Abstract
Background
Several studies have reported that metformin can reduce the risk of hepatocellular carcinoma (HCC) in diabetes patients. However, the direct anti-HCC effects of metformin have hardly been studied in patients, but have been extensively investigated in animal models of HCC. We therefore performed a systematic review and meta-analysis of animal studies evaluating the effects of metformin on HCC.
Methods
We collected the relevant studies by searching EMBASE, Medline (OvidSP), Web of Science, Scopus, PubMed Publisher, and Google Scholar. Studies were included according to the following inclusion criteria: HCC, animal study, and metformin intervention. Study quality was assessed using SYRCLE’s risk of bias tool. A meta-analysis was performed for the outcome measures: tumor growth (tumor volume, weight and size), tumor number and incidence.
Results
The search resulted in 573 references, of which 13 could be included in the review and 12 included in the meta-analysis. The study characteristics of the included studies varied considerably. Two studies used rats, while the others used mice. Only one study used female animals, nine used male, and three studies didn’t mention the gender of animals in their experiments. The quality of the included studies was low to moderate based on the assessment of their risk of bias. The meta-analysis showed that metformin significantly inhibited the growth of HCC tumour (SMD -2.20[-2.96,-1.43]; n=16), but no significant effect on the number of tumors (SMD-1.05[-2.13,0.03]; n=5) or the incidence of HCC was observed (RR 0.62[0.33,1.16]; n=6). To investigate the potential sources of significant heterogeneities found in outcome of tumor growth (I2=81%), subgroup analyses of scales of growth measures and of types of animal models used were performed.
Conclusion
Metformin appears to have a direct anti-HCC effect in animal models. Although the intrinsic limitations of animal studies, this systematic review could provide an important reference for future preclinical animal trials of good quality and clinical development.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer worldwide and the third leading cause of death from cancer. Surgical resection and liver transplantation are the only potentially curative treatment for a small proportion of the patients. However, disease recurrence hampers the ultimate success of the treatment [1]. Sorafenib, an oral multikinase inhibitor, was approved to treat advanced HCC. This however only increases patient survival with approximately 2–3 months [2,3]. Therefore, it is necessary to explore new strategies to improve the management of HCC.
Metformin is an oral drug widely used for treatment of type II diabetes. Interestingly, several studies, including observational studies and some randomized controlled trials (RCT), have reported that metformin can affect the risk of hepatocellular carcinoma (HCC) in diabetic patients [4–6]. Although these studies have suggested a preventive effect of metformin on the risk of HCC in these diabetic patients, there is still lacking of investigation whether metformin has direct anti-tumor effect in HCC patients. Nevertheless, substantial research has been performed in animal models of HCC, although the data are still inconclusive. Meta-analyses on data from animal studies can be used to explain clinical observation and to inform clinical trial design.
To better understand the direct effects of metformin on HCC and to pave the way for further prospective clinical study, we performed a systematic review and meta-analysis of currently available data from HCC animal models treated with metformin.
Materials and Methods
Review protocol
A protocol for this systematic review was prepared using SYRCLE’s protocol format (https://www.radboudumc.nl/Research/Organisationofresearch/Departments/cdl/SYRCLE/Pages/Protocols.aspx) [7].
Literature search
A systematic search (conducted on July 2014, without any restrictions on publication data or language) was conducted in Medline (OvidSP), Embase.com, Web of Science, and Scopus. Additional referenes were retrieved from Google Scholar, and unindexed references from PubMed. The searches were designed and executed by an experience information specialist (WB). The search strategy consisted of two main components: hepatocellular carcinoma, and metformin, and results were limited to animal studies. For each element multiple synonyms were searched in title and/or abstract, and when available thesaurus terms (Mesh for medline and Emtree for embase). The full strategy is available in S1 Table.
Study selection and inclusion criteria
The selection procedure was performed by two independent reviewers (J.L. and P.H.). The exclusion criteria for the title and abstract screening phase include: 1). not primary study; 2). not animal study; 3). not disease of interest (HCC), 4). not intervention of interest (metformin). The following additional criteria were used for full-text screening: 1). full-text not available; 2). double publication; 3). conference abstracts. In case of disagreement between the reviewers, consensus was reached.
Study characteristics and data extraction
Data was extracted from the full-text papers of the studies. The following items were extracted: author, year, language, species/strain, description of control group, animal gender, age and weight, number of animals in control and experimental group, the method for the establishment of the animal models, metformin dosage, timing, duration and route of metformin administration, and outcome measures (Table 1).
Table 1. Characteristics of the included animal studies.
| Study | Language | strain/Species | Experimental group | control group | Gender | Age | Weight | animal number: c/exp | Type of animal model | HCC Number/ animal | Dosage | Timing of metformin | Duration of metformin | Adminstration Route | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen 2013[9] | English | BALB/c nude mice | HCC+MET | HCC | F | 5–6 week | * | 5/5. | subcutaneous xenograft | 1/1 | 200 mg/ml in drinking water | 10 days after implanation | 30 days | orally | TW |
| Qu 2012[10] | English | BALB/c nude mice | HCC+MET | HCC+saline | M | 3–4 week | 14.0–16.0 g | 12/12; 12/12 | orthotopic xenograft | 1/1 | 125& 250 mg/kg /day | 10 days after implanation | 30 days | i.p. | TV |
| Miyoshi 2014[11] | English | BALB/c-nu/nu mice | HCC+MET | HCC+PBS | M | 8 weeks | 20–25 g | 10/10; 10/10 | subcutaenous xenograft | 1/1 | 1& 2 mg/body/day | after an identifiable mass > 6 mm | 14 days | i.p. | TV; cell cycle regulators; angiogenesis |
| Bhalla 2012[12] | English | C57BL/6J mice | HCC+MET | HCC | M | 2 week | * | 7/7. | DEN | 1/1 | 250mg/kg/day | 2 weeks after DEN | 168 days; 252 days | orally | TS, TN; AMPK activation |
| Kim 2013[13] | English | HBxTg mice | HCC+MET | HBx Tg mice | M | * | * | 20/26 | HBx Transgenic | 1/1 | 250mg/kg/day | 6 weeks of age | 462 days | orally | TN; Hepatic CRBP-1 protein level, Akt |
| DePeralta 2013**[14] | English | Rat (Wistar) | HCC+MET | DEN induction | M | 0 week | * | 9/9. | DEN | 1/1 | 250 mg/kg/day | 8 weeks of age; 12 weeks of age | 70 days; 42 days | orally | TI |
| Cai 2013[15] | English | BALB/c-nu mice | HCC+MET | HCC+PBS | M | 6–8 week | * | 10/10. | subcutaenous xenograft | 4/1 | 250 mg/kg/day | 1 week after transplantation | 49 days | i.p. | TV; cell cycle regulators; p-AMPK |
| Saito 2013[16] | English | NOD/SCID mice | HCC+MET | HCC | * | * | * | 5/5. | subcutaenous xenograft | 1/1 | 250 mg/kg/day | just after the transplantation | 56 days | i.p. | TV; ki-67, casp3. |
| Afzal 2012[21] | English | Wistar albino rat | MET+DENA; DENA+MET | DENA induction | M | Adult | 100–125g | 6/6. | DENA | 1/1 | 125mg/kg/day | Day 1; Day 7. | * | i.p. | animal weight, SGPT/ALT, SGOT/AST |
| Tajima 2013[17] | English | C57B1/6 mice | non-NAFLD+MET; NAFLD+MET | HFD-HFD | M | 8 week | * | 6/4; 17/16;4/7;4/8 | HFD | 1/1 | 250 mg/kg/day | 30 weeks after HFD | 210 days | orally | TS, TN; AMPK/mTOR/S6k |
| Cheng 2014[18] | English | BALB/c-nu mice | HCC+MET | HCC | M | 5 week | * | 28/10 # | subcutaenous xenograft | 4/1 | 30 Ag/g body weight | 1 week after transplantation | 49 days | i.p. | TW, TI; activity of AMPK; Ki-67 |
| Zheng 2013[19] | English | BALB/C nude mice | HCC+MET | HCC+vehicle | * | * | * | 8/8; 8/8. | subcutaenous xenograft § | 1/1 | * | * | 49–56 days | * | TV; AMPK activation |
| Xiong 2012[20] | English | BALB/c nude mice | HCC+MET | HCC+PBS | * | * | size of ~100mm3 | 5/5. | subcutaenous xenograft | 1/1 | 40 & 200mg/kg/day | 7 days after transplantation | 126 days | * | TV, TW |
* = not mentioned;
** = only abstract available;
# = indicate tumor number;
§ = include two different xenograft model (HCC-LM3 and SMMC7721); MET = metformin; DEN = diethylnitrosamine (liver-specific carcinogen); FBS = fasting blood glucose; HFD = high-fat diet; TV = tumor volume; TW = tumor weight; TS = tumor size; TI = tumor incidence; TN = tumor number.
The outcome measures including HCC growth, number and incidence were included in the meta-analysis. Mean value, standard deviation (SD) and the number of animals per group were extracted. If relevant data were not available in the text but only presented in graphic form, obtaining the data by measuring the graphs using Universal Desktop Ruler (Universal On-screen Digitizer, AVPSoft.).
Quality assessment of included studies
The SYRCLE’s Risk of Bias tool was used to assess the risk of bias of all included studies [8]. Two independent investigators (J.L. & P.H.) performed quality assessment of all included studies. Disagreements were resolved by discussion.
Data synthesis and statistical analysis
For the outcome measures of HCC growth and number, the standardized mean difference (SMD) was used as the effect measure. For the outcome measure of HCC incidence, Risk Ratio (RR) was used.
If studies contained multiple independent groups (e.g. different animal models or different time points), they were treated as separate experiments.
Because of expected heterogeneity, the statistical model of analysis used in this meta-analysis was a random effects model. I2 was used as a measure of heterogeneity. In order to explore potential causes of heterogeneity, predefined subgroup analyses were conducted for tumor volume, weight and size.
With assistance of RevMan5.3 (Cochrane Library) software, Forest Plots were established. In addition, sensitivity analyses were conducted as to evaluate whether the findings were robust enough to the decisions made. Visual inspection of funnel plots was used to detect publication bias. Our procedures accorded with PRISMA guidelines for reporting systematic review/meta-analysis (S2 Table).
Results
Description of the included studies
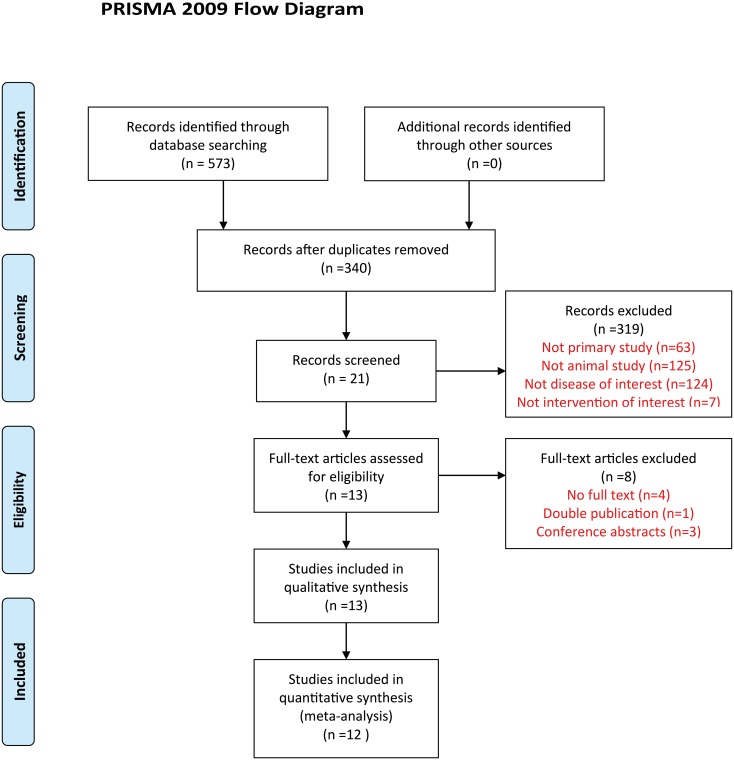
The comprehensive search strategy on the effects of metformin on HCC in animal models resulted in 573 records. After duplicates were removed, 340 studies were left. After title and abstract screening, 21 studies were screened full text. Ultimately, 13 studies were included in our systematic review [9–21], of which 12 studies (total 29 animal experiments and 311 animals involved) could be included in the meta-analysis (fig 1) [9–20].
Fig 1. Flow diagram showing literature search and selection results.
The characteristics of all included studies are described in Table 1. Since the investigation of metformin on HCC using animal models has only been started in recent years, the publication dates of the included studies ranged from 2012 to 2014. Apart from these, the characteristics among these studies varied considerably. The characteristics of animals themselves differed substantially between the studies. Seven of the studies used BALB/c nude mice, and others used C57BL/6J mice, NOD/SCID mice, HBxTg mice, and Wistar rat. Among these studies, seven used a subcutaneous xenograft model, while others used oncogenic compound inducing models. Only half of the studies used the same dosage of metformin (250 mg/kg). Administration timing and duration of metformin varied greatly. Besides, various time-points for outcome measurements were mentioned in the studies.
Risk of bias and quality of included studies
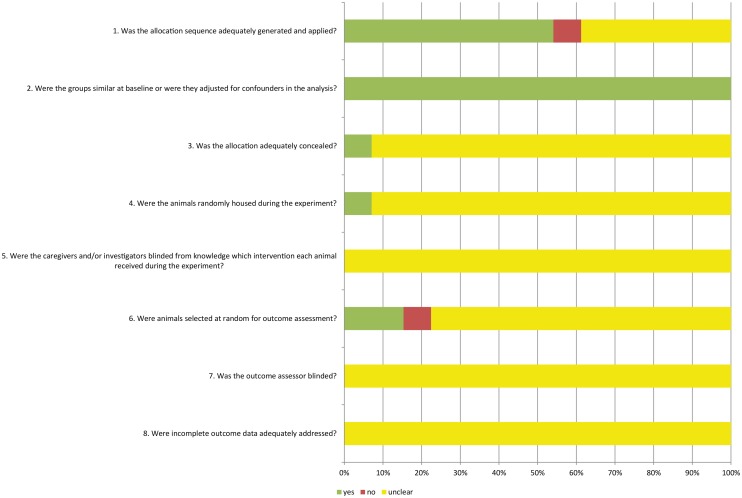
Fig 2 shows the results of the risk of bias assessment of the 13 studies included in this systematic review. Based on this assessment, 7 (54%) of the studies stated that the allocation was randomized. Since the background of animals were essentially homogenous, most of the studies didn’t describe the method of randomization. Besides, none of the studies mentioned whether the allocation was adequately concealed. As shown clearly in Fig 2, many items were scored as “unclear”, which indicates that reporting—and presumably experimental design—of these animal studies can be improved.
Fig 2. Risk of bias, score (%) per risk of bias item.
Yes = low risk of bias, no = high risk bias,? = unclear risk of bias.
Overall analysis of the effects of metformin on HCC growth
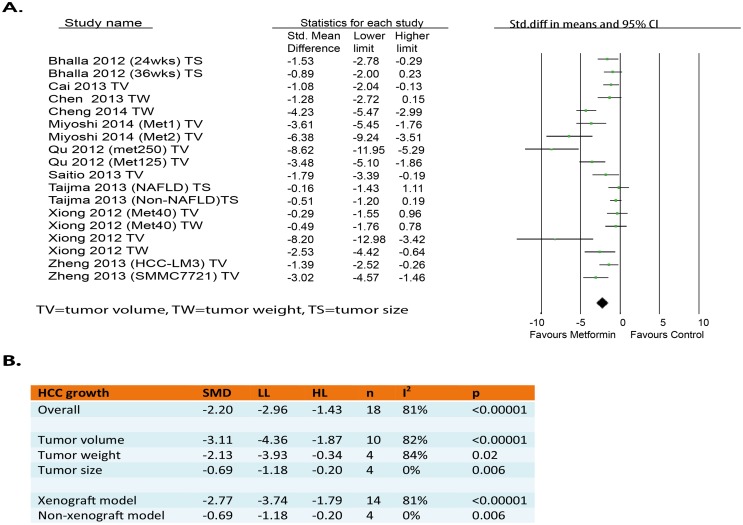
Ten out of the twelve studies reported outcomes related to tumor growth (tumor volume, tumor size or tumor weight). These 10 studies contained 18 independent experiments [9–12,15–20]. Of these 18 experiments, 12 showed a significant decrease of tumor growth. None of the experiments showed a significant increase of tumor growth. Meta-analysis of these experiments revealed that metformin intervention had a significant inhibiting effect on HCC growth (SMD-2.20[-2.96, -1.43]; n = 18) (Fig 3A). However, heterogeneity was quite high (I2 = 81%).
Fig 3. Effects of metformin on tumor growth in HCC animal models.
(A) Forest plot and (B) subgroup analysis of the 16 included studies. The forest plot displays the SMD, confidence interval, and effect weight for each study, plus the pooled effect estimate & confidence interval.
Subgroup analysis
To determine whether the effects differed per scale of measurement, the clinically relevant outcome measures including “tumor volume”, “tumor weight” and “tumor size” were analyzed separately in subgroups. As displayed in Fig 3B, even though all three subgroups showed a statistically significant inhibition of growth, the effect on volume (SMD-3.11[-4.36, -1.87]; n = 10) seemed to be larger than on size (SMD-0.69[-1.18, -0.20]; n = 4). Besides, heterogeneity levels significantly decreased in the subgroup analysis of “tumor size”(I2 = 0.0%) while still high heterogeneity level were observed in subgroups of “tumor volume”(I2 = 82%) and “tumor weight”(I2 = 84%).
In addition, a subgroup analysis was performed for the types of HCC model used. This analysis demonstrated that metformin had significant effect on both xenograft (SMD-2.77[-3.74, -1.79]; n = 14) and non-xenograft model (SMD-0.69[-1.18, -0.20]; n = 4), but the former group seemed to be affected by metformin more than the latter group. Subgroup analysis of “non-xenograft model” clearly reduced heterogeneity (I2 = 0.0%), while “xenograft model” subgroup analysis did not change high heterogeneity level.
Effects of metformin on HCC number
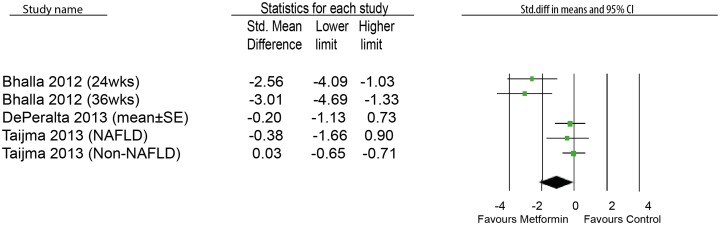
In addition to the analysis of the effect on tumor growth, we also did analysis on HCC number in five animal experiments. Of these five experiments, four showed a significant decrease of HCC number and none showed a significant increase. Although it was unclear how the data were presented in DePeralta’s study [14], we assumed they were presented as mean ± SE. The result didn’t show significant inhibitory effect on HCC tumor number by metformin (SMD-1.05[-2.13,0.03]; n = 5) (Fig 4). We found high heterogeneity (I2 = 78%).
Fig 4. Effects of metformin on tumor number in HCC animal models.
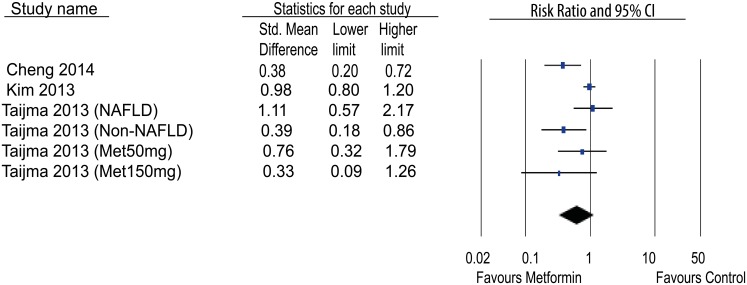
Effects of metformin on HCC incidence
The effects of metformin on the incidence of HCC in animal models was evaluated. Only two studies showed a significant decrease of HCC incidence; whereas the others showed trend of decrease but one showed a trend of increase. However, the meta-analysis didn’t show a significant effect of metformin on the incidence of HCC in comparison with non-treatment group (RR 0.62[0.33,1.16]; n = 6) (Fig 5). The heterogeneity was high (I2 = 83%).
Fig 5. Effects of metformin on tumor incidence in HCC animal models.
Sensitivity analysis
We performed sensitivity analysis to assess the robustness of our results on the effects of metformin on HCC number. In the subgroup analysis of “tumor number”, we assumed that DePeralta[14]presented the data as mean ± SE. For this sensitivity analysis we test the assumption that the data were presented as mean ± SD. With this assumption the meta-analysis showed that metformin could significantly reduce HCC tumor number (SMD-1.21[-2.29, -0.13];n = 5). This result differs from our previous findings in the subgroup analysis. Interpretation of this outcome measure should be done with extreme caution, as current available evidence is still inconclusive.
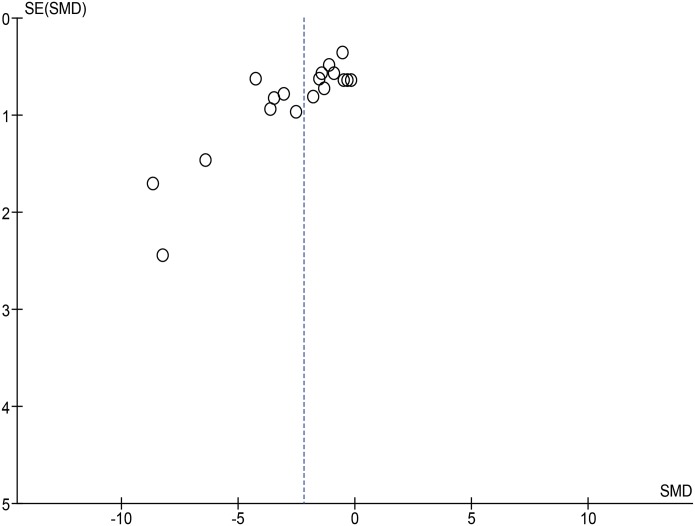
Publication bias
Publication bias was assessed for the outcome of overall tumor growth, since the analysis of this outcome included the highest number of studies. On visual inspection of the funnel plot (Fig 6), small studies with no or a negative effect seem to be missing. This asymmetry might indicate the presence of publication bias.
Fig 6. Funnel plot overseeing publication bias of included studies.
Discussion
Although there were already several clinical studies evaluating the effects of metformin on HCC risk in human population, all of them were on the chemo-preventive effect by metformin rather than the therapeutic effect and, moreover, the population were all diabetic patients. A meta-analysis of the effects of anti-diabetic medications on the HCC risk, in which both observational and RCT studies were included, has suggested a half reduction in HCC incidence when using metformin treatment [22]. However, our meta-analysis systematically analyzed all relevant animal studies to assess the therapeutic potential of metformin against HCC.
In this comprehensive systematic review and meta-analysis, we analyzed and described the effects of metformin on HCC growth and incidence. The overall analysis of the effect of growth showed that use of metformin was associated with a significant inhibitory effect (SMD = -2.20±0.76) on HCC growth, compared to untreated group. This therapeutic effect remained stable across subgroups of tumor volume, tumor weight and tumor size. It was most pronounced in “tumor volume” measurement while least in “tumor size” measurement. Besides, we performed subgroup analysis under the “tumor growth”. Both xenograft and non-xenograft studies showed significant inhibitory effect of metformin on HCC growth.
Mechanism underlying metformin anti-HCC
The anti-cancer effect of metformin was speculated to be associated with the activation of adenosine monophosphate-activated protein kinase (AMPK). An important upstream kinase of AMPK is LKB1, an very important tumor suppressor [23,24]. This signaling pathway was also discussed and explored in the included HCC animal trials. The coexistence of AMPK-dependent and AMPK-independent mechanisms for the effects of metformin on cancer was proposed[9,10,12,15]. But we should keep in mind of different study methodologies. For instance, two studies used cell lines in vitro[9,15]; one study used tumor harvested from xenograft[10]; whereas one study demonstrated their result based on observing AMPK level in liver[12]. In addition, metformin may also inhibit HCC cell growth by regulating cell-cycle regulatory proteins, such as cyclin D1 and cyclin E [10,14]. Of particular note, c-myc was suggested as a critical mediator in hepatocarcinogenesis [25]. Metformin treatment has been shown to inhibit c-myc expression by up-regulating let-7 family (tumor suppressor) [11]. However, no study among the included animal studies discussed such mechanism underlying specific HCC stage. Although we found one human study relevant to early stage of HCC, no clear mechanistic insight of metformin on early stage HCC was described[26].
Side effects of metformin in animal models
Being a classic antidiabetic medication, metformin is widely used among patients because of its relatively low cost and high safety profile. 10 out of 13 studies included in this systematic review mentioned tolerability of metformin treatment. These studies consistently showed that metformin didn’t change body weight and serum glucose level of animals. However, it should also be taken into consideration of further investigation on the appropriate therapeutic dosage rang of metformin for anti-cancer treatment. Evaluation of safety with these particular dosages is very necessary, especially when metformin is applied in non-diabetic patients.
Limitations
By using the risk of bias tool, we found out that reporting is poor and therefore the methodological quality of many studies is unclear. It shows that there is much room for improvement, since many items were shown “unclear” and only a few items were shown low risk only in very few studies. Besides, by visualizing the funnel plot, publication bias seems also to be present, probably due to the missing of studies with no or negative effects. Actually, in total, only 12 studies were included in the meta-analysis. Both the unclear methodological quality and publication bias might lead to under or overestimation of the overall effect size of metformin effect on anti-HCC, which is an additional threat to the robustness of the data especially the ones that are already inconclusive. What’s more, high heterogeneity was also seen among the studies, which is common in animal studies, although we tried to explore the potential factors contributing the heterogeneity. All of these potential limitations might influence us to draw concrete conclusions on the anti-HCC effect of metformin.
Besides, there were some methodological issues which might influence the translation of animal results to human trials. Firstly, the literature is unclear about which animal method is most representative for patient HCC. We found various HCC methods used in these studies: xenograft, DEN-induction, transgenic and dietary models. Secondly, there are two different administration routes (oral or i.p.) of metformin in the studies. However, metformin is usually an orally administrated drug in clinic, raising the question whether administration method could also affect the effect of metformin on tumor.
HCC invasion and metastasis are crucial factors related to poor prognosis. However, none of the animal studies described if metformin had potential effect on HCC metastasis, except one study indirectly mentioned correlation of metformin/p-AMPK with distant metastasis in human HCC cohort study [19].
Another limitation is that these animal studies did not study the HCC stage indicated for metformin. HCC stage could be an important factor for the therapeutic efficacy of metformin and has implication for selecting appropriate candidates for metformin treatment. Therefore, it’s very necessary to take the stage of HCC into account in the experimental design. As for xenograft animal model, which were used in most of the studies, however, it’s hard to define the cancer stage which didn’t discuss in the studies. In addition, although it’s possible to identify tumor stage in spontaneous animal tumor model, the authors did not report any information about HCC stage in their animal study[12,17].
Implications for practice
Based on the results of this meta-analysis, metformin could potentially have a therapeutic effect on HCC. Besides, the maximal dose of metformin used in all the included animal studies were consistent with human therapeutic dose in diabetics according to the calculation [10]. This furthermore supported the reliable and applicable results from animal models. Although several clinical studies reported that metformin could negatively modify the risk of HCC in diabetic patients [4–6], there are not yet any clinical trial investigating the therapeutic effect of metformin on HCC. Currently, several ongoing clinical trials (www.clinicaltrials.gov) are evaluating the effects of metformin on different cancers (breast cancer, colorectal cancer, pancreatic cancer, etc.), But not for HCC. Thus, the results of this systematic review provide an important reference for the future preclinical animal trials with high quality to aid the development of metformin for anti-HCC treatment in clinical trials. Considering the dominant risk factor for HCC in many western countries in particular United States--obesity and metabolic syndrome, metformin would be an appropriate choice for such a high-risk of population of HCC.
Conclusion
In summary, this systematic review of animal studies suggests that metformin potentially has a direct inhibitory effect on HCC growth, although the effects on tumor number and incidence are inconclusive. It supports the clinical observation that metformin is associated with lower risk of HCC in diabetic patients. Although these animal studies have some intrinsic limitations, these results do provide an important reference for future high-quality preclinical animal trials and potential clinical development.
Supporting Information
(DOCX)
(DOC)
Acknowledgments
We thank Dr. Rob BM de Vries for critically revising the manuscript. We also thank some authors of the referred articles for providing additional information of their animal experiments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Daniel den Hoed Foundation for a Centennial Award fellowship and the Netherlands Organization for Scientific Research (NWO/ZonMw) for a VENI grant (No. 916-13-032) (to Q. Pan), and the China Scholarship Council for funding PhD fellowship to J. Li (No. 201307720054). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chen K, Man K, Metselaar HJ, Janssen HL, Peppelenbosch MP, Pan Q (2014) Rationale of personalized immunosuppressive medication for hepatocellular carcinoma patients after liver transplantation. Liver Transpl 20: 261–269. 10.1002/lt.23806 [DOI] [PubMed] [Google Scholar]
- 2. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10: 25–34. 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 3. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378–390. 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 4. Oliveria SA, Koro CE, Ulcickas Yood M, Sowell M (2008) Cancer incidence among patients treated with antidiabetic pharmacotherapy. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2: 47–57. [Google Scholar]
- 5. Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, Viberti G, et al. (2010) Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 53: 1838–1845. 10.1007/s00125-010-1804-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang H, Gao C, Fang L, Zhao HC, Yao SK (2013) Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scand J Gastroenterol 48: 78–87. 10.3109/00365521.2012.719926 [DOI] [PubMed] [Google Scholar]
- 7. de Vries RBM, Hooijmans CR, Langendam MW, van Luijk J, Leenaars M, Ritskes-Hoitinga M, et al. (2015) A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evidence-based Preclinical Medicine 2: 1–9. [Google Scholar]
- 8. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW (2014) SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol 14: 43 10.1186/1471-2288-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. (2013) Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut 62: 606–615. 10.1136/gutjnl-2011-301708 [DOI] [PubMed] [Google Scholar]
- 10. Qu Z, Zhang Y, Liao M, Chen Y, Zhao J, Pan Y (2012) In vitro and in vivo antitumoral action of metformin on hepatocellular carcinoma. Hepatol Res 42: 922–933. 10.1111/j.1872-034X.2012.01007.x [DOI] [PubMed] [Google Scholar]
- 11. Miyoshi H, Kato K, Iwama H, Maeda E, Sakamoto T, Fujita K, et al. (2014) Effect of the anti-diabetic drug metformin in hepatocellular carcinoma in vitro and in vivo. Int J Oncol 45: 322–332. 10.3892/ijo.2014.2419 [DOI] [PubMed] [Google Scholar]
- 12. Bhalla K, Hwang BJ, Dewi RE, Twaddel W, Goloubeva OG, Wong KK, et al. (2012) Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev Res (Phila) 5: 544–552. 10.1158/1940-6207.CAPR-11-0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim JH, Alam MM, Park DB, Cho M, Lee SH, Jeon YJ, et al. (2013) The Effect of Metformin Treatment on CRBP-I Level and Cancer Development in the Liver of HBx Transgenic Mice. Korean J Physiol Pharmacol 17: 455–461. 10.4196/kjpp.2013.17.5.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DePeralta DK, Wei L, Lauwers GY, Fuchs BC, Tanabe KK (2014) Metformin Inhibits Hepatocellular Carcinoma in a Cirrhosis Model. Journal of Surgical Research 186: 633. [Google Scholar]
- 15. Cai X, Hu X, Cai B, Wang Q, Li Y, Tan X, et al. (2013) Metformin suppresses hepatocellular carcinoma cell growth through induction of cell cycle G1/G0 phase arrest and p21CIP and p27KIP expression and downregulation of cyclin D1 in vitro and in vivo. Oncol Rep 30: 2449–2457. 10.3892/or.2013.2718 [DOI] [PubMed] [Google Scholar]
- 16. Saito T, Chiba T, Yuki K, Zen Y, Oshima M, Koide S, et al. (2013) Metformin, a diabetes drug, eliminates tumor-initiating hepatocellular carcinoma cells. PLoS One 8: e70010 10.1371/journal.pone.0070010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tajima K, Nakamura A, Shirakawa J, Togashi Y, Orime K, Sato K, et al. (2013) Metformin prevents liver tumorigenesis induced by high-fat diet in C57Bl/6 mice. Am J Physiol Endocrinol Metab 305: E987–998. 10.1152/ajpendo.00133.2013 [DOI] [PubMed] [Google Scholar]
- 18. Cheng J, Huang T, Li Y, Guo Y, Zhu Y, Wang Q, et al. (2014) AMP-activated protein kinase suppresses the in vitro and in vivo proliferation of hepatocellular carcinoma. PLoS One 9: e93256 10.1371/journal.pone.0093256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng L, Yang W, Wu F, Wang C, Yu L, Tang L, et al. (2013) Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin Cancer Res 19: 5372–5380. 10.1158/1078-0432.CCR-13-0203 [DOI] [PubMed] [Google Scholar]
- 20. Xiong Y, Lu QJ, Zhao J, Wu GY (2012) Metformin inhibits growth of hepatocellular carcinoma cells by inducing apoptosis via mitochondrion-mediated pathway. Asian Pac J Cancer Prev 13: 3275–3279. [DOI] [PubMed] [Google Scholar]
- 21. Afzal M, Kazmi I, Gupta G, Rahman M, Kimothi V, Anwar F (2012) Preventive effect of Metformin against N-nitrosodiethylamine-initiated hepatocellular carcinoma in rats. Saudi Pharm J 20: 365–370. 10.1016/j.jsps.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh S, Singh PP, Singh AG, Murad MH, Sanchez W (2013) Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol 108: 881–891; quiz 892. 10.1038/ajg.2013.5 [DOI] [PubMed] [Google Scholar]
- 23. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, et al. (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A 101: 3329–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blandino G, Valerio M, Cioce M, Mori F, Casadei L, Pulito C, et al. (2012) Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat Commun 3: 865 10.1038/ncomms1859 [DOI] [PubMed] [Google Scholar]
- 26. Chen TM, Lin CC, Huang PT, Wen CF (2011) Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol 26: 858–865. 10.1111/j.1440-1746.2011.06664.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.