Stressful life events are risk factors for major depressive disorder (MDD) onset and recurrence (Hammen, 2005; Mazure, 1998). An estimated 20 to 50 percent of individuals develop depression after experiencing a recent major life stressor (Brown & Harris, 1989; Monroe & Simons, 1991). Psychosocial and biological factors may exist that create increased sensitivity to the effects of stressors, but the more proximal mechanisms underlying this diathesis-stress relationship have yet to be fully understood. One possible mechanism is dysregulation of the biological stress response system, in the form of altered hypothalamic-pituitary-adrenal (HPA) axis functioning (Gotlib et al., 2008). Alterations in the HPA axis have been linked to depression in past research (Carroll et al., 2007; Knorr et al., 2010; Vreeberg et al., 2009), and may also be related to several factors that could indicate risk for depression, such as the serotonin transporter gene (5-HTT) linked polymorphic region (5-HTTLPR) (Alexander et al., 2009; Chen et al., 2009; Goodyer et al., 2009; Gotlib et al., 2008), personality traits such as negative affect and neuroticism (Polk et al., 2005, Portella et al., 2005; Hauner et al., 2008), and family history of depression (Mannie et al., 2007; Vinberg et al., 2008). The current investigation uses a community sample to compare HPA functioning in currently depressed individuals and those believed to be at risk for depression as a result of one of these factors, trait levels of positive and negative affect.
Cortisol, the hormonal endpoint of the HPA axis, is one of the primary coordinators of the bodily response to a stressor and follows a circadian rhythm in concert with the sleep/wake cycle (Tsigos & Chrousos, 2002). Results of studies examining cortisol levels in depressed individuals appear to vary based on patient characteristics and severity of depression. Currently depressed individuals in inpatient samples typically have diurnal patterns characterized by high morning cortisol and a high, flat pattern of cortisol secretion across the day (Holsboer, 2000; Plotsky et al., 1998). However, there is some indication that this pattern may not hold true for less severely depressed populations outside of the hospital setting, nor for non-melancholic/psychotic depression (Carroll et al., 2007; Maes et al., 1994; Peeters et al., 2004; Strickland et al., 2002). Notably, a recent meta-analysis of twenty studies reported that the small observed difference in morning and evening cortisol did not reliably distinguish depressed and non-depressed persons, except for more severely depressed individuals (Knorr et al., 2010). In contrast, Vreeberg and colleagues (2009) reported that depressed participants in a large community-based study had higher morning and higher evening cortisol levels when compared to non-depressed participants, similar to the findings for depressed inpatients. Higher morning cortisol (Bhagwagar, 2005) and a larger cortisol awakening response (CAR: the peak in cortisol secretion occurring 30–45 minutes post awakening) (Pruessner, 2003) have also been found in some studies involving depressed populations, whereas other studies have reported a blunted CAR (Huber et al., 2006; Stetler & Miller, 2005). Thus, there appears to be support for higher morning cortisol levels in community samples of depressed individuals, but evidence remains inconclusive.
Similarly mixed results have been reported for individuals at risk for depression. However, certain findings have been consistently replicated over time. A number of prospective longitudinal studies have indicated that elevated waking cortisol (Goodyer et al., 2000; Goodyer et al., 2009; Harris et al., 2000; Halligan et al., 2007) or a larger CAR (Adam et al., 2010) in at-risk individuals predicts later depression onset. Cross-sectional studies have also reported higher waking cortisol in individuals at risk for depression based on family history (Mannie et al., 2007), genotype (Chen et al., 2009), and neurotic personality style (Portella et al., 2005). Taken together, the most robust findings suggest greater waking cortisol and a larger CAR in those at risk for depression.
Patterns of cortisol secretion in response to a psychosocial laboratory stressor or chemical challenge can also reveal HPA axis dysfunction (Adam and Kumari, 2009). Most studies of cortisol reactivity in depressed individuals have assessed cortisol secretion in response to a chemical challenge (i.e., the dexamethasone-/corticotropin-releasing hormone test; Holsboer, 2000) rather than the cortisol response to a laboratory stressor (Heim et al., 2000; Young et al., 2000), and therefore, research investigating the cortisol response to psychosocial laboratory stressors for depressed individuals is needed. Burke and colleagues (2005) conducted a meta-analysis of seven such studies, and reported that depressed individuals had higher cortisol levels during the recovery period following a stressor, although levels did not differ at baseline or immediately post-stressor. However, their meta-analysis only examined mean levels and not variation of cortisol secretion over time or total cortisol exposure. Moreover, only two of the seven studies involved social evaluative threat which has been shown to contribute to a reliable cortisol response (Dickerson & Kemeny, 2001).
There is also a paucity of research on the cortisol response to psychosocial laboratory stressors in individuals considered at-risk for depression due to behavioral or personality factors. Young and Nolen-Hoeksema (2001) found no difference between high and low ruminators on cortisol response to the Trier Social Stress Test (TSST: Kirschbaum et al., 1993). Shommer et al. (1999) reported no association between cortisol in response to the TSST and neuroticism. Tyrka and colleagues (2006) did report elevated cortisol response to the TSST based on temperament, specifically novelty seeking, but novelty seeking had no relationship to risk for depression. Kudielka and colleagues (2007), in a review of research involving the TSST, reported that personality variables were not related to the TSST on first exposure because of the effect of novelty (Brandstadter et al., 1991; Kirschbaum et al., 1992), but that high neuroticism, low self-esteem, and extraversion were associated with lack of habituation to the TSST upon repeated exposure (Kirschbaum et al., 1995; Pruessner, Gaabet al., 1997).
High negative affect (NA), a personality style defined as temperamental sensitivity to negative stimuli, and low positive affect (low PA; e.g., lack of enjoyment or energy) have been associated with both increased risk for depression and dysregulation of the HPA axis in past research (Clark et al., 1994; Gunderson et al., 2000; Hirschfeld et al., 1989). Additionally, NA and PA are reliably assessed via self-report and, if they are related to dysregulation of the biological stress system, may provide an easy means of identification of stress sensitive individuals. Clark et al. (1994) reported that individuals who later develop depression have NA scores that fall between those of normal controls and those with a current depressive episode. Hirschfeld et al. (1989) reported that individuals who went on to develop depression scored higher than those who did not on a measure of NA. Additionally, Gunderson et al. (2000) reported that neuroticism, which the authors state is essentially identical to NA, was associated with later onset of depression. The evidence for low PA as a risk factor for future depression is less clear, although it has been associated with current depression (Clark et al., 1994). Elevated diurnal cortisol production has been linked to high NA, most clearly for men, with a weaker relationship between elevated diurnal cortisol and low PA (Polk et al., 2005; Smyth et al., 1998; van Eck et al., 1996). There is currently no empirical evidence for greater cortisol response to a laboratory stressor for those high in NA and low in PA – the risk profile for depression according to Clark and Watson’s tripartite model (Clark & Watson, 1991) – although high NA appeared to mediate the relationship between naturalistic stressors and diurnal cortisol secretion in a study using experience sampling methodology (van Eck et al., 1996).
The current study is designed to identify distinct patterns of diurnal cortisol secretion and cortisol secretion in response to a psychosocial laboratory stressor for depressed and at-risk individuals. It is likely the first study to examine cortisol secretion in both contexts, and by using a community sample that has been rigorously evaluated for history of major depression, it represents an increasingly comprehensive investigation of HPA functioning in adults at risk for depression. It is hypothesized that depressed individuals, compared to control participants, will have a pattern of diurnal cortisol secretion characterized by higher mean levels of cortisol, a flatter downward slope over time, higher waking cortisol, and a larger CAR. Moreover, depressed individuals are hypothesized to demonstrate slower return to pre-stress cortisol levels following a psychosocial laboratory stressor. It is further predicted that individuals at risk for depression because of high trait NA and low PA will have features of HPA functioning similar to those of depressed individuals, including a greater waking cortisol, a larger CAR, and increased cortisol secretion in response to an environmental stressor compared to controls.
Method
Participants
Participants in this study were 57 women ranging in age from 17 to 23 (M = 18.60, SD = 0.90) recruited from the undergraduate subject pool at a large research university over the course of one academic year. The sample was ethnically representative of the student population (see Table 1). A total of 1,340 students were prescreened using a questionnaire including the trait form of the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) and two questions about depressed mood and anhedonia taken from the Structured Clinical Interview for DSM-IV (SCID-IV; First et al., 1995). Males were excluded from the study because gender differences in diurnal cortisol secretion and cortisol secretion in response to the TSST (Kudielka & Kirschbaum, 2005; Polk et al., 2005) would limit power given available recruitment resources.
Table 1.
Percentage Distribution of Descriptive Variables by Group
| Variable | Control (N = 22) |
At-Risk (N = 20) |
Depressed (N = 15) |
Total (N = 57) |
χ2 | p |
|---|---|---|---|---|---|---|
| Ethnicity | 12.2 | .596 | ||||
| Asian/Pacific Islander | 27.3 | 45.00 | 33.3 | 35.1 | ||
| White | 36.4 | 15.0 | 20.0 | 24.6 | ||
| Black | 0.0 | 0.0 | 6.7 | 1.8 | ||
| Hispanic | 18.2 | 15.0 | 26.7 | 19.3 | ||
| Middle Eastern | 9.1 | 10.0 | 0.0 | 7.0 | ||
| Indian | 4.5 | 10.0 | 0.0 | 5.3 | ||
| Biracial | 0.0 | 5.0 | 6.7 | 3.5 | ||
| Other | 4.5 | 0.0 | 6.7 | 3.5 | ||
| Oral Contraceptives | 36.4 | 20.0 | 6.7 | 22.8 | 4.6 | .100 |
| Medication Use | 9.1 | 5.0 | 14.3 | 8.8 | 0.8 | .688 |
In order to increase the likelihood of selecting those either currently depressed or at risk for depression, PANAS scores were used to identify those with high NA and low PA. Also, a control group of individuals with low NA and high PA was identified to include those without depression and at low risk for depression onset. Low and high PA and NA were defined as below and above the subject pool means respectively (PA: M = 34.0, SD = 5.82, NA: M = 22.3, SD = 7.01). Two hundred ninety three (21.9%) of the 1,340 students were called to screen for eligibility based on their PANAS scores. Seventy seven (26.3%) of the 293 participants contacted about the study met phone interview exclusion criteria and agreed to participate in the study. Exclusion criteria included regular smoking, regular stimulant medication use, current pregnancy, current anxiety disorder such as excessive worry, panic attacks, or obsessive-compulsive disorder, and any serious medical condition. These factors can have a significant effect on salivary cortisol secretion (Kirschbaum et al., 1993). Seventy three (24.9%) participants were not reachable by phone or failed to return messages. Thirteen (4.4%) were not interested in participating, eighty one (27.7%) already had their research credits, four (1.4%) dropped the class, 45 (15.3%) were not eligible based on exclusion criteria. Specific percentages for exclusion criteria were not available because the questions were asked at once and answered as a group to protect confidentiality.
Each of the 77 women was given a modified version of the SCID during the initial session. Twenty participants were screened out because of a clinical diagnosis of generalized anxiety disorder (GAD), dysthymic disorder, panic disorder, obsessive-compulsive disorder (OCD), psychotic symptoms, psychoactive substance use, post-traumatic stress disorder (PTSD), anorexia, or bulimia nervosa, because these disorders may have cortisol profiles that are distinct from depressed and non-disordered populations. Fifteen participants met DSM-IV diagnostic criteria for a current major depressive episode and were considered in the currently depressed group. Thirteen of the fifteen depressed participants had at least one prior episode of depression. Participants with high NA and low PA who had no current or past depression were considered to be at-risk (n = 20). Finally, 22 participants with no clinical diagnosis, high PA, and low NA were included in the study as a control group.
Procedure and Measures
Procedure
The prescreener and phone screener were administered first. Participants who met criteria for the study at this point were invited in for an intake interview including the modified SCID, the Beck Depression Inventory-II (BDI-II, Beck et al., 1996), and the UCLA Life Stress Interview (LSI; Hammen et al., 1987). At-risk, depressed, and control participants then completed diurnal cortisol collection the next week, and the TSST the week after.
Diagnosis
The SCID was shortened to focus on the criteria for major depressive episode (current and past) and on the exclusionary diagnoses noted above. Diagnosis of current MDD required meeting clinical criteria for a major depressive episode (MDE) within the past month. At-risk and control participants had no prior history of MDD or any other clinical diagnosis. A variable indicating presence or absence of anxiety symptoms based on the SCID was created to control for the effects of anxiety symptomatology. The interviews were conducted by a master’s level graduate student with 7 years of experience administering the SCID and other structured clinical interviews.
The Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) is a 20-item list of emotion adjectives that are empirically representative of positive and negative affectivity. The form administered at the screening and initial session had participant indicate whether each item described “To what extent do you generally feel this way?” on a Likert-type scale from 1 (very slightly or not at all) to 5 (extremely). During the TSST, participants indicated how much each item described their feeling at that moment. Each version of the PANAS has been widely used in mood research as a measure of stable characteristic affectivity and current mood state, respectively. The PANAS has been found to be have high internal consistency reliability both at the time of analysis (PA: α = .90, NA: α = .87) and over one year (PA: α = .86, NA: α = .84), and test-retest reliability over 8 weeks (Watson et al., 1988). In the current study the emotion adjective of “depressed” was added to the list to identify individuals who felt that they were characteristically depressed.
The Beck Depression Inventory-II (BDI-II, Beck et al., 1996) was administered at the initial interview to validate the group status of participants. The BDI-II consists of 21 items, rated on a 4-point scale from 0 to 3. A score of 0–13 reflects no or minimal depression; 14–19 is mild, 20–28 is moderate, and 29–63 is severe depressive symptomatology. Internal consistency has been estimated at .93 in a college student sample. The measure also has test-retest reliability of .93 over one week among outpatients (Beck et al., 1996).
Chronic stress
Chronic stress was assessed because both cortisol and depression have been related to chronic stress in past research, and it is important to clarify the association of depression and risk and cortisol secretion apart from chronic stress (Miller et al., 2007; Adam et al., 2010). Chronic stress was assessed using the UCLA Life Stress Interview (LSI; Hammen et al., 1987) administered by three trained masters-level graduate students. The interview is designed to assess the level of objective stress ongoing for at least the past 6 months across several domains of an individual’s life. The full interview with anchors was used for interpersonal areas of chronic stress including close friendships, social life, romantic relationships, and family relationships. The behavioral anchors for financial independence, work, individual health, family health, and school were used to form a questionnaire that participants completed. This semi-structured interview begins with a standard probe followed by queries when needed. There are several probes in each area. For example, in the best friend area the interview begins with “Do you have a best friend? Who would that be? How has this relationship been going?” This probe is followed by specific behavioral anchor questions in the areas of stability/proximity, supportiveness, and conflict resolution. Interviewers used behaviorally specific anchors to determine the objective level of severity of each area of chronic stress on a 1–5 scale. For example, a score of “1” in close friendships indicates a trusting, high quality, stable friendship that includes mutual disclosure in many areas. A “3” in the same category refers to a friendship that is close but sometimes unstable and conflictual, or a stable friendship that is only moderately close. Intra-class correlations for independent judges based on 57 participants in a previous study ranged from .82 to .91 (Davila et al., 1995). Stability and convergent validity of this chronic stress measure have been demonstrated in past research (Daley et al., 2000, Hammen et al., 2009). The ratings across all categories were summed together into a total chronic stress index.
Early Adversity Questionnaire (EAQ)
The EAQ, designed by Cohen et al. (2004), was administered as part of the initial interview to determine whether the adversity status of the participants could influence differences in cortisol above and beyond diagnosis. This instrument is designed to facilitate assessment of multiple adversity domains and assesses adverse events which occurred up to the age of 13 (12 and under). Adversity was assessed in eight areas: separation and loss involving the primary caretaker(s), significant loss involving non-caretaker (i.e., sibling or close friend), death and life-threatening illness or injury to self or others, physical neglect, emotional abuse or assault, physical abuse or assault, witnessing violence, sexual abuse or assault and peer victimization. Presence and absence of each area of adversity was rated by the participants by circling “yes” or “no.” A total early adversity score was calculated by summing the number of “yes” replies.
Diurnal cortisol assessment
Four to five time points over three to five days are characteristic of “moderately high intensity protocols” for diurnal salivary cortisol collection (Adam & Kumari, 2009). Thus, each participant provided four salivary cortisol samples upon awakening (before getting out of bed) and 30 minutes, 8 hours, and 11 hours after awakening on five consecutive weekdays using Salivettes (Sarstedt Rommelsdorf INC, Germany). Each subject was instructed not to drink or eat anything for one hour before sampling, as this may influence cortisol levels (Kudielka et al., 2007). They were also asked to record time of waking, hours slept, and quality of sleep (on a scale of 1=very badly to 4=very well) on each day of sampling. Electronic MEMS caps recorded the time and date that each bottle containing Salivettes was opened for 19 of the 57 participants in order to check that accurate time reporting occurred. The MEMS caps were randomly given out to participants without knowledge of diagnostic group or other study variables. Data were downloaded using MEMS software (MEMS View, version 161; Aprex Corporation). There was a total of 1,140 data points of cortisol collection. Of these, 34 were either missing or not valid. The time that the participants reported taking the samples closely corresponded to the MEMS data (ICC = .98, p < .001). 95% of the samples were reported taken within one hour of the MEMS recorded time, and 78% were within five minutes.
Biobehavioral Questionnaires
Participants completed a form asking them to report any regular medication use, current oral contraceptive use (yes/no), and the date of their last menstrual period. These variables have been shown to significantly affect cortisol secretion in response to the TSST in past research (Kudielka et al., 2007), as well as cortisol levels during the day. In addition, participants were asked to not take any over the counter medication, brush their teeth, or eat or drink anything for one hour before the cortisol sampling. They reported whether or not they performed any of these activities in addition to the time of day that each sample was collected. Finally, they filled out a questionnaire at the end of each day assessing frequency/level and typical intake of caffeine, nicotine, and sugar.
The Trier Social Stress Test
The TSST consists of an anticipatory period and a test period in which participants have to deliver a speech and perform mental arithmetic in front of an audience. The TSST was administered after the final day of diurnal cortisol collection in order to avoid the effects of an artificial stressor on diurnal cortisol rhythms. Participants were scheduled for an afternoon appointment between 4:00 pm and 6:00 pm and were told not to eat or drink for an hour before the scheduled appointment. Each participant gave a baseline saliva sample prior to the TSST. The participants were then informed that they would be participating in a task for the next hour and were ushered into a separate room with the audience. The audience consisted of three people, typically including at least one male and one female. Participants were told that they must deliver a five-minute impromptu speech as if to a judge and jury in response to being accused of shoplifting. The experimenter explained that the audience had been trained in behavior analysis and would take notes, and that the participant would be video- and audio-recorded. Also, participants were informed that these tapes would be coded for verbal and non-verbal behavior. The participant was given five minutes to prepare. Participants then spoke for five minutes and were prompted to continue if they stopped speaking. They were then given a new task in which they counted backwards by 13 as quickly as possible, starting at 6,233. If the participant gave an incorrect answer, they were corrected and told to begin from the correct number. Participants were interrupted after five minutes and immediately gave another salivary cortisol sample. They were then escorted to another room for the recovery period. Additional cortisol samples were taken 10 min, 25 min, and 40 min following the end of the TSST. The momentary form of the PANAS was administered at each sampling point to assess for mood changes in response to the task. Participants also filled out a questionnaire immediately following the task rating their emotional reaction to and their performance on the TSST (Gruenewald et al., 2004). They rated volume and surety of speech, fidgeting, eye contact, and overall difficulty of both the math and speech tasks using a 7-point likert-type scale (1 = not at all to 7 = very). Participants rated their emotional experience of the TSST (e.g., “to what extend did you feel nervous?”) using a 5-point scale (1 = not at all to 5 = extremely).
The original TSST involves a “job interview” paradigm before the “selection committee” (Kudielka et al., 2007). The shoplifting paradigm was first used by al’Absi et al. (1997). Buchanan et al. (2011) pilot tested both paradigms and found that the shoplifting paradigm elicited the greatest change in cortisol secretion. According to a meta-analysis by Dickerson and Kemeny (2004), social evaluative threat and uncontrollability are needed to reliably elicit a cortisol response. The shoplifting paradigm appears to have both of these components.
Sample Storage
Salivettes were stored in a refrigerator during each five day diurnal collection period, and were returned to the lab two days after sampling was completed. The samples from diurnal collection and the TSST were placed in a freezer at −20 °C. Following completion of the study, Salivettes were sent packed in dry ice to Trier, Germany to be assayed for cortisol. Cortisol samples are stable at room temperature for several weeks and have been shown to be unaffected by postal travel (Clements and Parker, 1998). Assays were conducted using time-resolved immunoassay with fluorescence detection (DELFIA; Dressendorfer et al., 1992). Intra-and interassay coefficients of variance have been reported at below 12% for the lab that conducted the assays.
Data Analysis Plan
Comparisons among the three groups on demographics, biobehavioral variables and subjective response to the TSST were conducted using one-way analyses of variance (ANOVAs). Due to severe positive skewness, raw cortisol values were natural log transformed for use as criterion variables. All analyses were performed in HLM 6.08 (Raudenbush et al, 2004) using robust standard errors.
Diurnal analyses
To control for other influences on cortisol while maximizing power, hypothesized control variables were evaluated using a series of models in which day-level (waking time, hours of sleep, caffeine use) and person-level (use of oral contraceptives, total chronic stress scores, menstrual cycle, early adversity) were individually tested for the effects on the pattern of cortisol secretion. Variables that significantly predicted rate of cortisol change or CAR in these analyses (results not shown) were included as control variables in the final analyses.
Diurnal cortisol was modeled using a three-level hierarchical linear model in which samples were nested within days, which in turn were nested within participants. At level-1, cortisol was modeled as a linear function of time (HOURS), time squared (HOURS2), and a dichotomous variable indicating whether the sample was taken within 1 hour of wakening, the coefficient of which was used to represent the CAR(MRISE). Thus, the level-1 equation for diurnal analyses took the form of:
Each level-1 coefficient was then predicted at level 2 with an intercept and any control variables as follows (with time of waking serving as a control):
Similar level 2 equations were constructed for each level-1 coefficient.
At level 3, group membership (CURRENT DEPRESSED, AT RISK) predicted the average levels of level-1 coefficients. In each analysis, the never depressed group was chosen as the comparison group and membership in the other two groups was represented using two dummy variables. Each of the four level-2 intercepts (and by extension, the level-1 coefficients adjusted for level-2 control variables) was predicted by the group membership variables and level-3 controls (OC):
In this case, for example, where β00k represents the initial cortisol level, γ002 represents the average difference between the currently depressed group’s initial cortisol level and that of the never depressed group, while γ003 represents the difference in initial cortisol between the never depressed and at-risk group. In all analyses, comparisons between the depressed and at-risk groups were performed by testing the linear hypothesis that the effects for their respective dummy variables were equal (e.g., in this example γ002 = γ003 for initial cortisol levels). Derivation of the chi-square distributed test statistic for such linear tests can be found in Raudenbush & Bryk (2002, pp. 58–60).
TSST analyses
As with the diurnal models, a series of models were run to individually test the effects of potential person-level control variables (use of oral contraceptives, day of menstrual cycle, total chronic stress, food consumed or drinking a beverage other than water in the hour before the task, early adversity). Variables that significantly predicted level-1 coefficients were included as control variables in the final TSST analyses.
Cortisol secretion across the TSST was modeled as a two-level hierarchical model in which samples were nested within individuals. At level 1, the natural log of cortisol was modeled as a piecewise regression with three predictor variables (see Llabre et al, 2001, for a similar analysis and extended discussion of variable coding), as follows:
The first variable, MIN12, was calculated as 0 for each baseline sample, and the number of minutes between the first, baseline sample and the second saliva sample for all remaining samples. As such, β 1j represents the slope of cortisol change between samples 1 and 2. The second variable, MIN23, was coded as 0 for samples 1 and 2, and the number of minutes between the second and third sample for all other samples. β2j thus represents the slope of change between samples 2 and 3. The third variable, MIN35, was coded as 0 for samples 1 through 3, and the number of minutes between sample 3 and 5 for all remaining samples. Thus, β3j represents the slope of change between samples 3 and 5. Because changes in cortisol secretion take approximately 20 minutes to be reflected in salivary free cortisol measurements (Kirschbaum et al., 2010), cortisol secretion in response to the task was also separately calculated as the slope of cortisol change from sample 1 to sample 3, 32 minutes later (Kirschbaum et al., 1993). At level 2, level 1 coefficients were modeled as a function of group membership:
in which CS represents chronic stress (as a control variable), and the group membership variables are calculated in the same manner as with the diurnal analyses.
Results
Descriptive Statistics
The three groups, control, at-risk, and currently depressed, were compared on multiple demographic variables. There were no significant differences among the three groups on age, ethnicity, height, weight, income level, and oral contraception use (Table 1). Medication use also did not differ among the three groups (p > .05). The medications used by depressed participants were fluoxetine and an amphetamine. The medication used by the at-risk participant was isotretinoin, and the medications used by the control participants were co-trimoxazole and fluticasone nasal spray. Several of these medications could potentially influence cortisol secretion, but medication use did not significantly predict cortisol levels in this study. As expected, there was a main effect of group on the BDI-II, F(2, 57) = 30.65, p < .01. The depressed group had a significantly higher score than both the at-risk (p < .001) and control groups (p < .001), and there was no significant difference between the at-risk and control groups (Table 2).
Table 2.
Means and Standard Deviations of Descriptive Data and Subjective Response to the TSST by Group
| Control (N = 22) |
At-Risk (N = 20) |
Depressed (N = 15) |
Total (N = 57) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | M | SD | M | SD | F | p |
| Descriptive Data | ||||||||||
| Age | 18.90 | 1.18 | 18.7 | 0.82 | 18.4 | 0.93 | 18.7 | 1.01 | 1.11 | .340 |
| PA | 40.10 | 2.53 | 29.0a | 3.56 | 31.6a | 6.45 | 34.0 | 6.52 | 40.2 | <.001 |
| NA | 15.30 | 1.93 | 29.4a | 3.93 | 29.3a | 8.71 | 23.9 | 8.56 | 50.4 | <.001 |
| Chronic Stress | 19.40 | 2.58 | 21.9a | 3.08 | 23.7a | 3.27 | 21.4 | 3.38 | 9.72 | <.001 |
| BDI-II | 4.39a | 3.30 | 6.10a | 5.48 | 18.0 | 7.67 | 8.57 | 7.86 | 30.7 | <.001 |
| Hours of Sleep* | 6.30 | 1.22 | 6.50 | 1.08 | 6.57 | 1.21 | 6.44 | 1.15 | 0.26 | .770 |
| Sleep Quality* | 2.75a | 0.36 | 3.15 | 0.36 | 2.73a | 0.28 | 2.89 | 0.39 | 9.27 | <.001 |
| Wake Time* | 8.79 | 1.16 | 8.76 | 1.23 | 8.64 | 1.02 | 8.74 | 1.13 | 0.08 | .923 |
| Self-Reported Performance on the TSST | ||||||||||
| Speech Task | ||||||||||
| Embarrassed | 2.95a | 1.99 | 4.94b | 1.91 | 4.25a,b | 2.06 | 3.93 | 3.93 | 4.79 | .013 |
| Eye Contact | 4.71a | 1.49 | 3.13 | 1.36 | 5.14a | 1.29 | 4.33 | 1.61 | 9.11 | .001 |
| Fidgety | 3.38a | 1.63 | 5.06 | 1.73 | 3.50a | 1.23 | 3.94 | 1.71 | 6.02 | .005 |
| Strong Voice | 4.48a,b | 1.17 | 3.63b | 1.20 | 4.75a | 1.45 | 4.28 | 1.32 | 3.38 | .042 |
| Math Task | ||||||||||
| Strong Voice | 4.81a | 1.17 | 3.56b | 1.63 | 4.64a,b | 1.65 | 4.37 | 1.54 | 3.64 | .034 |
| Overall Difficulty | 2.38a | 1.07 | 3.25b | 1.07 | 3.00a,b | 1.18 | 2.85 | 1.15 | 3.37 | .042 |
| Tense | 2.76a | 1.22 | 3.75b | 1.16 | 3.21a,b | 1.19 | 3.24 | 1.25 | 3.52 | .037 |
Sleep variables reported are grand means across 5 days of sampling for all individuals in the group.
Note. Shared alphabetical subscripts represent means that are not significantly different from each other.
There was a significant difference among the three groups on total chronic stress over the past 12 months, F(2, 57) = 9.72, p < .01. Both the at-risk and depressed groups reported significantly greater chronic stress over the past year compared to the control group (at-risk: p = .02; depressed: p < .001). The at-risk and depressed groups did not differ from each other in their amount of chronic stress (p > 0.05). There was also a significant difference among the three groups on whether or not they reported early adversity (χ2 (N = 57) = 6.54, p = .038). Specifically, more people in the at-risk group reported experiencing some form of adversity compared to the control group (p = .010).
Diurnal biobehavioral control variables were averaged across the five days of sampling. There was a significant difference among the three groups on their reported quality of sleep as reported in Table 2. The at-risk group reported significantly better sleep quality than both the control (p < .01) and depressed (p < .01) groups. There was no significant difference among the groups on time of waking (p > .05) or number of hours slept.
Diurnal Cortisol Analyses
Individual tests of the effects of potential control variables indicated that use of oral contraceptives (b = −.10, p = .05), sleep duration (b = −.01, p = .01), and waking time (b = −.02, p = .04) predicted faster decreases in cortisol over the course of the day; sleep duration (b = −.08, p = .03) and waking time (b = −.17, p < .01) predicted a smaller CAR. No other potential control variables (chronic stress, early adversity, day of menstrual cycle or caffeine intake) predicted CAR or rate of cortisol change (all p’s > .05). Because waking time and sleep duration were conceptually and empirically related (r = .35), only the stronger predictor of the two, waking time, was used as a control at level 2 to conserve power. Use of oral contraceptives was used as a control at level 3.
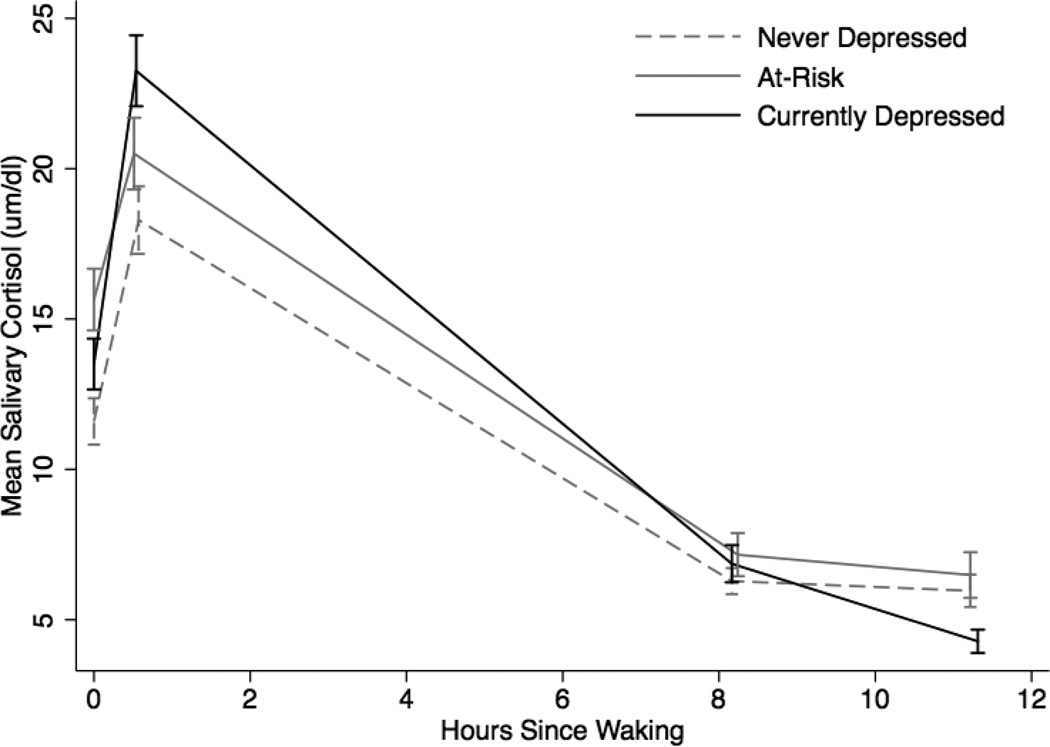
Figure 1 displays mean patterns of diurnal cortisol secretion for the control, at-risk, and currently depressed groups, and Table 4 shows the results of the multilevel model. Oral contraceptives were associated with lower mean cortisol (b = −0.31, p = .04). The depressed group had higher mean levels of cortisol than the control group (b = .35, p = .02), but the at-risk group did not (b = .16, p = .27). The at-risk group did have a higher mean level of cortisol at waking than the control group (b = .33, p = .02), although there was no significant difference on mean diurnal cortisol levels (b = .20, p = .15).
Figure 1.
Mean diurnal cortisol secretion across 5 days by group
Table 4.
Diurnal Cortisol Model Summary and Estimated Group Mean Parameters
| Estimates | Group Means | |||||
|---|---|---|---|---|---|---|
| b | SE | p | Control | At-Risk | Depressed | |
| Intercept | ||||||
| Intercept | 1.808 | 2.005 | 2.168 | |||
| Intercept | 1.808 | 0.109 | <.001 | |||
| Oral Contraceptives | −0.312 | 0.150 | .043 | |||
| Currently Depressed | 0.360 | 0.153 | .023 | |||
| At-Risk | 0.197 | 0.134 | .146 | |||
| Wake Time | ||||||
| Intercept | 0.085 | 0.048 | .080 | |||
| CAR | ||||||
| Intercept | 0.514 | 0.507 | 0.891 | |||
| Intercept | 0.514 | 0.125 | <.001 | |||
| Oral Contraceptives | −0.511 | 0.149 | .002 | |||
| Currently Depressed | 0.377 | 0.143 | .012 | |||
| At-Risk | −0.007 | 0.177 | .967 | |||
| Wake Time | ||||||
| Intercept | −0.173 | 0.048 | .001 | |||
| Time | ||||||
| Intercept | −0.085 | −0.098 | −0.084 | |||
| Intercept | −0.085 | 0.017 | <.001 | |||
| Oral Contraceptives | 0.012 | 0.021 | .576 | |||
| Currently Depressed | 0.001 | 0.024 | .966 | |||
| At-Risk | −0.013 | 0.018 | .485 | |||
| Wake Time | ||||||
| Intercept | −0.023 | 0.005 | <.001 | |||
| Time2 | ||||||
| Intercept | 0.003 | 0.003 | −0.008 | |||
| Intercept | 0.003 | 0.004 | .452 | |||
| Oral Contraceptives | 0.009 | 0.006 | .128 | |||
| Currently Depressed | −0.011 | 0.005 | .048 | |||
| At-Risk | <0.001 | 0.006 | .965 | |||
| Wake Time | ||||||
| Intercept | −0.003 | 0.002 | .068 | |||
| Variance Components | ||||||
| Intercept, r0 | 0.014 | .102 | ||||
| CAR, r1 | 0.005 | > .50 | ||||
| Time, r2 | 0.001 | .006 | ||||
| Level 1 | 0.258 | |||||
Note: The control group is the comparison group. Group means are calculated from estimates and do not include the influence of oral contraceptives.
The depressed group had a higher CAR than either the at-risk (χ2(1) = 4.48, p = .03) or control (b = .51, p = .01) groups, but there was no statistically significant difference between the at-risk or the control group (b = −.01, p = .97). Neither the depressed (b = .00, p = .97) nor the at-risk (b = −0.01, p = .49) group varied from the control group (b = −0.09, p < .01) with respect to rate of cortisol decline over the day, nor was there a statistically significant difference between the depressed and at-risk groups (χ2(1) = 0.36, p > .50). The quadratic time term was not significant for either the control (b = .00, p = .45) or at-risk (b = .00, p = .50) groups, but the depressed group’s rate of cortisol decrease accelerated over time (b = −.01, p < .05) relative to controls.
Trier Social Stress Test
In independent tests, activity level predicted cortisol change during the task phase (b = −.06, p = .01). Total chronic stress (b = −.001, p < .01), anxiety symptoms (b = −.01, p = .02), and early adversity (b = −.003, p = .02) were associated with steeper recovery slopes. Day of menstrual cycle was associated with a lower initial level of cortisol (b = −.03, p = .01). Each was thus included as a control variable in TSST analyses. Groups did not differ significantly on eating, drinking, activity or brushing of teeth in the hour prior to the TSST (all p’s > .05).
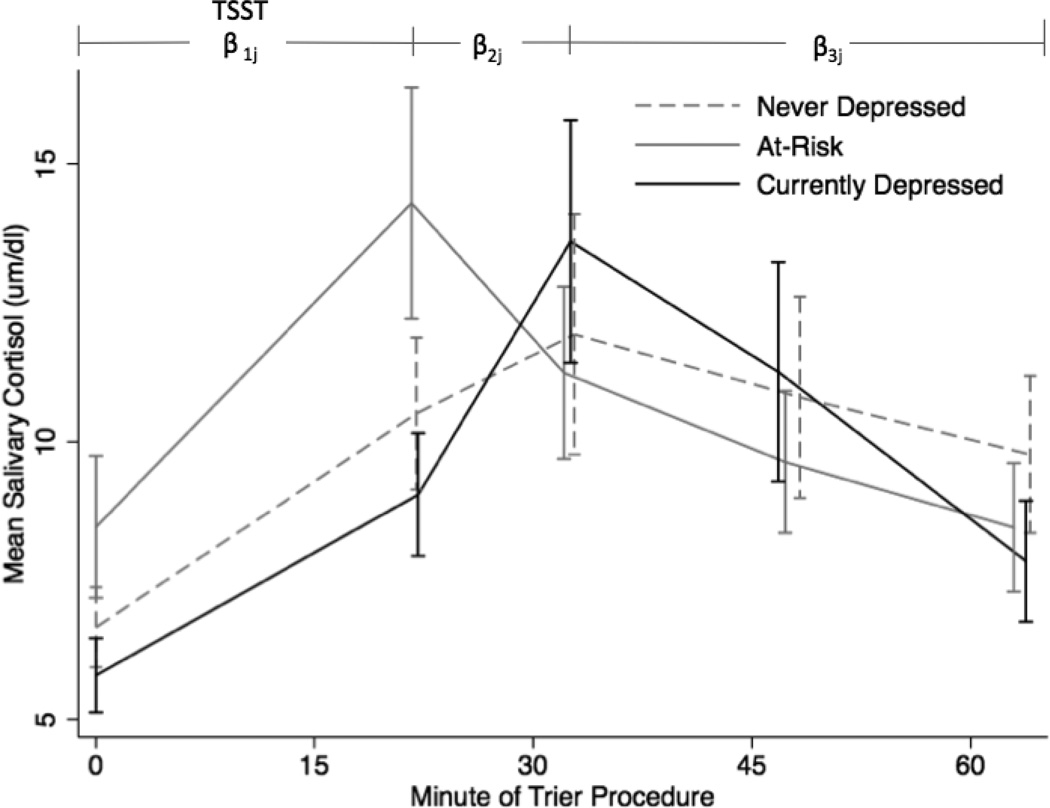
Group mean cortisol values from samples obtained during the TSST are illustrated in Figure 2. The results of the multilevel model are shown in Table 5. The depressed group had lower mean cortisol levels at baseline compared to the at-risk group (χ2(1) = 4.15, p = .04) and positive slope between samples 2 and 3, whereas the at-risk group had a negative slope (χ2(1) = 9.95, p < .01). The average rate of cortisol increase between the baseline and sample 3 (total reactivity to the task) also varied between the at-risk and depressed groups (χ2 (1) = 11.86, p < .01), with the depressed group having a higher net increase in cortisol secretion in response to the task. There were no group differences in the rate of decrease during the recovery period after sample 3 (all p’s > .05). Likewise, the control group was not significantly distinguishable from either the at-risk or depressed group on either mean levels of cortisol or trajectory of change (all p’s > .05).
Figure 2.
Mean cortisol secretion at each sampling point by group. TSST: Trier Social Stress Test, including the preparation, speech, and mental arithmetic.
Table 5.
Trier Social Stress Test model summary and estimated group means
| Estimates | Group Means | |||||
|---|---|---|---|---|---|---|
| b | SE | p | Control | At-Risk | Depressed | |
| Intercept, β0j | 2.421 | 2.716 | 2.358 | |||
| Intercept | 2.421 | 0.219 | 0.00 | |||
| Early Adversity | 0.130 | 0.051 | 0.02 | |||
| CSI | 0.003 | 0.027 | 0.92 | |||
| Anxiety Sx | −0.290 | 0.162 | 0.08 | |||
| Activity | −0.061 | 0.163 | 0.71 | |||
| In At-risk group | 0.296 | 0.181 | 0.11 | |||
| In CD Group | −0.063 | 0.189 | 0.74 | |||
| Day of cycle | −0.031 | 0.010 | 0.00 | |||
| Time 1 – Time 2 Slope, β1j | 0.008 | 0.008 | 0.017 | |||
| Intercept | 0.008 | 0.008 | 0.33 | |||
| Early Adversity | 0.001 | 0.002 | 0.70 | |||
| CSI | 0.000 | 0.001 | 0.84 | |||
| Anxiety Sx | −0.004 | 0.007 | 0.56 | |||
| Activity | 0.012 | 0.008 | 0.14 | |||
| In At-risk group | −0.001 | 0.008 | 0.93 | |||
| In CD Group | 0.009 | 0.008 | 0.27 | |||
| Day of cycle | 0.001 | 0.000 | 0.08 | |||
| Time 2 – Time 3 Slope, β2j | −0.005 | −0.012 | 0.035 | |||
| Intercept | −0.005 | 0.022 | 0.84 | |||
| Early Adversity | 0.005 | 0.005 | 0.29 | |||
| CSI | −0.003 | 0.002 | 0.11 | |||
| Anxiety Sx | 0.004 | 0.024 | 0.88 | |||
| Activity | −0.057 | 0.028 | 0.05 | |||
| In At-risk group | −0.007 | 0.023 | 0.75 | |||
| In CD Group | 0.040 | 0.025 | 0.12 | |||
| Day of cycle | 0.001 | 0.001 | 0.53 | |||
| Time 3 – Time 5 Slope, β3j | 0.006 | 0.003 | −0.004 | |||
| Intercept | 0.006 | 0.010 | 0.54 | |||
| Early Adversity | −0.003 | 0.002 | 0.14 | |||
| CSI | 0.000 | 0.001 | 0.71 | |||
| Anxiety Sx | −0.007 | 0.010 | 0.49 | |||
| Activity | 0.004 | 0.009 | 0.65 | |||
| In At-risk group | −0.002 | 0.009 | 0.79 | |||
| In CD Group | −0.010 | 0.010 | 0.29 | |||
| Day of cycle | −0.001 | 0.001 | 0.25 | |||
| Slope sample 1 to 3 (computed) | 0.004 | 0.002 | 0.023 | |||
| Variance Components | ||||||
| Intercept, r0 | 0.197 | <.001 | ||||
| Slope 1–2, r1 | <0.001 | .005 | ||||
| Slope 2–3, r2 | 0.002 | <.001 | ||||
| Slope 3–5, r3 | <0.001 | <.001 | ||||
| Level 1 | 0.063 | |||||
Note: The at-risk group is the comparison group. C = Control group. CD = Currently depressed group. Anxiety Sx = clinically significant anxiety symptoms (1 = present; 0 = absent). Task slope between measures 1 and 3 was calculated as mean of the slopes from samples 1 to 2 and 2 to 3, weighted by length of interval. Group means are calculated from estimates and do not include the influence of covariates.
Subjective Response to the Trier Social Stress Test
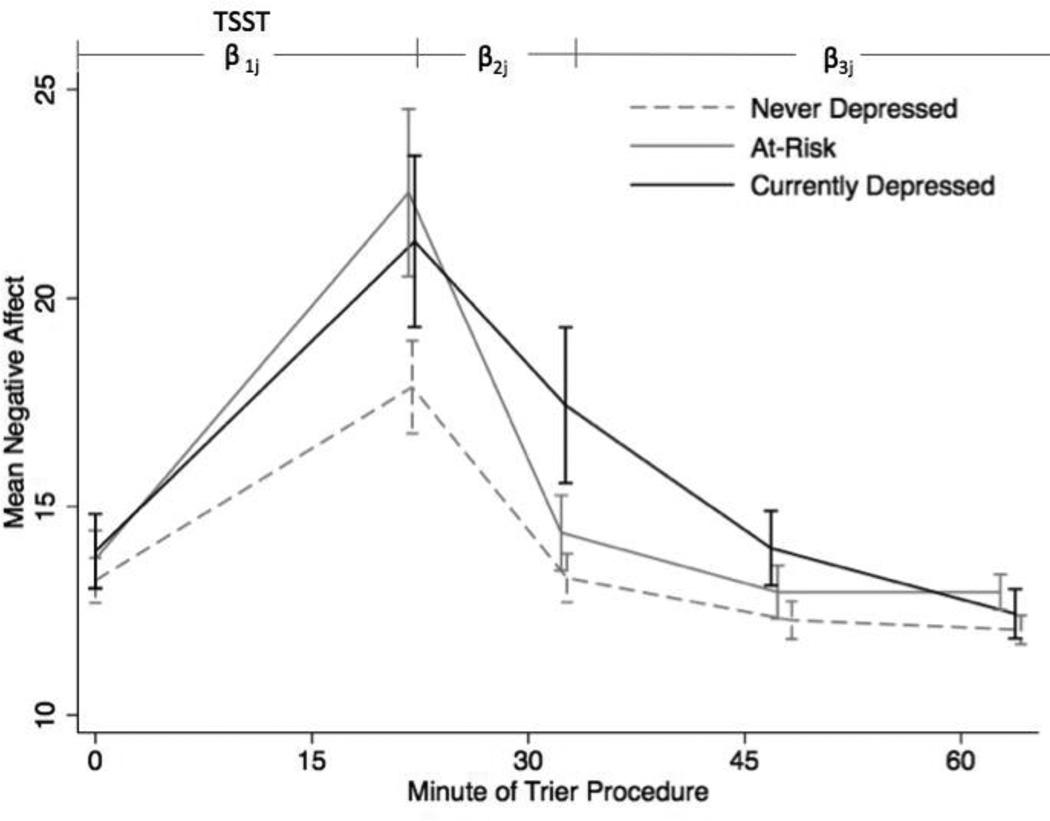
The three groups differed significantly on several self reported task responses to both the speech and math tasks (Table 2). The at-risk group generally reported a worse performance and more negative emotional experience than the control and depressed groups on the TSST. Self-reported negative affect based on the momentary mood form of the PANAS at each cortisol collection was subjected to a similar hierarchical analyses as was used for cortisol values, but without the biobehavioral control variables. Mean negative affect by group is displayed in Figure 3 and reported in Table 3. There were no differences among the groups at baseline (all p’s > .05), nor in the slope from baseline to the post-task collection point. However, there was a significant difference in how quickly the three groups’ negative affect levels fell between samples 2 and 3. The slope for the at-risk group (b = −.78) was significantly steeper than that of the depressed group (b = −.42; χ2(1) = 4.56, p = .03). Between samples 3 and 5, the at-risk group’s NA levels continued to fall at a rate comparable to the never depressed group (both b’s = −.05), but neither fell as quickly as those of the depressed group (b = −.16; vs. never depressed group: t(52) = 2.20, p = .03 ; vs. at-risk group: χ2(1) = 4.02, p = .04).
Figure 3.
Mean negative affect at each sampling point by group. TSST: Trier Social Stress Test, including the preparation, speech, and mental arithmetic.
Table 3.
Means and Standard Deviations Negative Affect Across the TSST by Group
| Control N = 21 |
At-Risk N = 20 |
Depressed N = 13 |
Total N = 54 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | M | SD | M | SD | F | p |
| Negative Affect | ||||||||||
| Baseline | 13.5 | 2.80 | 13.1 | 2.22 | 13.9 | 3.23 | 13.5 | 2.23 | 0.37 | .692 |
| Post Task | 18.6 | 6.27 | 21.9 | 8.05 | 21.4 | 7.69 | 20.5 | 7.34 | 1.16 | .320 |
| 10 min | 13.6a | 3.03 | 14.2a,b | 3.71 | 17.4b | 6.97 | 14.8 | 4.75 | 3.19 | .050 |
| 25 min | 12.5 | 2.38 | 12.7 | 2.56 | 14.0 | 3.35 | 13.0 | 2.74 | 1.43 | .250 |
| 40 min | 12.1 | 1.74 | 12.6 | 1.60 | 12.4 | 2.21 | 12.4 | 1.80 | 0.33 | .722 |
Note. Shared alphabetical subscripts represent means that are not significantly different from each other.
Discussion
The focus of the current study was to compare depressed, at-risk, and control participants’ patterns of cortisol secretion both in a naturalistic setting and in response to a laboratory stressor, the TSST. The differences among the three groups may indicate a distinct pattern of cortisol secretion for both depressed and at-risk participants that could shed light on a possible mechanism of risk for later depression onset. Compared to controls, the depressed group had a larger CAR and higher average cortisol across the day, although the slope of diurnal cortisol was not flatter than the slope for control participants as was predicted. Also, although depressed participants’ mean waking level of cortisol was higher than that of control participants, this comparison only approached significance. Thus, abnormalities in diurnal cortisol patterns of depressed individuals in the current community sample were generally in the same direction as those of depressed inpatients and melancholic individuals in previous studies (Carroll et al., 2007; Maes et al., 1994), though perhaps of lower magnitude. Similar to the depressed group, the at-risk participants had a higher waking cortisol secretion than control participants but, unlike the depressed group, they did not have a larger CAR, contrary to prediction. These findings suggest that diurnal cortisol secretion in at-risk individuals is similar to depressed individuals in direction, if not degree, of difference from controls. At-risk individuals’ patterns of diurnal cortisol secretion did not differ markedly from either group.
Findings comparing depressed versus control participants in this study are mainly consistent with research involving community samples. A large community study (Vreeberg et al., 2009) reported that depressed participants had higher morning and evening cortisol levels than controls. A larger CAR has also been associated with depression in an outpatient clinical sample (Bhagwagar et al., 2005) and a college community sample of young men (Pruessner et al., 2003). Other studies, however, have reported a smaller CAR and lower morning cortisol for depressed participants compared to control participants, or in some cases no differences (Huber et al., 2006; Stetler & Miller, 2005; Strickland et al., 2002). These mixed findings for depressed individuals are consistent with prior research reporting that both a larger and a flatter CAR have been associated with negative health outcomes, stress, and mood dysregulation (O’Connor et al., 2009, Adam & Kumari, 2009). Based on the current sample, it appears that it is not only severely depressed inpatients who have altered diurnal cortisol secretion, but also depressed individuals in the community who may not have sought treatment.
At-risk participants were predicted to have a pattern of diurnal HPA axis dysregulation similar to depressed participants, including greater waking cortisol and a larger CAR. Although the at-risk participants did not have a larger CAR than control participants, they did have significantly greater waking cortisol than controls. Cross-sectional research has found both a higher CAR and higher cortisol at waking for at-risk participants when compared to controls (Mannie et al., 20007; Halligan et al., 2004). Longitudinal studies have supported the predictive value of higher morning levels of cortisol for depression onset (e.g., cortisol measured at 8:00am regardless of waking time; Goodyer et al., 2000; Goodyer et al., 2009; Halligan et al., 2007; Harris et al., 2000), but whether waking cortisol predicts similar risk for depression onset is unclear. Adam and colleagues (2010) conducted the only prospective study measuring both waking cortisol and CAR in individuals at risk for depression. They reported that a larger CAR, not waking cortisol, prospectively predicted onset of depression. Thus, it is unclear to what extent the at-risk group’s higher waking cortisol levels are indicative of risk for depression.
Unexpectedly, depressed individuals did not differ from controls in cortisol reactivity to the TSST, but they did differ from at-risk participants. Depressed individuals had a significantly lower baseline cortisol level and steeper rise in cortisol secretion in response to the TSST than at-risk participants. It was predicted that the two groups would be similar in their patterns of cortisol reactivity to the TSST, so this finding was contrary to prediction. This difference in slope may be due to the early peak in cortisol secretion in response to the TSST for the at-risk group. Changes in cortisol take 10–20 minutes to show up in saliva, therefore the early peak for the at-risk group may mean that they are having a stronger response to the anticipation of stress than to the task itself. It is also possible that the at-risk group is particularly sensitive to the stressor, because of this early peak in cortisol secretion.
The concept of allostasis may be helpful in understanding this finding. Allostasis is “achieving stability through change” (McEwen & Wingfield, 2003), and accounts for the recovery to baseline following the high levels of cortisol secretion triggered by a stressful event. Quick response to a stressor followed by rapid recovery is healthy and vital to survival (McEwen & Seeman, 1999; Epel et al., 1998). At-risk participants may be sensitive to threat in the environment (definition of negative affect), but have not yet developed a disease (depression). Therefore, a quick response to the threat of entering a laboratory and preparing for a stressor, followed by a rapid shutdown of the stress response (see Figure 2), may indicate a resilient response to threat (Epel et al., 1998). However, this finding may also be indicative of sensitivity to threat in the form of a more rapid and strong emotional and biological response to a psychosocial laboratory stressor. In keeping with the sensitivity-to-threat hypothesis, the at-risk group had a more negative self reported performance on the TSST in comparison to both of the other groups including less eye contact, more vocal shaking, and increased embarrassment, and greater reported difficulty and tension when compared to the control group. At-risk individuals may have greater sensitivity to stress in the form of increased emotional and behavioral reactivity, which may be a mechanism of risk for later onset of depression. However, even though they reported a subjectively negative experience, they recovered from it rapidly based on reported negative mood. The rapid decrease in negative mood following the stressor (Figure 3) for the at-risk group compared to the depressed group may support the proposed “resilience” of the at-risk group in the face of a stressor, although their increased sensitivity to the stressor is also apparent.
Based on the meta-analysis conducted by Burke et al. (2005), it was predicted that depressed individuals would have a slower recovery following the TSST compared to controls, which was not upheld. Notably, only one study in that meta-analysis involved a laboratory public speaking task; the rest involved cognitive tasks or naturalistic stress. The divergent findings may therefore result from different task demands.
The current study is one of the first comparisons of depressed and at-risk participants on cortisol reactivity to the TSST. Young and Nolen-Hoeksema (2001) compared individuals high and low in rumination (a risk factor for depression) on the TSST and found no difference in cortisol secretion between the two groups. However, they did not include a depressed comparison group. Heim and colleagues (2002) did compare currently depressed participants with women with early adversity on the TSST and found no significant differences between the two groups. In the current study, depressed participants had lower baseline cortisol and a steeper cortisol rise in response to the task when compared to at-risk participants. Although individuals with high NA and low PA are believed to be vulnerable to depression onset following life stress (Clark et al., 1995), at-risk participants in the present study had not yet developed a major depressive episode. More research is needed to determine if this early response and rapid recovery is indeed a replicable difference between depressed individuals and those at risk for depression, and whether it is indicative of increased sensitivity to stress, resilience, or both.
Differentiation of at-risk and depressed participants based on mood reactivity to psychosocial stress and dysregulated cortisol secretion may allow for the identification of a group of stress sensitive individuals who, when confronted with life stress, are more likely to develop depression. Individuals who are sensitive to stress may be characterized by high levels of negative affect, increased mood reactivity to stress, and a distinct pattern of diurnal cortisol secretion. However, based on the results of this study, that pattern is not necessarily a heightened CAR, a high flat pattern of cortisol secretion, and increased cortisol secretion in response to a psychosocial laboratory stressor. Although depressed individuals differed significantly from healthy controls in both the CAR and average diurnal cortisol secretion, they did not differ significantly from the at-risk group. The difference observed between clinically depressed and at-risk participants was in their reactivity to a laboratory stressor. The at-risk group may have a strong, rapid biological and psychological response to stress that puts them at risk for later onset of mood disorders. Additionally, their elevated morning levels of cortisol in comparison to controls are in keeping with past studies that have found higher morning cortisol to be associated with risk for depression onset.
Stress sensitivity, and therefore risk, may involve greater cortisol response to daily stress, or psychosocial laboratory stressors than non-sensitive individuals, accompanied by a greater change in mood. If, over time, stress sensitive individuals encounter repeated stressors, and they are responding intensively both biologically and emotionally, this repeated stimulation could lead to later onset of depression through the mechanism of ever increasing biological and emotional sensitivity. Elevated diurnal cortisol secretion and cortisol secretion in response to a stressor have been associated with both greater self reported stress and negative mood in past research (Adam et al., 2006; van Eck et al., 1996; van Eck et al., 1996; Smyth et al., 1998; Polk et al., 2005), although both a larger and smaller CAR have been associated with self reported stress (O’Connor et al., 2009; Wust et al., 2000). In fact, two studies reported that negative affect mediated the relationship between daily stress and cortisol for both depressed and healthy individuals (Smyth et al., 1998; Peeters et al., 2003). Adams et al., (2006) hypothesized that individuals who have increased cortisol reactivity and mood reactivity to daily stress will eventually have greater basal cortisol dysregulation and likelihood of negative health outcomes, although longitudinal research is needed to test this proposed mechanism of risk. Therefore, increased mood reactivity to stress and increased cortisol secretion in response to daily stressors, or psychosocial laboratory stressors, may be a mechanism of risk for depression onset.
More research is needed at this point to determine the specific mechanisms involved in how reactivity of the HPA axis to acute distress eventually becomes the chronic HPA axis dysregulation seen for depressed individuals (Ehlert et al., 2001). Speculation about the meditational role of increased self-reported and biological sensitivity to stress is supported by the results of this study. It is important to note that at-risk individuals responded and recovered rapidly in their cortisol secretion in response to a psychosocial laboratory stressor, and they did not have a heightened CAR. Therefore, they do not yet have the level of dysregulation of the HPA axis that can be seen for depressed individuals in the current study. A daily diary study looking at naturalistic stress and cortisol secretion using ESM would help to determine if indeed cortisol reactivity is the main form of dysregulation that differentiates at-risk and depressed individuals.
The present study has several strengths. It study is the only comparison of depressed and at-risk individuals including both diurnal cortisol secretion and cortisol reactivity to a psychosocial laboratory stressor. Its daily cortisol sampling plan allowed explicit modeling of waking cortisol, CAR, average cortisol, and diurnal slope, as well as consideration of a large number of potentially confounding variables. Hierarchical linear modeling of both diurnal variation and TSST response was used, which better simultaneously models features of the cortisol response compared to traditional methods. Finally, both depressed and at-risk participants were included in this study, whereas most cortisol studies include one or the other of these groups.
The study also has several limitations. Notably, although significance testing generally agreed with visual inspection of the data, sample sizes at each level of analysis may have led to limited power. Additionally, this study was cross-sectional in nature and thus cannot address whether the at-risk participants would go on to develop depression. Other limitations of this study include the limited age range of the sample, the exclusion of males, and the lack of an extensive baseline waiting period at the start of the TSST. Finally, disparity in CAR findings may be due to methodological difficulties inherent in sampling cortisol immediately upon waking (O’Connor et al., 2009, Thorn et al., 2006). The sample size of the groups in the current study did not allow for assessing responders and non-responders as has been suggested in past research (Thorn et al., 2006). Larger groups should be included in future research so that responders and non-responders for both the CAR and the TSST can be examined separately.
In conclusion, the study clearly indicated a distinct pattern of cortisol secretion for a community sample of depressed individuals involving a larger CAR and higher average cortisol across the day. There was also support for the prediction that the at-risk group would have greater waking cortisol when compared to controls, although the magnitude of the CAR did not differ. By contrast, group responses to an environmental laboratory stressor were unexpected and suggest the need for further investigation in larger samples. It is possible that the at-risk group is currently subjectively sensitive on a behavioral and emotional level to a stressor, but recovers emotionally from that stressor on a rapid basis. This psychological response may be mirrored by a rapid rise in cortisol to anticipated stress, followed by an earlier recovery, perhaps indicating either a resilient or a stress-sensitive cortisol response when compared to depressed participants. Ultimately, it is hoped that further longitudinal investigation of HPA functioning in those believed to be at risk for depression may help to clarify the nature of risk for depression and suggest avenues for prevention.
Acknowledgments
This research was supported in part by a Sigma Xi Grant in Aid of Research, a Dissertation Fellowship, and an Excellence in Research Award from UCLA awarded to Kimberly A. Dienes. Support in manuscript preparation was provided to Nicholas A. Hazel by T32 MH015442.
Footnotes
This data was previously presented in Kimberly A. Dienes’ doctoral dissertation.
Contributor Information
Kimberly A. Dienes, Department of Psychology, Roosevelt University
Nicholas A. Hazel, Department of Psychiatry, University of Colorado Denver
Constance L. Hammen, Department of Psychology, University of California, Los Angeles
References
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large scale epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Bongard S, Buchanan TW, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–275. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene-environment interactions predict cortisol responses after acute stress: implications for the etiology of depression. Psychoneuroendocrinology. 2009;34:1294–1303. doi: 10.1016/j.psyneuen.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories–IA and –II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen PJ. Increased salivary cortisol after waking in depression. Psychopharmacology. 2005;182:54–57. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- Brandstadter J, Balter-Gotz B, Kirschbaum C, Hellhammer D. Developmental and personality correlates of adrenocortical activity as indexed by salivary cortisol: observation in the age range of 35 to 65 years. J. Psychosom. Res. 1991;35:173–185. doi: 10.1016/0022-3999(91)90072-v. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Depression. In: Brown GW, Harris TO, editors. Life Events and Illness. New York: Guilford Press; 1989. pp. 49–93. [Google Scholar]
- Buchanan TW, Bagley SL, Stansfield RB, Preston SD. The empathic, physiological resonance of stress. Social Neuroscience iFirst. 2011:1–11. doi: 10.1080/17470919.2011.588723. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Cassidy F, Naftolowitz D, Tatham NE, Wilson WH, Iranmanesh A, Veldhuis JD. Pathophysiology of hypercortisolism in depression. Acta Psychiatr. Scand. 2007;115:90–103. doi: 10.1111/j.1600-0447.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- Chen MC, Joormann J, Hallmayer J, Gotlib IH. Serotonin transporter polymorphism predicts waking cortisol in young girls. Psychoneuroendocrinology. 2009;34:681–686. doi: 10.1016/j.psyneuen.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. J. Abnorm. Psychol. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. J. Abnorm. Psychol. 1994;103:103–116. [PubMed] [Google Scholar]
- Clements AD, Parker CR. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Cohen AN, Hammen C, Henry RM, Daley SE. Effects of stress and social support on recurrence in bipolar disorder. J. of Affect. Dis. 2004;82:143–147. doi: 10.1016/j.jad.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Daley SE, Hammen C, Rao U. Predictors of first onset and recurrence of major depression in young women during the 5 years following high school graduation. J Abnorm Psychol. 2000;109:525–533. [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annu. Rev. Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Davila J, Hammen C, Burge D, Paley B, Daley SE. Poor interpersonal problem solving as a mechanism of stress generation in depression among adolescent women. J. Abnorm. Psychol. 1995;104:592–600. doi: 10.1037//0021-843x.104.4.592. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol—biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J. Steroid Biochem. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biol. Psychology. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Eppel ES, McEwen BS, Ickovics JR. Embodying psychological thriving: Physical thriving in response to stress. J. Soc. Issu. 1998;54:301–322. [Google Scholar]
- Erickson K, Drevets W, Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neurosci. Biobehav. Rev. 2003;27:233–246. doi: 10.1016/s0149-7634(03)00033-2. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, D.C.: American Psychiatric Press; 1995. [Google Scholar]
- Foley P, Kirschbaum C. Human hypothalamus–pituitary–adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci. Biobehav. Rev. 2010;35:91–96. doi: 10.1016/j.neubiorev.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Bacon A, Ban M, Croudace T, Herbert J. Serotonin transporter genotype, morning cortisol and subsequent depression in adolescents. Br. J. Psychiatry. 2009;195:39–45. doi: 10.1192/bjp.bp.108.054775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, Altham PME. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. Br. J. Psychiatry. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA-Axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol. Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson JG, Shea T, Skodol AE, McGlashan TH, Morey LC, Stout RL, et al. The collaborative longitudinal personality disorders study: Development, aims, design, and sample characteristics. J. Personal. Disord. 2000;14:300–315. doi: 10.1521/pedi.2000.14.4.300. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biol. Psychiatry. 2007;62:40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Hammen C, Kim EY, Eberhart NK, Brennan PA. Chronic and acute stress and the prediction of major depression in women. Depression and Anxiety. 2009;26:718–723. doi: 10.1002/da.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annu. Rev. Clin. Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hammen C, Adrian C, Gordon D, Burge D, Jaenicke C, Hiroto D. Children of depressed mothers: maternal strain and symptom predictors of dysfunction. J. Abnorm. Psychol. 1987;96:190–198. doi: 10.1037//0021-843x.96.3.190. [DOI] [PubMed] [Google Scholar]
- Harris TO, Borsanyi S, Messari S, Stanford K, Cleary SE, Shiers HM, Herbert J. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. Br. J. Psychiatry. 2000;177:505–510. doi: 10.1192/bjp.177.6.505. [DOI] [PubMed] [Google Scholar]
- Hauner KKY, Adam EK, Mineka S, Doane LD, DeSantis AS, Zinbarg R, Craske M, Griffith JW. Neuroticism and introversion are associated with salivary cortisol patterns in adolescents. Psychoneuroendocrinology. 2008;33:1344–1356. doi: 10.1016/j.psyneuen.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM, Klerman GL, Lavori P, Keller MB. Premorbid personality assessments of first onset of major depression. Arch. Gen. Psychiatry. 1989;46:345–350. doi: 10.1001/archpsyc.1989.01810040051008. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Huber TJ, Issa K, Schik G, Wolf OT. The cortisol awakening response is blunted in psychotherapy inpatients suffering from depression. Psychoneuroendocrinology. 2006;31:900–904. doi: 10.1016/j.psyneuen.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol. In: Fink G, editor. Encyclopedia of Stress. Vol. 3. San Diego: Academic Press; 2000. pp. 379–383. [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer DH. The 'Trier Social Stress Test': a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wüst S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom. Med. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Knorr U, Vinberg M, Kessing LV, Wetterslev J. Salivary cortisol in depressed patients versus control persons: a systematic review and meta-analysis. Psychoneuroendocrinology. 2010;35:1275–1286. doi: 10.1016/j.psyneuen.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C. Ten years of research with the Trier Social Stress Test-Revisited. In: Harmon-Jones E, Winkielman P, editors. Social Neuroscience: Integrating Psychological and Biological Explanations Of Social Behavior. New York: Guilford Press; 2007. pp. 56–83. [Google Scholar]
- Llabre MM, Spitzer SB, Saab PG, Scheiderman N. Piecewise latent growth curve modeling of systolic blood pressure reactivity and recovery from the cold pressor test. Psychophysiology. 2001;38:951–960. doi: 10.1111/1469-8986.3860951. [DOI] [PubMed] [Google Scholar]
- Maes M, Calabrese J, Meltzer HY. The relevance of the in- vs outpatient status for studies on HPA-axis in depression: spontaneous hypercortisolism is a feature of major depressed inpatients and not of major depression per se. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1994;18:503–517. doi: 10.1016/0278-5846(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Mannie ZN, Harmer CJ, Cowen PJ. Increased waking salivary cortisol levels in young people at familial risk of depression. Am. J. Psychiatry. 2007;164:617–621. doi: 10.1176/ajp.2007.164.4.617. [DOI] [PubMed] [Google Scholar]
- Mazure C. Life stressors as risk factors in depression. Clin. Psychol.: Sci. Pract. 1998;5:291–313. [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Ann. N Y Acad. Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis stress theories in the context of life stress research: Implications for the depressive disorders. Psychol. Bull. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- O’Connor DB, Hendrickx H, Dadd T, Elliman TD, Willis TA, Talbot D, Mayes AE, Thethi K, Powell J, Dye L. Cortisol awakening rise in middle-aged women in relation to psychological stress. Psychoneuroendocrinology. 2009;34:1486–1494. doi: 10.1016/j.psyneuen.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J. Levels and variability of daily life cortisol secretion in major depression. Psychiatry Res. 2004;126:1–13. doi: 10.1016/j.psychres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicholson NA, Berkhof J. Cortisol responses to daily events in major depressive disorder. Psychosom. Med. 2003;65(5):836–841. doi: 10.1097/01.psy.0000088594.17747.2e. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression: hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Polk DE, Cohen S, Doyle WJ, Skoner DP, Kirschbaum C. State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinology. 2005;30:261–272. doi: 10.1016/j.psyneuen.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Portella MJ, Harmer CJ, Flint J, Cowen P, Goodwin GM. Enhanced early morning salivary cortisol in neuroticism. The Am. J. Psychiatry. 2005;162:807–809. doi: 10.1176/appi.ajp.162.4.807. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosom. Med. 2003;65:92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon RT. Computer software manual. Chicago, IL: Scientific Software International; 2004. HLM 6: Hierarchical linear and nonlinear modeling. [Google Scholar]
- Schommer NC, Kudielka BM, Hellhammer DH, Kirschbaum C. No evidence for a close relationship between personality traits and circadian cortisol rhythm or a single cortisol stress response. Psychol. Rep. 1999;84:840–842. doi: 10.2466/pr0.1999.84.3.840. [DOI] [PubMed] [Google Scholar]
- Smyth J, Ockenfels MC, Porter L, Kirschbaum C, Hellhammer DH, Stone AA. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology. 1998;23:353–370. doi: 10.1016/s0306-4530(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Blunted cortisol response to awakening in mild to moderate depression: regulatory influences of sleep patterns and social contacts. J. Abnorm. Psychol. 2005;114:697–705. doi: 10.1037/0021-843X.114.4.697. [DOI] [PubMed] [Google Scholar]
- Strickland PL, Deakin JFW, Percival C, Dixon J, Gater RA, Goldberg DP. Bio-social origins of depression in the community: interactions between social adversity, cortisol and serotonin neurotransmission. Br. J. Psychiatry. 2002;180:168–173. doi: 10.1192/bjp.180.2.168. [DOI] [PubMed] [Google Scholar]
- Thorn L, Hucklebridge F, Evans P, Clow A. Suspected nonadherence and weekend versus week day differences in the awakening cortisol response. Psychoneuroendocrinology. 2006;31:1009–1018. doi: 10.1016/j.psyneuen.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Mello AF, Mello MF, Gagne GG, Grover KE, Anderson GM, Carpenter LL. Temperament and hypothalamic-pituitary-adrenal axis function in healthy adults. Psychoneuroendocrinology. 2006;31:1036–1045. doi: 10.1016/j.psyneuen.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- van Eck MM, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states and stressful daily events on salivary control. Psychosom. Med. 1996a;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- van Eck MM, Nicolson NA, Berkhof H, Sulon J. Individual differences in cortisol responses to a laboratory speech task and their relationship to responses to stressful daily events. Biol. Psychol. 1996b;43:69–84. doi: 10.1016/0301-0511(95)05159-7. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress. I. Preclinical studies. Depress. Anxiety. 2002;15:126–147. doi: 10.1002/da.10016. [DOI] [PubMed] [Google Scholar]
- Vinberg M, Bennike B, Kyvik KO, Andersen PK, Kessing LV. Salivary cortisol in unaffected twins discordant for affective disorder. Psychiatry Res. 2008;161:292–301. doi: 10.1016/j.psychres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJG, Pelt J, DeRijk RH, Verhagen JCM, van Dyck R, Penninx BWJH. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch. Gen. Psychiatry. 2009;66:617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. J. Abnorm. Psychol. 1988;97:346–353. doi: 10.1037//0021-843x.97.3.346. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32:358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharmacology. 2000;23:411–418. doi: 10.1016/S0893-133X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Young EA, Nolen-Hoeksema S. Effects of ruminations on the saliva cortisol response to a social stressor. Psychoneuroendocrinology. 2001;26:319–329. doi: 10.1016/s0306-4530(00)00059-7. [DOI] [PubMed] [Google Scholar]