ASD in the child. The current study is a population-based cohort study using data from the Danish National Birth Cohort (DNBC). We estimated whether different types of common infectious diseases, febrile episodes, or use of antibiotics during pregnancy are associated with diagnosis of ASD and infantile autism in the offspring.
METHODS
Study Population
From 1996 to 2002, a total of 101 033 pregnant women were recruited to the DNBC.8 The women were recruited through their general practitioner at the first prenatal visit. Overall, the participation rate at enrollment was 31% of all pregnant women in Denmark during this time period.9 A total of 96 736 DNBC children ages 8 to 14 years were included in the study (Fig 1). All live-born children in Denmark are assigned a personal identification number10 that is used as a key to individual information in all national registers as well as the DNBC.
FIGURE 1.
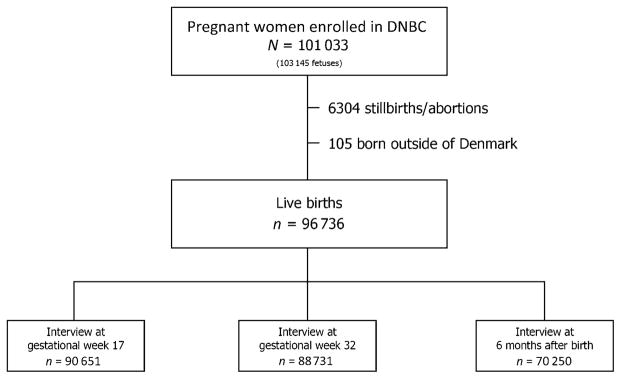
Flow diagram of the number of women recruited to the DNBC, the number of women found eligible for this study, and the number of children whose mother participated in each DNBC interview.
Data on Febrile Episode, Maternal Infection, and Antibiotic Use
The exposure data were collected through telephone interviews with the mother at an average ± SD 17 ± 4 weeks of gestation, 32 ± 3 weeks of gestation, and when the child was 6 ± 1 months of age. However, there were no questions concerning febrile episodes, cough, herpes infections, and venereal warts after gestational week 32; thus, the information on these diseases during the third trimester is incomplete. Figure 1 displays the number of children whose mother participated in each interview. In each interview, the questions were related to a specific time period, and generally the women were asked to specify in which gestational weeks they were affected by the exposure.
Questions regarding infections or febrile episodes were generally formulated as: “Have you had cystitis?” or “Have you had any episode of fever?” The women had the choice of answering “yes,” “no,” “don’t know,” or “don’t wish to answer.” For analyses concerning the whole pregnancy, children were considered exposed if the mother answered yes in 1 or more interviews to have had a specific disease. The reference group was the group of women who answered no to the same question in all relevant interviews. There was no specific question regarding respiratory disease and influenza; when available, information on these infectious diseases was extracted from answers (text strings) to a more general question: “Have you had other infections?”
A question regarding antibiotic use was formulated as: “Have you taken any medication against infection or inflammation (for example, penicillin, sulfa drug, other antibiotics or medicine against fungus)?” Similar to the questions regarding the infections, we excluded from the analysis children with mothers who either chose not to answer or were not sure of the answer. If the woman answered yes to have taken antibiotics, the follow-up question specified 66 different types of antibiotics as well as an option of “other,” in which case the answer was entered as a text variable. A dichotomous variable was created describing whether the woman reported taking any antibiotic. The different types of antibiotics were subdivided into 4 categories: penicillins, macrolides, sulfonamides, and cephalosporins. The questionnaire did not include a question concerning the direct disease indication for the antibiotic use. Table 1 indicates which interview supplied the self-reported information, total number of exposed children and unexposed children, and number of missing values.
TABLE 1.
Infection, Febrile Episodes, and Antibiotic Use During Pregnancy Reported in the DNBC (N = 96 736)
| Factor | Interview Supplying Exposure Informationa | No. (%) of Exposed Children | No. (%) of Unexposed Children | No. (%) of Missing Valuesa |
|---|---|---|---|---|
| Febrile episode | I17gw,b I32gwb | 23 128 (24) | 61 482 (64) | 12 126 (13) |
| Urinary tract infection | I32gw,b I6ppb | 11 559 (12) | 57 017 (59) | 28 160 (29) |
| Cystitis | I32gw,b I6ppb | 11 367 (12) | 57 218 (59) | 28 151 (29) |
| Pyelonephritis | I32gw,b I6ppb | 435 (0) | 65 724 (68) | 30 577 (32) |
| Respiratory tract infection | I32gw,c I6ppc | 7392 (8) | 60 496 (63) | 28 414 (29) |
| Influenza | I32gw,c I6ppc | 808 (1) | 65 496 (68) | 30 432 (31) |
| Cough | I32gwb 14 | 012 (15) | 73 660 (76) | 9064 (9) |
| Vaginal yeast infection | I17gw,b I32gw,b I6ppc | 18 140 (19) | 66 574 (69) | 12 022 (12) |
| Venereal warts | I32gwb | 800 (1) | 86 891 (90) | 9045 (9) |
| Genital herpes | I32gwb | 1386 (1) | 86 295 (89) | 9055 (9) |
| Labial herpes | I32gwb | 10 899 (11) | 76 565 (79) | 9272 (10) |
| Use of any antibiotic | I32gw,b I6ppb | 18 076 (19) | 51 726 (53) | 26 277 (27) |
| Penicillins | I32gw,b,c I6ppb,c | 10 310 (11) | 51 726 (53) | 34 700 (36) |
| Macrolides | I32gw,b,c I6ppb,c | 1531 (2) | 51 726 (53) | 43 479 (45) |
| Tetracycline | I32gw,b,c I6ppb,c | 1193 (1) | 51 726 (53) | 43 479 (45) |
| Sulfonamides | I32gw,b,c I6ppb,c | 2314 (2) | 51 726 (53) | 42 696 (44) |
| Cephalosporin | I32gw,b,c I6ppb,c | 1133 (1) | 51 726 (53) | 43 877 (45) |
I17gw, interview at 17 gestational weeks; I32gw, interview at 32 gestational weeks; I6pp, interview at 6 months’ postpartum.
Missing value due to loss to follow-up, mother chose not to answer question, or mother did not know answer.
Information retrieved from specific questions.
Information retrieved from open-ended questions.
Comparing Maternal-Reported Data and Hospital Data
We were able to investigate, in part, the validity of the DNBC maternal reports of infectious diseases by comparing the mother’s answers in the interviews with the recorded inpatient or outpatient hospital contact of the mother for the same infection during the relevant time period. Data on hospital contact were found in the Danish National Hospital Register11 by identifying diagnoses given at discharge. The DNBC data were validated as follows: if a mother (included in the DNBC) was recorded in the Danish National Hospital Register as having received a primary or secondary discharge diagnosis of a specific infection during the relevant time period, we would expect the mother to have answered yes in the DNBC interview when asked if she had experienced this infection during the same time period. Thus, we calculated an agreement percent defined as the number of DNBC mothers in contact with the hospital for a relevant infection during a specific time period where the mother said yes to having had the disease during that time, divided by the number of DNBC mothers in contact with the hospital for the infection during the relevant time period where the mother said yes or no to having had the disease during that time.
Data on ASD
Diagnoses of ASD were found in the Danish Psychiatric Central Register.12 All diagnoses are assigned by psychiatrists. International Classification of Diseases, 10th Revision diagnoses of ASD included: F84.0 (infantile autism), F84.1, F84.5, F84.8, and F84.9. The positive predictive value of the infantile autism diagnosis found in the DCPR has been reported to be 94%.13 The prevalence of ASD in the Danish register14 is comparable to ASD prevalence observed in the United States15 and a prevalence estimate for ASD observed in a screening study conducted in Denmark.16
Analytical Strategy
The association between various self-reported infections, febrile episodes, and use of antibiotics during pregnancy and ASD in the offspring were studied. If the DNBC questionnaires included information on time of exposure, the associations were stratified according to trimester of exposure. To distinguish the effects of mild self-reported infections from more severe ones, additional analyses were conducted by using proxy measures of disease severity (eg, duration of illness, treatment received) when the relevant data were available.
Adjustments were made for gender, maternal age, parity, and maternal smoking during pregnancy retrieved from the Danish medical birth registry, 17 paternal age retrieved from the Danish civil registration system,10 parental psychiatric history (dichotomous variable indicating whether either parent had a history of psychiatric diagnosis before the birth of the child [International Classification of Diseases, 10th Revision code F00-F99]) retrieved from the Danish Psychiatric Central Register, 12 and parents’ educational status retrieved from the DNBC interview at 17 gestational weeks; the parent with the highest educational status determined to which group the parents were assigned. All covariates were included as categorical variables (Table 2). The missing values were dealt with by using listwise deletion. To adjust for changes in the prevalence of ASD over calendar time, separate baseline diagnostic rate functions were included for birth year groups (1996–1998, 1999–2000, and 2001–2003). Preterm birth is potentially an intermediary variable between maternal infection and ASD; therefore, it was not included in the main analyses as a potential confounder. However, sensitivity analyses were conducted excluding from the analyses all children born before week 37.
TABLE 2.
Characteristics of ASD (n = 976) and Non-ASD (n = 96 736) Children Included in the DNBC and Characteristics of the Background Population (N = 529 465)
| Characteristic | DNBC, ASD, n (%) | DNBC, Non-ASD, n (%) | All Children Born in Denmark, 1996–2003, n (%) |
|---|---|---|---|
| Offspring characteristics | |||
| Gender | |||
| Male | 792 (81.1) | 48 808 (51.0) | 271 898 (51.3) |
| Female | 184 (18.9) | 46 952 (49.0) | 257 567 (48.7) |
| Gestational age, wk | |||
| <37 | 56 (5.7) | 5971 (6.2) | 33 960 (6.4) |
| ≥37 | 916 (93.9) | 89 497 (93.5) | 492 062 (92.9) |
| Missing | 4 (0.4) | 292 (0.3) | 3443 (0.7) |
| Year of birth | |||
| 1996–1998 | 364 (37.3) | 32 643 (34.1) | 201 654 (38.1) |
| 1999–2000 | 432 (44.3) | 41 192 (43.0) | 133 490 (25.2) |
| 2001–2003 | 180 (18.4) | 21 925 (22.9) | 194 321 (36.7) |
| Parental characteristics | |||
| Mother’s age, y | |||
| <21 | 13 (1.3) | 553 (0.6) | 8671 (1.6) |
| 21–25 | 120 (12.3) | 8480 (8.9) | 70 823 (13.4) |
| 26–30 | 344 (35.3) | 36 444 (38.1) | 191 579 (36.2) |
| 31–34 | 344 (35.3) | 35 765 (37.4) | 180 062 (34.0) |
| ≥35 | 155 (15.9) | 14 518 (15.2) | 78 330 (14.8) |
| Father’s age, y | |||
| <26 | 58 (5.9) | 4126 (4.3) | 36 078 (6.8) |
| 26–30 | 258 (26.4) | 25 327 (26.5) | 139 493 (26.4) |
| 31–35 | 360 (36.9) | 37 622 (39.3) | 192 215 (36.3) |
| ≥35 | 291 (29.8) | 28 264 (29.5) | 156 804 (29.6) |
| Missing | 9 (0.9) | 421 (0.4) | 4875 (0.9) |
| Parity | |||
| 1 | 499 (51.1) | 43 497 (45.4) | 222 357 (42.0) |
| 2+ | 447 (45.8) | 49 739 (51.9) | 291 892 (55.1) |
| Missing | 30 (3.1) | 2524 (2.6) | 15 216 (2.9) |
| Parental psychiatric disorder | |||
| Yes | 77 (7.9) | 3688 (3.9) | 22 089 (4.2) |
| No | 899 (92.1) | 92 072 (96.2) | 507 376 (95.8) |
| Maternal smoking during pregnancy | |||
| Yes | 205 (21.0) | 16 258 (17.0) | 114 465 (21.6) |
| No | 734 (75.2) | 76 155 (79.5) | 390 805 (73.8) |
| Missing | 37 (3.8) | 3347 (3.5) | 24 195 (4.6) |
| Parental educational status | |||
| Academic level | 548 (56.2) | 57 126 (59.7) | — |
| Beyond compulsory school | 284 (29.1) | 24 939 (26.0) | — |
| Compulsory school | 45 (4.6) | 3430 (3.6) | — |
| Missing | 99 (10.1) | 10 265 (10.7) | — |
—, data not available.
By use of Cox proportional hazards regression, crude and adjusted hazard ratios (aHRs) were estimated. Age of the child was used as the time scale. The proportional hazards assumption was evaluated by assessing log-minus-log survivor curves. The hazard ratio (HR) may be interpreted as a relative risk because ASD, for this purpose, is a rare disease (ie, prevalence of <10%). Follow-up time ended at the first date of reported ASD diagnosis, death, or on December 31, 2010, whichever came first. Data on death came from the Danish Register of Causes of Death.18 To account for the lack of independence of children within the same family, a robust (Huber-White) variance estimator was used that allowed for clustering of outcomes within a family. To avoid unreliable and uncertain estimates, we only estimated HRs if at least 5 subjects with a diagnosis of ASD had been exposed. No adjustments for multiple comparisons were made.
RESULTS
A total of 976 DNBC children were diagnosed with ASD (1%); 342 had infantile autism (0.4%). Table 2 displays the distribution of confounder characteristics among ASD and non-ASD children. The mean follow-up time was 10 years, and 484 cohort children died during follow-up. The characteristics of the DNBC population were similar to the background population.
We observed no association between specific types of self-reported common infection during pregnancy and ASD in the offspring. The estimates ranged from an aHR of 0.7 (95% confidence interval [CI]: 0.4–1.4) for genital herpes to an aHR of 1.8 (95% CI: 0.9–3.9) for pyelonephritis (Table 3). Also, we observed no increased risk for ASD after common infections at specific trimesters (Table 4). The results were similar for infantile autism. However, we report an increased risk of infantile autism after influenza infection (aHR: 2.3 [95% CI: 1.0–5.3]).
TABLE 3.
aHRs of Offspring Being Diagnosed With ASD or Infantile Autism, Specifically After Self-Reported Infection During Pregnancy
| Type of Infection | ASD
|
Infantile Autism
|
|||
|---|---|---|---|---|---|
| No. Exposed ASD/Non-ASD | Crude HR (95% CI) | aHRa (95% CI) | No. Exposed Infantile Autism/Non–Infantile Autism | aHRa (95% CI) | |
| Pyelonephritis | 9/426 | 2.1 (1.1–4.0) | 1.8 (0.9–3.9) | 4/431 | NE |
| Cystitis | 123/11 244 | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 49/11 318 | 1.2 (0.9–1.7) |
| Respiratory tract infection | 70/7322 | 0.9 (0.7–1.2) | 1.0 (0.7–1.3) | 32/7360 | 1.2 (0.8–1.8) |
| Influenza | 9/799 | 1.1 (0.6–2.1) | 1.1 (0.6–2.3) | 7/801 | 2.3 (1.0–5.3) |
| Coughb | 150/13 862 | 1.1 (0.9–1.3) | 1.0 (0.8–1.2) | 53/13 959 | 1.1 (0.8–1.5) |
| Vaginal yeast infection | 194/17 946 | 1.1 (0.9–1.3) | 1.2 (1.0–1.4) | 56/18 084 | 0.9 (0.7–1.3) |
| Treatment of yeast infectionc | 139/12 646 | 1.1 (0.9–1.3) | 1.2 (1.0–1.4) | 35/12 750 | 0.8 (0.6–1.2) |
| Venereal wartsb | 12/788 | 1.5 (0.9–2.6) | 1.6 (0.9–2.8) | 2/798 | NE |
| Genital herpesb | 14/1372 | 1.0 (0.6–1.7) | 0.7 (0.4–1.4) | 5/1381 | 0.9 (0.3–1.5) |
| Labial herpesb | 105/10 794 | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) | 37/10 862 | 1.0 (0.7–1.5) |
NE, not estimated due to limited number of exposed cases (<5).
Adjusted for maternal age, paternal age, maternal smoking during pregnancy, parity, gender, parents’ educational status, and the parents’ psychiatric condition. Data were analyzed in strata according to year of birth.
Data included only information until week 32 in pregnancy.
Treatment includes suppository, tablets, and cream.
TABLE 4.
aHRs of Offspring Being Diagnosed With ASD After Febrile Episode, Infection, or Use of Antibiotics During Pregnancy Stratified by Trimester
| Variable | First Trimester
|
Second Trimester
|
Third Trimester
|
|||
|---|---|---|---|---|---|---|
| aHRa (95% CI)
|
aHRa (95% CI)
|
aHRa (95% CI)
|
||||
| ASD | Infantile Autism | ASD | Infantile Autism | ASD | Infantile Autism | |
| Febrile episode | 1.1 (0.9–1.4) | 1.2 (0.8–1.7) | 1.0 (0.8–1.3) | 1.4 (1.0–2.0) | NEb | NEb |
| Febrile episode ≥7 d | 1.6 (0.9–2.9) | 2.9 (1.4–6.1) | 1.8 (0.8–3.8) | 4.2 (1.8–9.5) | NEb | NEb |
| Cystitis | 1.0 (0.7–1.5) | 1.1 (0.6–2.0) | 0.8 (0.6–1.2) | 0.8 (0.5–1.5) | 1.1 (0.8–1.5) | 1.4 (0.9–2.2) |
| Pyelonephritis | NEc | NEc | NEc | NEc | 1.8 (0.7–5.0) | 1.3 (0.3–9.2) |
| Respiratory tract infection | 1.3 (0.8–2.2) | 1.3 (0.5–3.1) | 1.0 (0.7–1.5) | 1.3 (0.7–2.2) | 1.0 (0.8–1.3) | 1.3 (0.8–1.8) |
| Cough | 1.1 (0.7–1.7) | 0.8 (0.3–1.8) | 1.1 (0.9–1.4) | 1.2 (0.8–1.8) | NEb | NEb |
| Vaginal yeast infection | 1.0 (0.8–1.3) | 0.7 (0.4–1.1) | 1.2 (0.9–1.4) | 0.9 (0.6–1.4) | 1.2 (1.0–1.6) | 1.2 (0.8–1.8) |
| Use of penicillins | 1.0 (0.6–1.6) | 1.1 (0.5–2.3) | 1.4 (1.1–1.8) | 1.6 (1.0–2.4) | 1.4 (1.0–1.8) | 1.5 (1.0–2.3) |
| Use of sulfonamides | 1.5 (0.7–2.9) | NEc | 1.5 (0.9–2.6) | NEc | 1.4 (0.8–2.6) | NEc |
Adjusted for maternal age, paternal age, maternal smoking during pregnancy, parity, gender, parents’ educational status, and the parents’ psychiatric condition. Data were analyzed in strata by year of birth.
Not estimated (NE) due to incomplete information in third trimester (data included only information until week 32).
NE due to limited number of exposed cases (<5).
No association was observed between febrile episodes before week 32 of pregnancy and ASD/infantile autism when data were analyzed according to the occurrence of a febrile episode in general, number of febrile episodes, highest temperature measured during an episode, symptoms accompanying the febrile episode, or when studying febrile episodes within different trimesters (Tables 4 and 5). However, we observed a statistically significant increased risk of ASD (aHR: 1.6 [95% CI: 1.0–2.5]) and infantile autism (aHR: 3.2 [95% CI: 1.8–5.6]) after febrile episodes lasting ≥7 days.
TABLE 5.
aHRs of Offspring Being Diagnosed With ASD After Febrile Episode Until Week 32 in Pregnancy
| Febrile Episode | ASD
|
Infantile Autism
|
|||
|---|---|---|---|---|---|
| No. Exposed ASD/Non-ASD | Crude HR (95% CI) | aHRa (95% CI) | No. Exposed Infantile Autism/Non–Infantile Autism | aHRa (95% CI) | |
| Febrile episode | 234/22 894 | 1.0 (0.8–1.1) | 1.0 (0.9–1.2) | 101/23 027 | 1.4 (1.0–1.8) |
| No. of episodes | |||||
| 1–3 | 163/15 864 | 1.0 (0.8–1.2) | 1.0 (0.9–1.2) | 93/20 552 | 1.4 (1.1–1.8) |
| ≥4 | 71/7030 | 1.0 (0.7–1.5) | 1.0 (0.6–1.6) | 8/2475 | 0.7 (0.3–2.0) |
| Length of the longest episode, d | |||||
| 1–2 | 140/14 028 | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) | 59/14 109 | 1.3 (0.9–1.8) |
| 3–6 | 73/7282 | 1.0 (0.8–1.2) | 1.0 (0.8–1.3) | 28/7327 | 1.2 (0.8–1.8) |
| ≥7 | 20/1355 | 1.4 (0.9–2.2) | 1.6 (1.0–2.5) | 14/1361 | 3.2 (1.8–5.6) |
| The highest temperature | |||||
| <38.5°C | 33/3280 | 1.0 (0.7–1.4) | 1.1 (0.8–1.6) | 12/3301 | 1.3 (0.7–2.3) |
| ≥38.5°C | 105/10 040 | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) | 47/10 098 | 1.3 (0.9–1.8) |
| Febrile episode with | |||||
| Respiratory symptomsb | 122/10 697 | 1.1 (0.9–1.4) | 1.1 (0.9–1.4) | 55/10 764 | 1.5 (1.1–2.0) |
| Gastrointestinal symptomsc | 65/5371 | 1.2 (0.9–1.5) | 1.2 (0.9–1.6) | 24/5412 | 1.1 (0.7–1.9) |
| General symptomsd | 112/10 658 | 1.0 (0.8–1.2) | 1.0 (0.8–1.3) | 44/10 726 | 1.2 (0.8–1.6) |
Adjusted for maternal age, paternal age, maternal smoking during pregnancy, parity, gender, parents’ educational status, and the parents’ psychiatric condition. Data were analyzed in strata by year of birth.
Include cold, cough, sore throat, and pain in ears.
Include diarrhea, pain in stomach, and vomiting.
Include headache, pain in joints, pain in muscles, pain behind ears, and tiredness.
There was a small increased risk of ASD/infantile autism after the use of various antibiotics. The use of sulfonamides anytime during pregnancy as well as use of penicillin during the second and third trimesters increased the risk of ASD/infantile autism by ~50% (Tables 4 and 6). In addition, the use of macrolides anytime during pregnancy increased the risk for infantile autism in the offspring (aHR: 2.2 [95% CI: 1.1–4.4]) (Table 6).
TABLE 6.
aHRs of Offspring Being Diagnosed With ASD After Self-Reported Use of Antibiotics During Pregnancy
| Use of Antibiotics | ASD
|
Infantile Autism
|
|||
|---|---|---|---|---|---|
| No. Exposed ASD/Non-ASD | Crude HR (95% CI) | aHRa (95% CI) | No. Exposed Infantile Autism/Non–Infantile Autism | aHRa (95% CI) | |
| Any antibiotic | 214/18 519 | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 79/18 654 | 1.2 (0.9–1.7) |
| Penicillins | 121/10 189 | 1.2 (1.0–1.5) | 1.3 (1.0–1.6) | 48/10 262 | 1.4 (1.0–2.0) |
| Macrolides | 17/1514 | 1.3 (0.8–2.1) | 1.3 (0.7–2.2) | 9/1522 | 2.2 (1.1–4.4) |
| Sulfonamides | 34/2280 | 1.5 (1.1–2.2) | 1.5 (1.0–2.2) | 12/2302 | 1.6 (0.9–3.0) |
| Cephalosporins | 12/1121 | 1.3 (0.7–2.3) | 1.1 (0.6–2.3) | 6/1127 | 1.9 (0.8–4.7) |
Adjusted for maternal age, paternal age, maternal smoking during pregnancy, parity, gender, parents’ educational status, and the parents’ psychiatric condition. Data were analyzed in strata according to year of birth.
Restricting the analyses to children born at term (≥37 gestational weeks) did not essentially change the results (data not shown).
The overall agreement between maternal reports of infection episodes and a corresponding hospital contact record was fairly good for most infections for which the women were asked directly: cystitis (65%), pyelonephritis (61%), and vaginal yeast infection (77%). There was a very low agreement between maternal-reported infection and hospital-registered infection when the self-reported information was retrieved from open-ended questions (ie, respiratory infection [6%] and influenza [7%]).
DISCUSSION
In the current study, there was little evidence that self-reported common infections during pregnancy are risk factors for ASD in the child. This lack of association was consistent when studying infectious diseases occurring at specific trimesters. In a previous study, by using Danish hospital data,7 we estimated the risk for ASD after hospital admission for specific prenatal infections. In these previous analyses, we found no association between ASD and maternal respiratory infection, urinary tract infection, or genital infection specifically, which coincides with the current findings by using self-reported data. We reported in our previous study that viral infection during the first trimester gave rise to an almost threefold increased risk of ASD, an association possibly driven by infection with influenza virus. In the current study, we found almost a twofold increased risk of infantile autism in the child after self-reported infection with influenza virus during pregnancy. Infection with influenza virus during the first trimester has been suggested to be associated with development of schizophrenia in the unborn child,19 and animal studies have raised concern that prenatal infection with influenza virus is associated with development of ASD in the offspring.3
We found an approximate threefold increased risk of infantile autism if the mother suffered from a febrile episode lasting >1 week before gestational week 32. We do not know whether a febrile episode in our study is acting as a proxy for a specific infectious illness, specific severity of illness, a specific immune response, or if the direct action of hyperthermia on the fetus is potentially harmful. The association between febrile episodes during pregnancy and ASD could be a coincidental finding caused by multiple testing and requires further study. The relatively long period of fever could, in some cases, be a proxy for influenza.
We observed a small increased risk for ASD and infantile autism after the use of different antibiotics during pregnancy. We do not know whether the antibiotic treatment itself caused the observed association or whether the antibiotic use functioned as a proxy variable for an underlying disease, disease severity, a maternal immune response to a disease, or whether this was a chance finding. The association between antibiotics and autism is a novel finding, which requires confirmation. However, sulfonamides are known folate antagonists, and impaired folate status has been implicated as a risk factor for ASD.20 In addition, periconceptual folate supplementation has been found to be associated with a reduced risk for ASD.21,22
This population-based cohort study included several important strengths: for example, the amount and novelty of data, the availability of hospital records in addition to self-reported data, the prospective data collection, reliable autism diagnoses, and the examination of the associations stratified by trimester. A great limitation of the study is that we made 106 adjusted comparisons; thus, the few statistically significant associations observed could be chance findings. The study is explorative, and we thus made no adjustments for multiple testing. Noteworthy, none of the statistically significant findings would have survived any correction for multiple comparisons. Another main limitation of the study concerns the quality of exposure data, which we elaborate on in the following discussion.
The overall low participation at recruitment to the DNBC has raised concern about possible biased enrollment, 23 as it has been reported that single women with little or no education and low income were less likely to participate.9 However, a previous study investigated the potential effects of selection bias in the DNBC on the association between variables that are highly dependent on socioeconomic status and concluded that the effects of differential participation on these estimated associations were small.23 Some 14% of women enrolled in the cohort did not participate in the interview ~6 months’ postpartum, which could introduce bias. However, the cumulative incidence proportion for ASD for all participants in the DNBC and for participants in various interviews were similar, indicating that loss to follow-up within the cohort unlikely resulted in severe bias.
Each interview of the DNBC included >200 questions for the mother, and the questionnaire covered a broad range of topics on maternal and child health and daily routines. The information was not gathered specifically for the current study, but instead the questions were general and meant to be used in a variety of different studies. Consequently, information concerning types of infection and circumstances concerning the infection/febrile episode and antibiotic use was limited.
Exposure was reported by the mother, and we did not have any formal information on the validity of the reported exposure information. The exposure information was collected prospectively and thus before the woman was aware of the child’s potential developmental problem, which limited the likelihood of differential misclassification of exposure information. If the maternally reported data were misclassified, the misclassification was most likely nondifferential. Such misclassification usually drives the estimates toward the null, which could explain the general lack of positive associations.
We do not have any information on the completeness of information on infectious diseases or febrile episodes. Collier et al24 reported the prevalence of self-reported infection among pregnant women from 10 different sites in the United States. The proportions of women reporting any infection, fever, or urinary tract infection were comparable between our study and the study by Collier et al. We observed a very low proportion of women reporting respiratory infections and influenza, which is most probably due to the fact that women were not specifically asked about respiratory infections; instead, the information was extracted from an open-ended question (text-string variable). Misreporting of influenza is likely to be considerable; any episode of fever may be mistaken for influenza, and not all women infected with influenza virus might have been aware of this. Moreover, the agreement between hospital admission data and maternal reports regarding influenza was very poor.
Olesen et al25 studied the use of dispensed medications among pregnant women in Northern Denmark, by using the information from the DNBC as the reference and comparing that with the Danish prescription database. They found that only 52% (95% CI: 47–58) of women who were prescribed and purchased antibiotics reported actually taking the drugs. The proportion of women reporting usage of prescribed drugs was similar, whether recall was 30 days (50%), 60 days (49%), 90 days (47%), or 120 days (43%), suggesting poor compliance rather than underreporting at the time of the interview. Thus, the results by Olesen et al support the usage of self-reported data instead of prescription databases when studying use of medication during pregnancy.
CONCLUSIONS
Our results do not suggest that mild infections, febrile episodes, or use of antibiotics during pregnancy are strong risk factors for ASD and infantile autism. Due to multiple testing, the few statistically significant findings were possible chance findings. We experienced several methodologic limitations, and the results of this study thus cannot solely negate a possible association. We emphasize the need for further research on this important topic.
WHAT’S KNOWN ON THIS SUBJECT
It has been suggested that maternal immune activation during pregnancy is associated with cardinal behaviors of autism in the offspring. Epidemiologic studies have yielded conflicting results concerning the association between any infection during pregnancy and the development of autism.
WHAT THIS STUDY ADDS
This population-based cohort study investigated the association between specific common infectious diseases, febrile episodes, or use of antibiotics during pregnancy by using maternal population-based self-reported data.
Acknowledgments
FUNDING: Funding for this study was provided by the Aarhus University Research Foundation, the Aase and Ejnar Danielsen Foundation, and the Augustinus Foundation. The funding sources did not participate in any part of the performance of the study. The Danish National Research Foundation has established the Danish Epidemiology Science Centre that initiated and created the Danish National Birth Cohort. The cohort is furthermore a result of a major grant from this foundation. Additional support for the Danish National Birth Cohort is obtained from the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Augustinus Foundation, and the Health Foundation.
ABBREVIATIONS
- aHR
adjusted hazard ratio
- ASD
autism spectrum disorder
- CI
confidence interval
- DNBC
Danish national birth cohort
- HR
hazard ratio
Footnotes
Dr Atladóttir conceptualized and designed the study, participated in acquisition of data, participated in analysis and interpretation of data, drafted the article and revised it critically for important intellectual content, and approved the final version to be published. Dr Henriksen participated in analysis and interpretation of data, revised the article critically for important intellectual content, and approved the final version to be published. Dr Schendel participated in designing the study, participated in analysis and interpretation of data, drafted the article and revised it critically for important intellectual content, and approved the final version to be published. Dr Parner participated in designing the study and acquisition, analysis, and interpretation of data; revised the article critically for important intellectual content; and approved the final version to be published.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.World Health Organization. International Classification of Diseases, 10th Revision. Geneva, Switzerland: World Health Organization; 1993. [Google Scholar]
- 2.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17(7):389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23(1):297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry. 2009;195(1):7–14. doi: 10.1192/bjp.bp.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchmayer S, Johansson S, Johansson A, Hultman CM, Sparén P, Cnattingius S. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics. 2009;124(5) doi: 10.1542/peds.2008-3582. Available at: www.pediatrics.org/cgi/content/full/124/5/e817. [DOI] [PubMed] [Google Scholar]
- 7.Atladóttir HO, Thorsen P, Østergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 8.Olsen J, Melbye M, Olsen SF, et al. The Danish National Birth Cohort—its background, structure and aim. Scand J Public Health. 2001;29(4):300–307. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen TN, Nohr EA, Frydenberg M. Selection by socioeconomic factors into the Danish National Birth Cohort. Eur J Epidemiol. 2010;25(5):349–355. doi: 10.1007/s10654-010-9448-2. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449. [PubMed] [Google Scholar]
- 11.Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263–268. [PubMed] [Google Scholar]
- 12.Munk-Jørgensen P, Mortensen PB. The Danish Psychiatric Central Register. Dan Med Bull. 1997;44(1):82–84. [PubMed] [Google Scholar]
- 13.Lauritsen MB, Jørgensen M, Madsen KM, et al. Validity of childhood autism in the Danish Psychiatric Central Register: findings from a cohort sample born 1990–1999. J Autism Dev Disord. 2010;40(2):139–148. doi: 10.1007/s10803-009-0818-0. [DOI] [PubMed] [Google Scholar]
- 14.Parner ET, Schendel DE, Thorsen P. Autism prevalence trends over time in Denmark: changes in prevalence and age at diagnosis. Arch Pediatr Adolesc Med. 2008;162(12):1150–1156. doi: 10.1001/archpedi.162.12.1150. [DOI] [PubMed] [Google Scholar]
- 15.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2006 Principal Investigators; Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58(10):1–20. [PubMed] [Google Scholar]
- 16.Petersen DJ, Bilenberg N, Hoerder K, Gillberg C. The population prevalence of child psychiatric disorders in Danish 8- to 9-year-old children. Eur Child Adolesc Psychiatry. 2006;15(2):71–78. doi: 10.1007/s00787-006-0488-9. [DOI] [PubMed] [Google Scholar]
- 17.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45(3):320–323. [PubMed] [Google Scholar]
- 18.Juel K, Helweg-Larsen K. The Danish registers of causes of death. Dan Med Bull. 1999;46(4):354–357. [PubMed] [Google Scholar]
- 19.Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61(8):774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 20.Main PA, Angley MT, Thomas P, O’Doherty CE, Fenech M. Folate and methionine metabolism in autism: a systematic review. Am J Clin Nutr. 2010;91(6):1598–1620. doi: 10.3945/ajcn.2009.29002. [DOI] [PubMed] [Google Scholar]
- 21.Roth C, Magnus P, Schjølberg S, et al. Folic acid supplements in pregnancy and severe language delay in children. JAMA. 2011;306 (14):1566–1573. doi: 10.1001/jama.2011.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt RJ, Hansen RL, Hartiala J, et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology. 2011;22(4):476–485. doi: 10.1097/EDE.0b013e31821d0e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nohr EA, Frydenberg M, Henriksen TB, Olsen J. Does low participation in cohort studies induce bias? Epidemiology. 2006;17(4):413–418. doi: 10.1097/01.ede.0000220549.14177.60. [DOI] [PubMed] [Google Scholar]
- 24.Collier SA, Rasmussen SA, Feldkamp ML, Honein MA National Birth Defects Prevention Study. Prevalence of self-reported infection during pregnancy among control mothers in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2009;85(3):193–201. doi: 10.1002/bdra.20540. [DOI] [PubMed] [Google Scholar]
- 25.Olesen C, Søndergaard C, Thrane N, Nielsen GL, de Jong-van den Berg L, Olsen J EuroMAP Group. Do pregnant women report use of dispensed medications? Epidemiology. 2001;12(5):497–501. doi: 10.1097/00001648-200109000-00006. [DOI] [PubMed] [Google Scholar]


